Translate this page into:
Hot methanol extract of Leea macrophylla (Roxb.) manages chemical-induced inflammation in rodent model
⁎Corresponding author at: Department of Biochemistry & Molecular Biology, University of Chittagong, Chittagong 4331, Bangladesh. atiar@cu.ac.bd (Md. Atiar Rahman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
This study investigated how a chemical-induced analgesia and inflammation could be managed by hot methanol extract of Leea macrophylla (Roxb.) root (LM).
Methods
Nature of secondary metabolites and the phytochemicals was studied by the established qualitative tests and GC–MS analysis. The analgesic potential was tested by acetic acid-induced writhing model, formalin test and tail immersion model. In contrast, carrageenan and histamine-induced paw edema models were applied to estimate the impact on inflammatogenic agents in Wistar albino rats.
Results
The LM was found to contain cardiac glycosides, flavonoids, tannins and triterpenoids. A list of about thirty compounds from the GC–MS study of LM has been recorded. The LM significantly (p < 0.05) minimized the writhing responses in acetic acid-induced writhing assay. The LM100 (LM 100 mg / kg b.w.) and LM200 (LM 200 mg / kg b.w.) reduced the licking period both in early and late phases of formalin-induced animal studies. The LM50 (LM 50 mg/kg b.w.) demonstrated the analgesic effects at 180 min, and in the tail immersion test diclofenac sodium displayed a substantial latency time at 120 and 150 min. The paw edema inhibition of LM100 and LM200 was statistically significant compared to diclofenac sodium of carrageenan-induced test. Also, the paw edema size in histamine-induced paw edema model was significantly decreased by LM50.
Conclusion
Results demonstrated that L. macrophylla (Roxb.) root extract could be very potential source of therapeutic formulation in pain and inflammation with further clarification.
Keywords
Inflammatogenic agents
Leea macrophylla
Acetic acid
Formalin
- LM
Hot methanol extract of Leea macrophylla (Roxb.) root
- GC–MS
Gas Chromatography-Mass spectrometry
- NF-κB
Nuclear Factor kappa light chain enhancer of activated B cells
- TNFR
Tumor Necrosis Factor Receptor
- TRADD
TNFR1-associated death domain protein
Abbreviations
1 Introduction
Pain is defined as a sensory and emotional unpleasant sensation, helping to alert a person to real or possible tissue damage. This damage may be caused by exposure to harmful chemical, thermal or mechanical stimuli or by a pathological process such as muscle spasm, inflammation, tumour, nerve damage, etc. (McPhee et al., 2014). Pain situation needs different treatment schemes, since each form of pain is related to specific mechanisms, such as nerve damage, noxious stimulation or inflammation (McPhee et al., 2014). Analgesics address peripheral pain or central nervous system pain without affecting the consciousness substantially (Olukunle et al., 2011). Analgesics decrease the sensitivity of the pain-sensing systems to external stimulation to control the pain sensation (Pal and Pawar, 2011).
Inflammation is the immediate reaction of the body to pathogens, harmful chemical substances or physical damage to its cells and tissues (Weiss, 2008). It has pain, heat, swelling, redness and physiological disruptions (Niranjan et al., 2010). Injured tissue and migratory cells release inflammatorry agents such as histamine, bradykinin, leukotrienes, nitric oxide, and prostaglandins (Chaudhari et al., 2013) can participate in inflammation induction (Niranjan et al., 2010). Increase of blood flow causes vascular permeability to increase eventually produces heat and redness, swelling, and activation of afferent nerve fibres leading to pain (Bellik et al., 2012). Nevertheless, excessive use of these treatments may have many unfavourable effects, including gastrointestinal discomforts such as gastrointestinal lesions, bleeding and peptic ulcers, immunodeficiency and humoral disorders (Narayanan et al., 2018). Recently, many alternative medicines derived from plants are used in treating various illnesses, including inflammatory diseases, due to their complex biological properties and pleasant safety profile (Ghasemian et al., 2016). Triterpenoids derived from plants are important in reducing inflammation (Vivek et al., 2010). Plant flavonoids prevent oxidative stress and inflammation, pain induced by inhibiting prostaglandin production from plasma membrane arachidonic acid precursor (Li et al., 2003). Therefore, we have conducted L. Macrophylla, a Bangladeshi plant for assessing its analgesic and anti-inflammatory effects based on the plant's traditional uses.
Leea macrophylla (Roxb.) is a herb, locally known as Hatikana because of its elephant-ear like leaf. It belongs to the Leeaceae family with a height of 90 cm or more, with alternating branches, and perennial tuberous roots. It is distributed mainly to hotter parts of India, China, central and eastern Nepal, Bhutan, Thailand, Myanmar, Cambodia, and Laos (Singh and Singh, 1981). Several areas in Bangladesh like Rajshahi, Jessore, and Natore are noteworthy for LM's habitat (Ferdousy et al., 2016). It has been documented as fruitful in amnesia (Akhter et al., 2015) hepatic damage (Bulbul et al., 2020), urolithiasis (Nizami et al., 2012), diabetic complications (Rahman et al., 2018), goiter and tetanus (Islam et al., 2013), microbial infection, cancer and sexual debility (Choudhary et al., 2008). This research investigated the phytochemicals groups present in Leea macrophylla root hot methanolic extract (LM), and evaluated its analgesic and anti-inflammatory activities.
2 Materials and methods
2.1 Plant collection and identification
Leea macrophylla (Hatikana) roots are collected from the Rajshahi centre of Bangladesh Council of Scientific and Industrial Research (BCSIR). Dr. Sheikh Bokhtear Uddin, taxonomist and Professor at the Department of Botany, University of Chittagong has authenticated the herb. A sample voucher with the accession number ACCU-2011/07 has been retained for future reference.
2.2 Preparation of plant extract
The fresh roots L. macrophylla samples were cleaned, cut into small pieces and oven-dried at 45 °C. The dried sample from mechanically prepared Willy mill was ground into powder and placed in an airtight container. Root powder (450 g) was macerated with pure n-hexane at room temperature (23 ± 0.5) °C with intermittent stirring for 2 days, and filtered through Whatman filter paper. The resulting defatted extract was extracted with hot methanol using a Soxhlet apparatus with 99% distilled methanol as an extraction solvent filtered and concentrated at 45 °C by RE200 rotatory evaporator (Bibby sterling Ltd, UK) using reduced pressure. The condensed extract was collected in a Petri dish for complete evaporation of methanol residue. Finally, 9 g (yield 2%, W/W) of the green-black crude of hot methanol extract of L. macrophylla (Roxb.) root (LM) was obtained and preserved at 4 °C for further use.
2.3 Experimental animals
Thirty-six-week-old (body weight 140–160 g) male Wistar albino rats were collected from Bangladesh Council of Scientific and Industrial Research (BCSIR), Chittagong-4220, Bangladesh. They were kept individually in a polycarbonated cage at 23 ± 2 °C and humidity 55–60% ensuring a light–dark cycle of 12 h. During the entire experimental period all animals were provided with reference rat pellet diet and drinking water ad libitum. Animals were randomly divided into control, reference control, and three treatment groups (LM50, LM100 and LM200) each comprising of six animals. All animal experimentations were conducted according to OECD guidelines in compliance with the regulations of Animal Ethical Review Board of Faculty of Biological Sciences, University of Chittagong (approval no. AERB/FBS/UC/05, 2015).
2.3.1 Acute toxicity evaluation and selection of appropriate dose
Wistar Albino rats (n = 5) maintained in standard laboratory condition received a single oral dose of 0.5, 1.0, 1.5, and 2.0 g/kg b.w. of LM. They were fasted over-night prior to the dosing. Once the dosing was completed, food was withheld for next 3 to 4 h. Individual animals were closely observed for the first 30 min, attended particularly for the first 24 h, after which the delayed toxicity was reported for 3 days. Once daily, cage side was observed to record changes in the fur and skin, eyes and mucous membrane, respiratory and circulatory rate, autonomic and central nervous system. One tenth of the median lethal dose was administered as the effective dose (LD50 > 2.0 g/kg) (Zaoui et al., 2002).
2.4 Phytochemical screening
Established methods were followed to know the occurrence of carbohydrate, reducing sugars, alkaloids, flavonoids, glycoside, saponin, triterpenoids and tannins in LM (Harborne, 1998; Evans, 1997; Wagner, 1993; Raaman, 2006; Kokate, 2001; Evans, 1997).
2.5 GC–MS analysis of root extract
An electron impact (EI) ionization-based gas chromatograph on GC-17A (Shimadzu Corporation, Japan) coupled with a mass spectrometer on GC–MS TQ 8040 (Shimadzu Corporation, Japan) was accomplished by a thin layer chromatographic (TLC) separation. A fused silica capillary column of 0.25 m film-thickness ((Rxi-5 ms), coated with DB-1 (J&W), was adhered with the facilty. The inlet was fixed at 260 °C and oven was set at 70 °C (0 min) to 220 °C (5 min). The flow rate (0.6 mL/min Helium) of column was maintained at the constant pressure of 90 KPa. The temperature of the aux for GC to MS interface was fixed at 280 °C. The scanned mode MS set with the scanning range 40–350 amu was ensured between 50 and 550 m/z. The prepared sample (one μL for split less modes) was then run for 35 min to complete GC/MS analysis. All peaks obtained were compared with the GC–MS library database (version NIST 08-S).
2.6 Analgesic activity
2.6.1 Acetic acid-induced writhing test
Inhbition of acetic acid-induced writhing was investigated using the proven method defined by Koster et al. (1959). Among wistar rats, intraperitoneal injection of 1 per cent acetic acid (v/v, 100 μl/10 g) caused the abdominal constrictions. Every group of animals was administered orally with three separate doses denoted as LMs (LM50, LM100 and LM200) just 30 min prior to injection of acetic acid. Diclofenac sodium (DS, 40 mg / kg) was administered as a reference drug 30 min before the chemical stimulus. The number of writhings was counted over a 20-minute period. The percentage inhibition of analgesic activity was determined using the following formula:
2.6.2 Formalin-induced test
The formalin-induced test was undertaken in experimental rats with slight modification of the Gaertner et al. (1999) developed method. Briefly, formalin injection was used to administer DS and LMs just 30 min before the induction pain. Twenty μL of 2.5 percent formalin produced in phosphate buffer was subcutaneously injected into the animals' right hind paw. Each rat was observed at an early (5 min after injection with formalin) and late (20–30 min after injection of formalin) stage to determine the response. The amount of paw licking time spent on the injected paw by rodents was reported using a stop watch and considered to be pain indicative.
2.6.3 Tail immersion test
Toma et al. (2003) implemented the tail immersion model as depicted. The animals were fasted on drinking water for 16 h. The time for basal reaction was determined by immersing the animal's tail tips (last 2–3 cm) in warm water at 55 ± 1 °C following treatment with LMs and reference drug DS for each animal group. The tail tip withdrawal time taken from hot water system was calculated in second was considered as the flick response. To prevent injury to rodent, the cut-off point was set to a latency time of 15 s. The latent time was measured in 30 min before and 30, 60, 90, 120, 150 and 180 min after the drug administration of each experimental group.
2.7 Anti-inflammatory activity
2.7.1 Carrageenan-induced paw edema test
Carrageenan-induced paw edema model was applied by following the modified method of Winter et al. (1962). Briefly, the basal volume of right hind paw of each rat was measured before the treatment of any drug. LMs and DS were orally ingested just 60 min before chemical stimulus. Paw edema was induced by a 50 µl subplantar injection of 1% carrageenan at the right hind paw just 1 h immediate after an oral administration of reference and test drugs. The volume of the paw was assessed after carrageenan injection at 1st, 2nd, 3rd and 4th and 5th h and the paw edema was calculated in mm by using cotton thread around the paw. We used the the following formula to measure the inhibitory activity:where Ct and Co were designated as paw size after and before the carrageenan injection.
2.7.2 Assay of histamine-induced paw edema
The histamine-induced paw edema model was accomplished following Perianayagam et al. (2006). Briefly, LMs and reference anti-inflammatory drug DS was administered 60 min before histamine injection and distilled water were given equal volume to control group. Acute inflammation (paw edema) was induced by an injection of 100 µl 0.1% (w/v) histamine in albino rats subcutaneously after the initial volume of right-hind paw for each rat is measured. The right-hind paw volume was calculated at 1st, 2nd, 3rd and 4th and 5th h after histamine injection and inhibitory activity was estmated with the same formula used for carrageenan-induced model.
2.8 Statistical analysis
All data for six animals were presented as a Mean ± SEM. The results were analyzed by independent variable tests T-test with the aid of SPSS (Statistical Package for Social Science) for Windows (version 22.0, IBM Corporation, New York) and the values at *p < 0.05 were considered to be significantly different. All graphs have been completed using graph pad Prism 6.
3 Results
3.1 Phytochemical status
Phytochemical status of LM has been revealed with the presence of secondary metabolite-types summarized in the Table1. A list of phytoconstituents obtained from the GC–MS data (Fig. 1) has been presented as Table 2. N.B. The signs (+) and minus (−) respectively indicates the Presence and Absence of phytochemical groups.
Phytochemical constituents
Name of the tests
Observation
Carbohydrates
Molisch’s test
−
Fehling’s test
−
Alkaloids
Mayer’s test
−
Wagner’s test
−
Dragendorff’s test
−
Cardiac glycosides
Baljet test
+
Flavonoids
Alkali test
+
Saponin
Frothing test
−
Triterpenoids
Salkowski’s test
+
Tannins
FeCl3 test
+
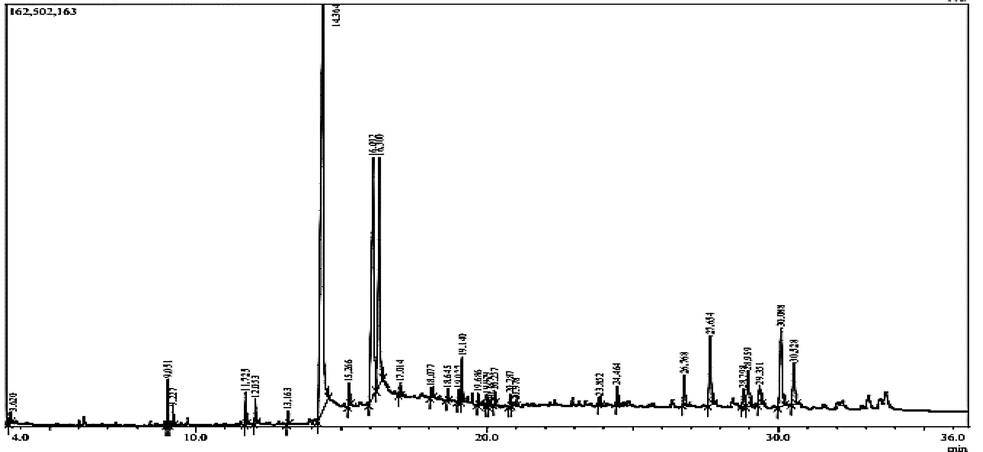
GC–MS analysis of LM produced with the combination of electron-impact ionization and gas chromatograph in association with a a fused silica capillary column maintaining the inlet temperatue 260 °C and oven temperatures 70 °C (0 min)–220 °C (5 min). The flow rate for column was 0.6 mL/min Helium gas with a constant 90 KPa pressure. The MS was set in a scan mode (40–350 amu) for mass range 50–550 m/z.
S.N.
Name of the compounds
Retention Time
Peak area (%)
1.
2,2-Bis (chloromethyl)-1-propanol
3.620
0.43
2.
2H-Pyran-2-one, tetrahydro-4-hydroxy-6-pentyl-
9.051
1.36
3.
Butylated Hydroxytoluene
9.227
0.59
4.
Benzaldehyde, 3-ethoxy-
11.725
0.99
5.
Tetradecanoic acid
12.053
0.76
6.
Pentadecanoic acid
13.163
0.44
7.
n-Hexadecanoic acid
14.364
37.15
8.
l-(+)-Ascorbic acid 2,6-dihexadecanoate
15.266
0.72
9.
9-Octadecenoic acid, 1,2,3-propanetriyl ester, (E,E,E)-
16.092
18.87
10.
Octadecanoic acid
16.300
12.56
11.
12,13-Epoxy-octadec-9-enoic acid, DMOX derivative
17.014
0.38
12.
Eicosanoic acid
18.077
0.45
13.
Docosanal
18.645
0.32
14.
(2,3-Diphenylcyclopropyl)methyl phenyl sulfoxide, trans-
19.027
0.45
15.
2-Hydroxy-4-methoxy-7-methyl-7,8,9,10,11,12,13,14-octahydro-6-oxabenzocyclododecen-5-one
19.140
1.40
16.
Bis(2-ethylhexyl) phthalate
19.686
0.32
17.
(2,3-Diphenylcyclopropyl)methyl phenyl sulfoxide, trans-
19.979
0.42
18.
(2,3-Diphenylcyclopropyl)methyl phenyl sulfoxide, trans-
20.117
0.52
19.
7-Methoxy-3-(3,4-dimethoxyphenyl)-4H-chromen-4-one
20.257
0.35
20.
Tetrapentacontane, 1,54-dibromo-
20.787
0.63
21.
2,2-Dimethyl-6-methylene-1-[3,5-dihydroxy-1-pentenyl]cyclohexan-1-perhydrol
20.978
0.31
22.
Stigmasta-4,7,22-trien-3.beta.-ol
23.852
0.29
23.
Cholesta-4, 6-dien-3-ol, (3.beta.)-
24.464
0.72
24.
Stigmasterol
26.768
1.58
25.
γ-Sitosterol
27.654
4.13
26.
Ergosta-4,6,8(14),22-tetraen-3-one
28.798
1.15
27.
4,22-Cholestadien-3-one
28.959
2.64
28.
Cyclopropa[5,6]-33-norgorgostan-3-ol, 3′,6-dihydro-, (3.beta.,5.beta.,6.alpha.,22.xi.,23.xi.)-
29.351
1.21
29.
γ-Sitostenone
30.088
5.88
30.
Cholesterol epoxide
30.528
2.95
3.2 Analgesic effect of LM
3.2.1 Acetic acid-induced writhing assay
LM displayed an analgesic effect in a dose-dependent approach, while the treatment and reference drug decreased the writhing numbers significantly (P < 0.05) as shown in Fig. 2. LM200 was found to have the highest amount of writhing inhibition (62.39 ± 3.08%), significant compared to DS, and the lowest number of writhing (21.50 ± 1.76%) in acetic acid-induced writhing experiment.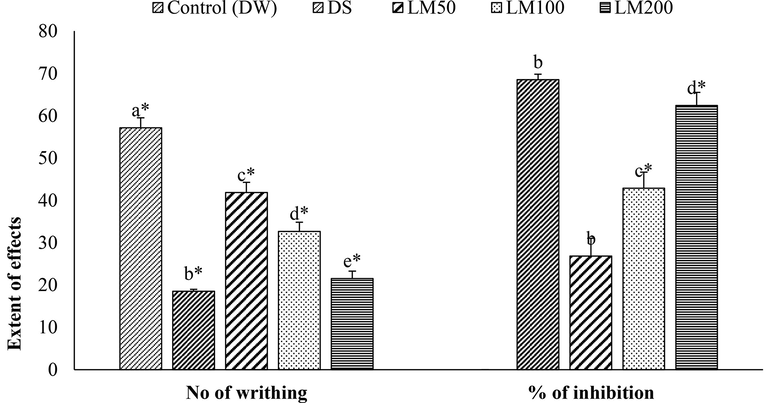
Effect of LM on writhing number and inhibition of writhing in acetic acid-induced test. Data for six animals is reported Mean ± SD. Data analysis was accomplished by SPSS, the statistical software, (Statistical Package for Social Science, 22 version of IBM corporation, NY) using ANOVA (One-Way Analysis of Variance) followed by Tukey’s multiple regression post Hoc test. The alphabetes(a-d) over the graphs indicate the level of significance of the groups in experimental environment. Values at p < 0.05 were considered significant.
3.2.2 Formalin-induced pain test
LM reduced the licking span of formalin-induced pain in a dose-dependent relationship at early and late phases. The reference drug DS significantly inhibited the licking time against both stages of formalin-induced pain, which is illustrated in Fig. 3. The maximum degree of licking inhibition, 46.02 ± 1.26% at the early phase and 59.95 ± 1.49% at the late phase, was gained by LM200, which was significant at p < 0.05 compared with DS.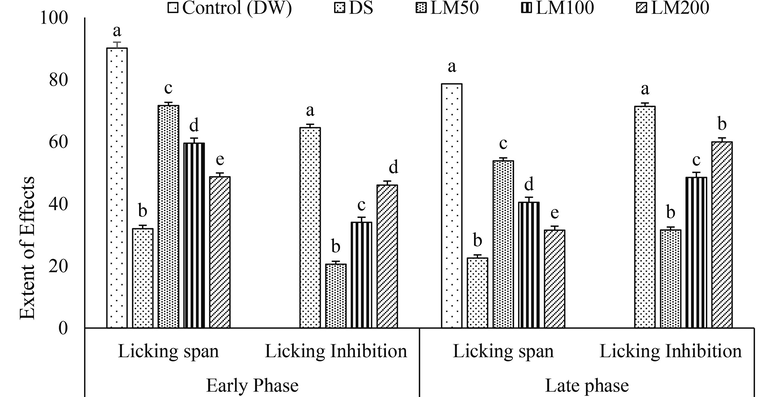
Effect of LM on the paw licking span and paw licking inhibition in formalin induced test. Data for six animals is reported Mean ± SD. Statistical software SPSS (Statistical Package for Socialc Science, 22 Version of IBM corporation, NY) using ANOVA (One Way Analysis of Variance) followed by Tukey’s multiple regression post Hoc test. The alphabetes(a-e) over the graphs denote the level of significance of the groups in an experimental environment. Values at p < 0.05 were considered significant.
3.2.3 Tail immersion test
The maximum effects for all the doses of LM were achieved at 30–150 min in the tail immersion study. The major effects were observed for LM50 at 150 min, LM100 at 90 and 180 min. Compared to the reference drug, LM50 substantially increased the latency duration. Table 3 summarizes the effects of all doses in the tail immersion test. Data for six animals is presented as Mean ± SD. Data analysis was accomplished by SPSS using ANOVA followed by Tukey’s multiple regression test. Values at p < 0.05 are considered significant. Asterisks values denote the level of significance.
Groups (n = 6)
Latency period (Sec.)
0 min
30 min
60 min
90 min
120 min
150 min
180 min
Control (DW)
3.00 ± 0.36
3.16 ± 0.40
3.53 ± 0.35
3.66 ± 0.42
3.83 ± 0.11*
3.66 ± 0.21*
3.66 ± 0.42
DS
2.50 ± 0.22
4.33 ± 0.42
5.00 ± 0.36
5.33 ± 0.55
6.00 ± 0.56*
5.33 ± 0.49*
4.66 ± 0.55
LM50
2.33 ± 0.21
3.66 ± 0.21*
4.66 ± 0.21
5.00 ± 0.36
5.66 ± 0.49
7.77 ± 0.76
4.66 ± 0.21
LM100
2.33 ± 0.21
4.33 ± 0.21*
4.66 ± 0.21
5.00 ± 0.00**
7.00 ± 0.51
5.66 ± 0.333
4.66 ± 0.21*
LM200
2.66 ± 0.21
4.66 ± 0.21*
5.33 ± 0.71
5.66 ± 0.42
6.33 ± 0.71
7.1 ± 0.428
6.50 ± 0.42
3.3 Anti-inflammatory effect
3.3.1 Carrageenan-induced paw edema
The anti-inflammatory effect at all three doses is summarized in Table 4 with the paw edema’s average size. The induced paw size was restored and decreased at different observation hours, where DS began decreasing the edema by 5 h, and treatments by 3 h. Fig. 4, summarized for the decrease of inflammation, shows that LM200 exhibited the highest anti-inflammatory effect 98.33 ± 1.66% at the final hour and it was also higher than that of DS within the same time-interval. Data for six animals is presented as Mean ± SD. Data analysis was accomplished by SPSS using ANOVA followed by Tukey’s multiple regression test. Values at p < 0.05 are considered significant. Asterisks values denote the level of significance.
Group (n = 6)
NPS
Reading of paw edema size after carrageenan induction (mm)
1 h
2 h
3 h
4 h
5 h
Control (DW)
22.83 ± 0.79
24.00 ± 0.73
26.16 ± 0.52
27.08 ± 0.53
26.75 ± 0.76
25.91 ± 0.71
DS
20.91 ± 0.32
25.33 ± 0.44
24.16 ± 0.28
23.16 ± 0.35
22.50 ± 0.44
22.16 ± 0.27*
LM50
21.33 ± 0.33
26.58 ± 0.20
24.50 ± 0.40
23.08 ± 0.61
22.58 ± 0.30
23.16 ± 0.30
LM100
22.33 ± 0.70
25.58 ± 0.47
26.33 ± 0.71**
23.33 ± 0.57
22.41 ± 0.70
22.58 ± 0.61*
LM200
22.33 ± 0.45
26.33 ± 0.16
26.41 ± 0.67*
23.83 ± 0.55
22.83 ± 0.40
22.41 ± 0.43
Effect of LM on paw edema inhibition in carrageenan-induced paw-edema test. Data for six animals is reported Mean ± SD. Statistical software SPSS (Statistical Package for Social Science, 22 nd Version, IBM corporation, NY) analyzed the data using ANOVA (One Way Analysis of Variance) followed by Tukey’s multiple regression post Hoc test. The alphabetes over the bar-graph denote the level of significance of the groups in a particular environment. Values at p < 0.05 were considered significant.
3.3.2 Histamine-induced paw edema
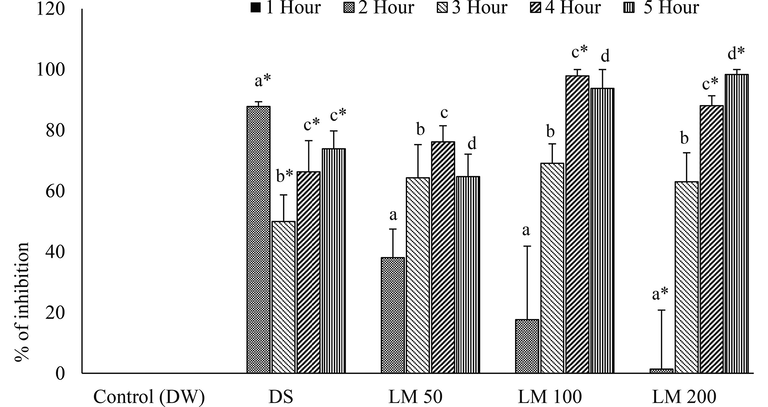
The histamine injection in paw produced inflammatory edema that was decreased slowly by all treatment doses except LM50 by 2 and 3 h (Fig. 5). However, DS and LM strikingly reduced paw edema by 4 h and 5 h and suppressed the effect of histamine in several hours consecutively and the highest significant (P < 0.001) inhibition was recorded by LM200 at the finishing hour of administration (Fig. 5). Although all doses of LM inhibited the histamine-caused inflammation, the LM50 conspicuously showed the negative effect at 2nd and 3rd hr (Fig. 6).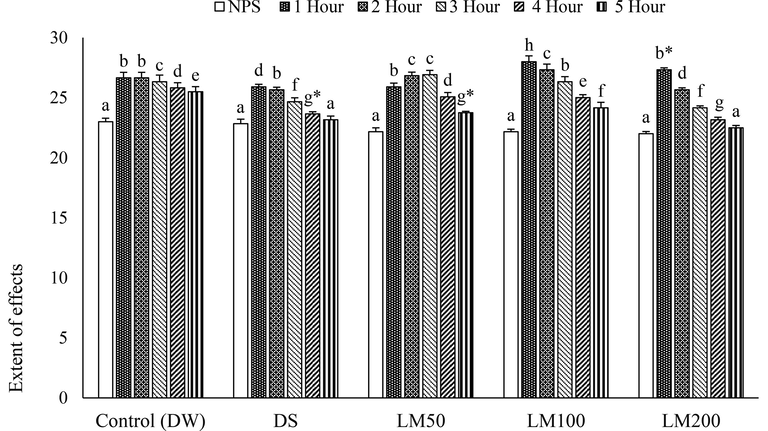
Effects of LM at different time interval in histamine-induced paw edema model. Data for six animals is reported as Mean ± SD. Data analysis was made by SPSS (Statistical Package for Social Science, Version 22, IBM corporation) using ANOVA (One Way Analysis of Variance) by Tukey’s multiple regression Post Hoc test. The superscript letters(a-h) over the bar-graph denote the level of significance of the groups in a experimental environment. Values at p < 0.05 were considered significant.
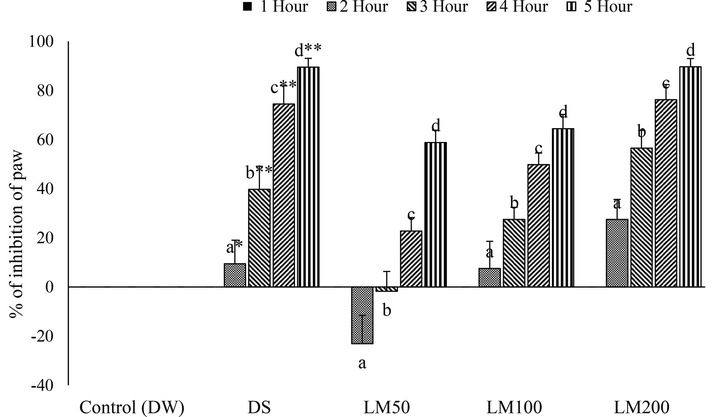
Percentage inhibition of histamine induced paw edema at different time interval by different doses of LM. Data for six animals is reported as Mean ± SD. Data analysis was accomplished by SPSS (Statistical Package for Social Science, version 22, IBM Corporation) using ANOVA (One Way Analysis of Variance) followed by Tukey’s multiple regression post Hoc test. The superscript letters(a-d) over the bar-graph denote the level of significance of the groups in experimental environment. Values at p < 0.05 were considered significant.
4 Discussion
Qualitative phytochemical tests of the LM revealed the presence of medicinally important phytochemicals especially cardiac glycosides, flavonoids, triterpenoids and tannins. The treatment of inflamed or ulcerated tissues is greatly assisted by tannins which have remarkable anti- inflammatory and anticancer activity. Three major fatty acids n-Hexadecanoic acid (37.15%), Octadecanoic acid (12.56%), and 9-Octadecenoic acid, 1, 2, 3-propanetriyl ester, (E, E, E) (18.87%), according to previous investigations, explained the role of saturated fatty acids at the anti-inflammatory pathway regulation. Additionally, n-Hexadecanoic acid, which is an anti-inflammatory, antioxidant, hypocholesterolemic and antiandrogenic agent acting as a competitive inhibitor of phospholipase A2 and controls cytokine synthesis by modifying arachidonic acid release (Aparna et al., 2012).
The extract containing these compounds might have played significant role to cure inflammation. The terpenoids are significantly anti-inflammatory and several plant-derived triterpenoids play significant anti-inflammatory role by downregulating NF-κB (Vivek et al., 2010). Flavonoids, because of its anti-inflammatory actions through regulation of membrane permeability and inhibition of ATPase, phospholipase and other membrane-bound enzymes, work as a health promoting agent (Vivek et al., 2010; Li et al., 2003). Cardiac glycosides inhibit TNF-α, TNF-kβ signaling by blocking recruitment of TRADD to the TNFR that ultimately controls the inflammation (Muhammad et al., 2012).
Assessmesnt of analgesic properties of medicinal agents by acetic acid induction is well known and the protocol is long existing. A local inflammatory response elicited by pain sensation is generated in this method because of arachidonic acid release caused by cyclooxygenase enzyme. Subsequently prostaglandins particularly PGE2 and PGF2α some and lipoxygenase products are also released (Sireeratawong et al., 2012). These lipoxygenase products and prostaglandins increase capillary permeability and eventually cause inflammation and pain. Medicinal plants or plant-products capable of inhbiting the writhing might have retarded prostlandin-sysnthesis in a peripheral pain-inhibition mechanism to proclaim analgesic effect (Sireeratawong et al., 2012). A reliable extent of inhibition of writhing reflux showed by LM strongly claims its peripheral antinociceptive activity achieved through cyclooxygenase inhibition.
Both peripheral and central nociceptions are revealed by formalin test which generates a distinctive biphasic nociception. The early phase (neurogenic nociceptive response) starts immediately by formalin having a clear impact on nociceptors and continues for 0–5 min whereas the nociceptive response (late phase) began from 15 to 30 min. Substance P is imparts in early phase while bradykinin, histamine, prostaglandins, and serotonin participate in the late phase (Reeve and Dickenson, 1995). The inhibition of licking by LM at both phases, nonetheless, ascribes the analgesic activity mediated by suppressing both neurogenic and inflammatory nociception.
Tail immersion is considered as very effective method to determine the effect of centrally acting anti-nociceptive and opioid receptor agonists. The effect on spinal (δ2, σ2 and k1) and supraspinal (δ1, σ1 and k3) receptors is a good indicator for assessing analgesic properties of opioid agents. The opioid μ receptor agonists are more sensitive to thermal nociceptive assays such as the tail immersion test. Prolonging of the latency time by our samples, not unambiguously proven, can explicit an effect of LM on spinal and supraspinal receptors indicating its prospect on both central and peripheral antinociception (Muzammil et al., 2013).
In carrageenan and histamine-induced acute edema models, the volume of rat’s paw usually increases due to the recruitment of leukocytes and rapid release of inflammatory agents (Loram et al., 2007). Apart from this, production of bradykinin, 5-hydroxytryptamine (5- HT), and histamine, in the first stage of edema, and IL-1β, COX-2, PGs and TNF-α in the second phase results an increased production of prostaglandin and COX-2 at the late phase of carrageenan-induced paw edema. The achieved normalization of paw edema at LM50 and LM200 followed a U-shaped Hormesis (a subtype of a broad array of dose response relationships) (Ricciotti and FitzGerald, 2011) which is consistent with our previous observation for Alpinia calcarata indicating a prostaglandin-inhibited way to decrease the activity or expression of COX-2 at least in this experimental procedure (Rahman et al., 2012).
Histamine, one of the potent vasodilators and inflammatory mediators, causes to increase the vascular permeability (Abdulkhaleq et al., 2018) and subsequent edema with injectable administration. Evaluation of anti-histaminic effect of medicinal phytochemicals using histamine-induced paw edema model is well known and it is well co-opted with a dose-dependent antihistaminic potential of LM advising a competitive inhibition of histamine receptor to regulate edema formation. Our study suggests that LM can exert both central and peripheral effects in mitigation of inflammation due to its phytochemical components.
5 Conclusion
The L. macrophylla root extract demonstrated promising analgesic and anti-inflammatory effects which are evidently exterted by the reported phytoconstituents although further investigation of the chemical constituents of this plant, relevant molecular markers and quantification of anti-inflammatory agents are necessary to determine the advanced mechanism for observed activities.
Acknowledgements
The authors wish to thank Professor Dr. Sheikh Bokhtear Uddin for identification of the plant.
Disclosure of funding
This research is partially funded by Chittagong University Research and Publication Cell (Gr No. 15/Res/Dir/Pub/Off/CU/2014).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The crucial roles of inflammatory mediators in inflammation: a review. Vet. World.. 2018;11:627-635.
- [Google Scholar]
- Antioxidative role of Hatikana (Leea macrophylla Roxb.) partially improves the hepatic damage induced by CCl4 in Wistar albino rats. BioMed Res. Int.. 2015;356729:2015.
- [Google Scholar]
- Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol. Drug. Des.. 2012;80:434-439.
- [Google Scholar]
- Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: an update. Molecules. 2012;18:322-353.
- [Google Scholar]
- Leea macrophylla (Roxb.) root extract reverses CCl4 induced liver injury through upregulation of antioxidative gene expression: a molecular interaction for therapeutic inception. Adv. Traditional Med.. 2020;20:35-52.
- [Google Scholar]
- In vitro anti-diabetic and anti-inflammatory activity of stem bark of Bauhinia purpurea. Bull. Pharm. Med. Sci.. 2013;1:139-150.
- [Google Scholar]
- Ethnobotanical survey of Rajasthan – an update. American-Eurasian J. Bot.. 2008;1:38-45.
- [Google Scholar]
- Pharmacology. Singapore: Harcourt Brace and Company; 1997. p. :226.
- Antioxidative and neuroprotective effects of Leea macrophylla methanol root extracts on diazepam-induced memory impairment in amnesic Wistar albino rat. Clin. Phytoscience.. 2016;2:1-11.
- [Google Scholar]
- Analgesic triterpenes from Sebastianiaschottiana roots. Phytomedicine.. 1999;6:41-44.
- [Google Scholar]
- Review of anti-inflammatory herbal medicines. Adv. Pharmacol. Sci.. 2016;2016:1-11.
- [CrossRef] [Google Scholar]
- Phytochemical Methods, a Guide to Modern Techniques of Plant Analysis (second ed.). London: Chapman and Hall; 1998. p. :54-84.
- Phytochemical screening and anti-microbial activity studies on Leea macrophylla seed extracts. J. Sci. Res.. 2013;5:399-405.
- [Google Scholar]
- Practical pharmacognosy. New Delhi: Vallabh Prakarshan; 2001. p. :45-49.
- Review in the studies on tannins activity of cancer prevention and anticancer. Zhong. Yao. Cai.. 2003;26:444-448.
- [Google Scholar]
- Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J. Pain.. 2007;8:127-136.
- [Google Scholar]
- Current Medical Diagnosis & Treatment 2014 (53rd ed.). New York: McGraw-Hill Medical; 2014.
- Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement. Altern. Med.. 2012;12:59.
- [Google Scholar]
- Muzammil, A.S., Farhana, T., Salman, A., 2013. A algesic activity of leaves extracts of Samaneasaman Merr., and Prosopis cineraria Druce. Int. Res. J. Pharm. 4, 93–95.
- Peptic ulcer disease and helicobacter pylori infection. Mol. Med.. 2018;115:219-224.
- [Google Scholar]
- Comparative adverse effects of Cox-1 and Cox-2 inhibitors in rat liver: an experimental study. J. Anatom. Soc. India.. 2010;59:182-186.
- [Google Scholar]
- Whole Leea macrophylla ethanolic extract normalizes kidney deposits and recovers renal impairments in an ethylene glycol-induced urolithiasis model of rats. Asian Pac. J. Trop. Med.. 2012;5:533-538.
- [Google Scholar]
- Studies on the anti-inflammatory and analgesic properties of Jatropha curcasleaf extract. Acta Vet. Brno.. 2011;80:259-262.
- [Google Scholar]
- A study on Ajuga bracteosa Wall ex. Benth for analgesic activity. Int. J. Curr. Biol. Med. Sci.. 2011;1:12-14.
- [Google Scholar]
- Anti-inflammatory activity of Trichodesma indicum root extract in experimental animals. J. Ethnopharmacol.. 2006;104:410-414.
- [Google Scholar]
- Phytochemical techniques. Pitam Pura. New Delhi: New India Publishing Agency; 2006. p. :22.
- Leea macrophylla Roxb. leaf extract potentially helps normalize islet of β- cells damaged in STZ- induced albino rats. Food Sci. Nutr.. 2018;6:943-952.
- [Google Scholar]
- Essential oil of Alpinia calcarata Rosc. Rhizome: heals inflammation and nociception in animal models. J. Biologically Active Products Nat.. 2012;2:365-376.
- [Google Scholar]
- The roles of spinal adenosine receptors in the control of acute and more persistent nociceptive responses of dorsal horn neurons in the anaesthetized rat. Br. J. Pharmacol.. 1995;116:2221-2228.
- [Google Scholar]
- Prostaglandins and Inflammation. Arterioscler Thromb. Vasc. Biol.. 2011;31:986-1000.
- [Google Scholar]
- On the identity and economic medical uses of Hastikarnapalsa (Leea macrophylla Roxb., Family Ampelidaceae) as evinced in the ancient texts and traditions. Indian J. Hist. Sci.. 1981;16:219-222.
- [Google Scholar]
- Anti-Inflammatory, Analgesic, and Antipyretic Activities of the Ethanol Extract of Piper interruptum Opiz. and Piper chaba Linn. ISRN Pharmacology. 2012;2012:1-6.
- [CrossRef] [Google Scholar]
- Evaluation of the analgesic and antiedematogenic activities of Quassiaamara bark extract. J. Ethnopharmacol.. 2003;85:19-23.
- [Google Scholar]
- Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins (Basel). 2010;1:2428-2466.
- [Google Scholar]
- PharmazeutischeBiologie (fifth ed.). Stuttgart: Gustav Fisher Verlag; 1993. p. :184.
- Inflammation. Nature. 2008;454:427.
- Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med.. 1962;111:544-547.
- [Google Scholar]
- Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine. 2002;9:69-74.
- [Google Scholar]







