Translate this page into:
Host Range and Pathogenicity Potential of Helicoverpa armigera Nucleopolyhedrovirus (HaNPV) to Lepidopterous Pests of Cotton
⁎Corresponding authors at: Department of Entomology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan 60800, Pakistan. adabid5@gmail.com (Allah Ditta Abid), shafqat.saeed@mnsuam.edu.pk (Shafqat Saeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Farmers rely on chemical control for managing insect pests which cause various human and environmental health hazards. So, biopesticides like Nucleopolyhedrovirus (NPVs) can be a suitable alternative due to their less toxic effects on non-target organisms and environment. As a matter of fact, NPVs have been proven very effective against lepidopteran larvae. However, defining the host range of NPV was required according to the ecological conditions of Pakistan. So, this study was planned in which NPV isolated from Helicoverpa armigera, HaNPV, was used to infect H. armigera, Spodoptera litura, S. exigua, Pectinophora gossypiella and Trichoplusia ni under controlled conditions (temperature = 25 ± 2 °C; RH = 60 ± 5%). Higher mortality was observed in H. armigera among all the tested hosts. Additionally, mortality of all the other tested species showed no significant difference as compared to their untreated control. Similarly, a higher peak was achieved in H. armigera after two days of infection. Due to the multiplication of virions inside the host body, host tissues are destroyed. Many factors like larval body size, temperature and environmental conditions affect the rate of response and pathogenicity. Thus the pathogenicity varies with time of infection. Decrease in infection and mortality can be attributed to resistance and increase in the size of larvae with the passage of time. Non significant mortality in non-hosts species by HaNPV suggests its highly specific nature. Thus, it can be successful highly specific ecofriendly management tool for H. armigera. However, in extreme environmental conditions, NPV should be used with sunblock.
Keywords
Cotton
Lepidopteran larvae
Mortality
NPV infection
morbidity
1 Introduction
Bollworms complex of cotton consists of Helicoverpa armigera Hübner (American bollworm), Earias vittella/insulana Fabricius (spiny bollworms) and Pectinophora gossypiella Saunders (Pink bollworm). Though due to Bt cotton, bollworms were managed for longer time but these bollworms have started developing resistance against Bt cotton (Tabashnik et al., 2013). Among bollworms, American bollworm (ABW) is one of the important pest which attacks to a range of agricultural crops, such as fiber crops, horticultural plants, and vegetables (Gajbe 2020; Mishra and Omkar, 2021). In Pakistan, it has been considered the most dangerous and yield decliner over field crops from the 1990s to the early 21st century (Karim, 2000). Additionally, polyphagous nature, several generations, high fertility rate, migratory behavior, and development of resistance are important reasons that have made the pest destructive (Katsikis et al. 2020; Kora and Teshome, 2021). It is estimated that this pest caused losses of about 3 billion dollars annually in cotton (Riaz et al. 2021).
Baculoviruses (Baculoviridae) consist of a diverse group of double-stranded DNA viruses with about 1000 described species. Two Baculovirus genus, Nucleopolyhedrovirus (NPVs) and the Granulovirus (GVs) (Rodriguez et al. 2012) are good alternative to synthetic insecticides for agricultural and forest pests (Nawaz et al., 2019). The NPVs, similar to other groups of viruses, are obligate pathogens which utilize the host cell machinery for its replication. The NPVs need oral intake for infection of their larval hosts, although vertical diffusion and inoculation via parasitoids are also documented (Yasin et al. 2020). Vertical diffusion or infection from parents to offspring can occur; on egg surfaces, inside the egg or via dormant infections (Fuxa, 2004). The virus is in a non-replicative state and can change to an infective and replicative state when a host is under stress (Beldomenico and Begon, 2010). Vertical diffusion was documented in some insect NPV systems (Fuxa, 2004) but does not appear to take place in all of them (Akhanaev et al. 2020). The NPV have been proven effective against lepidopterans in Pakistan under controlled conditions and in field (Abid et al. 2020; Ahmad et al. 2020).
The NPVs have specifically restricted host range and infect only certain species within a genus (Tanuja et al., 2019). But studies have shown that their host ranges vary from relatively wide (e.g. NPVs of Autographa californica Speyer and Mamestra brassicae Linnaeus belonging to Noctuidae family) (Huang et al. 2019) to apparently monospecific (e.g. NPVs of Lymantria dispar and Euproctis chrysorrhoea) (Gani et al. 2017).
A specific host range of NPVs has been documented. Seven orders of insects and some crustaceans have been associated with NPV, but apart from hymenopterans and lepidopterans insect pests of other orders are less favorable hosts. The NPVs have specifically restricted host range and infect only certain species within a genus (Tanuja et al., 2019; Sajid et al., 2021). But studies have shown that their host ranges vary from relatively wide (e.g. NPVs of Autographa californica Speyer and Mamestra brassicae Linnaeus belonging to Noctuidae family) (Huang et al. 2019) to apparently monospecific (e.g. NPVs of Lymantria dispar and Euproctis chrysorrhoea) (Gani et al. 2017). The H. armigera Nucleopolyhedrovirus (HaNPV) is a specific type of Baculoviruses that mostly infects and kills larvae of H. armigera and few closely related species (Williams et al. 2017). The information of the host range of a particular NPV is very necessary for its use against a particular pest or a range of pest species (Abid et al. 2020). Additionally, information of host range of Baculoviruses is very important for the registration and commercialization of as microbial pesticides. In the current study, we aimed to find the host range of HaNPVs in five lepidopteran species that are found on cotton. These pest species include H. armigera, Spodoptera litura, S. exigua, Pectinophora gossypiella and Trichoplusia ni.
2 Materials and methods
2.1 Insects and their rearing
The larval species chosen for the cross-infection assays were congeneric of H. armigera, S. litura, S. exigua, P. gossypiella and T. ni. The eggs of H. armigera, S. litura, S. exigua, and T. ni were collected from the field and shifted to a laboratory with great care. After hatching from eggs, first instar larvae were shifted to their specific host. The S. litura and T. ni were reared on cabbage, S. exigua on tobacco leaves, while H. armigera on gram/sunflower. The fresh leaves of each respective host were washed with distilled water, dried in laminar flow and placed in a glass jar. However, P. gossypiella larvae were collected from cotton flowers and premature bolls and reared on cotton bolls in jars.
The jars were placed in a laboratory under controlled temperature (25 ± 2 °C) and relative humidity (60 ± 5%). They were reared until pupation. After that, the pupae were identified as male and female and shifted to a glass jar containing napiliner for egg-laying. In each glass jar, one pair of male and female was released with honey 2% solution as diet. The eggs were collected from napiliner and shifted to their natural diet as described above. After hatching, 2nd instar larvae were used in the experiment.
2.2 NPV isolates
The H. armigera larvae showing symptoms of NPV infection (blackish and found hanging upward) were collected from mungbean, tomato, okra, cotton, sunflower, grams, wheat and berseem crops located at Kabirwala, Muzaffargarh, Dera Ghazi Khan, Layyah, Mianwali, Rajan Pur, Bhakkar and Bahawalpur (Table 1). The Helicoverpa armigera NPV (HaNPV) was isolated and maintained in laboratory for further trials.
Sr.#
Place of Collection
Name of Crop
No. of Larvae collected
No. of Diseased Larvae
No. of Un-diseased Larvae
Strains of NPV
1
Moza Jhoke Vains Multan
Barseem, Tomato, Wheat, Sunflower
56
2
54
HaNPV
2
Moza Salar Wahin Kohna, Kabir Wala
Sunflower, Okra
11
1
10
HaNPV
3
M. Garh
Moong, Sunflower
9
0
9
–
4
Kot Addu
Okra, Cotton
7
0
7
–
5
Layyah
Sunflower
72
12
60
HaNPV
6
D.G. Khan
Gram, Wheat, Barseem
124
23
101
HaNPV
7
Mian Wali
Gram
33
2
31
HaNPV
8
Rajan Pur
Sunflower
8
0
8
HaNPV
9
Bhakkar
Gram
41
6
35
HaNPV
10
Bhalwal
Gram
55
11
44
HaNPV
2.3 Treatment of NPV to insects
Second instar larvae of each pest species were treated with HaNPV @ 10 million polyhedral inclusion bodies per mL (PIB/mL) at the dose of 1 mL/L of water by introducing on natural diet (as used for rearing) (Vanderzant et al. 1962). The experiments were conducted in August 2013. A group of 40 larvae were used in each treatment. Equal numbers of insects of each species were used for control treatments. Insects that had eaten the virus- or water-treated diet were transferred onto a fresh natural diet after 24 h and maintained at 25 °C temperature with a 16-h photoperiod until death or pupation. All the larvae which died before pupation were ice-covered. Larvae which died prior to pupal formation were examined for the presence of virus by checking infected larval tissues tarnished on microscopic slides. Briefly, to determine occlusion bodies (OBs) present in cadavers, larval tissues were smeared, fixed (in 100% methanol for 4 min) followed by staining with 5% Giemsa for 45 min and examined with dark-field microscopy (Olympus CX30) at 1000X magnification (Smits and Vlak, 1988). The experiment was set in CRD to determine the interaction between hosts and NPV. The mortality data were recorded after every 48 h for a total of 12 d. Only those which could be dependably identified as virus-infected or virus free were utilized for data analysis.
2.4 Data analysis
The mortality data of each treatment were subjected to paired t-test along with the control for comparison. All the data were analyzed using Statistix 8.1 (Analytical Software, 2005).
3 Results
3.1 Mortality among different hosts
There was a greater difference in larval mortality of different lepidopteran species after exposure to NPV. Relatively higher mortality was observed in H. armigera than other hosts. The larval mortality of H. armigera treated with NPV was 58.00% which was significantly higher than the control mortality (10%) as shown in Fig. 1A (t = 8.22, DF = 4, p < 0.05). The larval mortality of S. litura larvae (12.00%) treated with NPV was statistically similar to the 8.00% mortality observed in the control (t = 0.99, DF = 4, P > 0.05) (Fig. 1B).
Percent mortality of larvae of Helicoverpa armigera (A), Spodoptera litura (B), Spodoptera exigua (C), Pectinophora gossypiella (D) and Trichoplusia ni (E) after exposure to NPV compared with the control. *The larval percent mortality differed significantly based on t-test.
Similarly, the larval mortality i.e. 14.00% recorded in S. exigua treated with NPV was statistically similar to the control where 10.00% mortality was recorded (t = 0.49, DF = 4, p > 0.05) (Fig. 1C). The mortality of P. gossypiella larvae treated with NPV was not significantly different compared to the control without NPV (t = 0.77, DF = 4, p > 0.05) (Fig. 1D). The mortality of T. ni larvae treated with NPV was also statistically similar to the control (t = 0.99, DF = 4, p > 0.05) (Fig. 1E).
3.2 Daily mortality of different host species
Daily mortality of H. armigera in NPV treatment and its respective control has been shown in Fig. 2A. Maximum mortality (20.00%) was observed on 5th day of NPV treatment. After four days, it started decreasing again and eventually, no mortality was observed after 9th day. Similarly, daily percent mortality for S. litura in NPV treatment and its respective untreated control has been represented in Fig. 2B. Where, there was no mortality during the initial six days of NPV treatment. While, comparatively higher mortality (6.00%) was observed in NPV treatment on 11th day of treatment.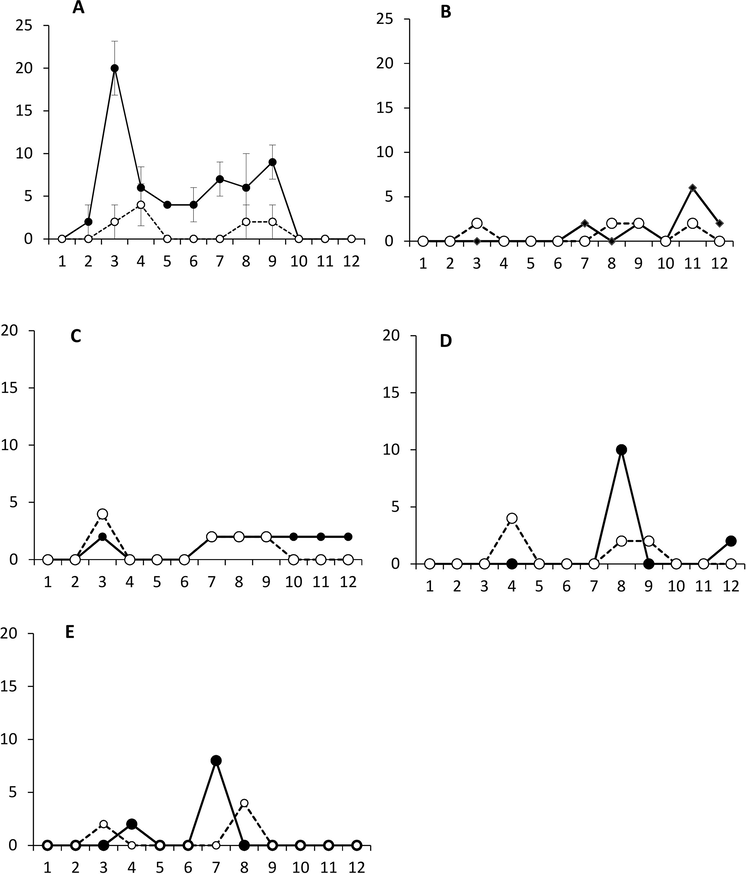
Percent daily mortality of larvae of Helicoverpa armigera (A), Spodoptera litura (B), Spodoptera exigua (C), Pectinophora gossypiella (D) and Trichoplusia ni (E) after exposure to NPV compared with the control. Black circles = NPV Treatment, White circles = Control.
Percent mortality in NPV treated S. exigua and the untreated control has been depicted in Fig. 2C. The NPV treated larvae and control larvae both approached only 2.00% on occasional days during the experiment. Similarly, daily mortality (%) for treated and untreated P. gossypiella is shown in Fig. 2D. Treated larvae showed no mortality during the initial seven days of treatment. Mortality reached the peak of 10.00% mortality after eight days of treatment as compared to the peak (4.00%) in the control approached on day 4. The daily mortality percentages for treated and control T. ni larvae have been shown in Fig. 2E. The NPV treated T. ni larvae approached the peak of 8.00% on day 7 and then no mortality was observed after the peak.
4 Discussion
Entomopathogenic viruses have also been recognized as intrinsically environment friendly as compared to synthetic insecticides (Gramkow et al. 2010). So, entomologists and pest managers encourage their use as bio-control agents due to their host specificity and less hazardous effects on the environment and non-target organisms (Sivakumar et al. 2020). Nuclear polyhedrosis virus (NPV) plays an important role in controlling insect pests. Host suitability of NPV is necessary to be employed as biological control. In the present study, the range of different hosts was tested and results revealed that H. armigera was the most preferable host of NPV regarding mortality among the five tested lepidopteran species.
Previously, it had been reported that the host range of NPV is extremely narrow (Murillo et al. 2003, Wu et al. 2016, Simón et al. 2017). In our results, 58.00% larval mortality was recorded for H. armigera. These findings are comparable to the previous studies conducted by Nawaz et al. (2019) and Abid et al. (2020), who reported more than 50.00% mortality in NPV treated H. armigera larvae. Similarly, percent mortality first increased with an increase in the number of days and then started decreasing. The viruses replicate in the host body, thus destroying the host tissues. The rate of response and pathogenicity are highly dependent on the larval body size and temperature (Jones et al., 1994). Thus the pathogenicity varies with treatment and time. Decrease in infection and mortality can be attributed to resistance and increment in the size of larvae with the time.
In our findings, no significant difference was observed between the infected larvae and their respective control except H. armigera. The NPV could be used for the management of H. armigera larvae in the field. Mono-specific NPVs are highly specific and have little risk of infecting non-target species, thus suggesting their suitability as pest control agents, predominantly when used for natural reserves and urban areas. Therefore, host range testing of NPV needs to carry out on other species which are found in the fields where the control agents are going to be released. Concluding, highly specific NPV strain can be successfully utilized in the field by merely adding sunblock/protector like Tinopal LPW to make a successful viral formulation which can be useful not only to decrease the negative effect of solar UV on the virus, but also to decrease the quantity of viral inoculum needed to accomplish an adequate degree of management of these species.
5 Conclusion
This experiment was conducted to explore the host specificity of HaNPV. Infecting non-hosts viz. S. litura, S. exigua, P. gossypiella and T. ni with HaNPV resulted in lethal or sub-lethal effects. However, these lethal or sub-lethal effects were approximately equal to that of control. Several internal factors (larval body size and host) and external factors (environmental conditions) affect the infection rate. As a result, pathogenicity varies with infection time. By simply applying sunblock/protector to a highly specific NPV strain, it can be successfully used in the field.
Acknowledgement
Authors are thankful to the Dr. Hasnain Ali Syed (late), Institute of Pure and Applied Biology for his technical support in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Manifold passages in an assorted infection in a host could improve virulence of Helicoverpa armigera Nucleopolyhedrovirus (HaNPV) Saudi J. Biol. Sci.. 2020;27(6):1419-1422.
- [Google Scholar]
- Sub-lethal dose reponses of native polyhydroviruses and spinosad for economical and sustainable management of Spodoptera litura in Pakistan. Pakistan J. Zool.. 2020;52(3):989-999.
- [Google Scholar]
- A Comparison of the Vertical Transmission of High-and Low-Virulence Nucleopolyhedrovirus Strains in Lymantria Dispar L. Insects. 2020;11(7):455.
- [Google Scholar]
- Analytical Software, 2005. Statistix 8.1 for Windows. Analytical Software, Tallahassee, Florida.
- Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol.. 2010;25(1):21-27.
- [Google Scholar]
- Ecology of insect nucleopolyhedroviruses. Agric. Ecosyst. Environ.. 2004;103(1):27-43.
- [Google Scholar]
- Gajbe, P.U., 2020. Integrated Pest Management Strategies for the Management of Global Pest, Helicoverpa armigera. Recent Trends in, 1, p.45.
- Molecular identification and phylogenetic analyses of multiple nucleopolyhedrovirus isolated from Lymantria obfuscata (Lepidoptera: Lymantriidae) in India. Appl. Entomol. Zool.. 2017;52(3):389-399.
- [Google Scholar]
- Insecticidal activity of two proteases against Spodoptera frugiperda larvae infected with recombinant baculoviruses. Virol. J.. 2010;7(1):1-10.
- [Google Scholar]
- Genomic sequencing of Troides aeacus nucleopolyhedrovirus (TraeNPV) from golden birdwing larvae (Troides aeacus formosanus) to reveal defective Autographa californica NPV genomic features. BMC Genomics. 2019;20(1):1-16.
- [Google Scholar]
- Application rate trials with a nuclear polyhedrosis virus to control Spodoptera littoralis (Boisd.) on cotton in Egypt. Crop Prot.. 1994;13(5):337-340.
- [Google Scholar]
- Management of Helicoverpa armigera: a review and prospectus for Pakistan. Pak. J. Biol. Sci.. 2000;3(8):1213-1222.
- [Google Scholar]
- Life history traits of a key agricultural pest, Helicoverpa armigera (Lepidoptera: Noctuidae): are laboratory settings appropriate? Austral. Entomol.. 2020;59(1):189-201.
- [Google Scholar]
- Effect of integrating chickpea varieties with insecticides for the management of pod borer (Helicoverpa armigera Hubner)(Lepidoptera: Noctudae) Int. J. Agric. Sci. Food Technol.. 2021;7(1):081-085.
- [Google Scholar]
- Mishra, G., 2021. Gram Pod Borer (Helicoverpa armigera). In Polyphagous Pests of Crops (pp. 311-348). Springer, Singapore.
- Host range and biological activity of three Spodoptera nucleopolyhedrovirus genotypic variants and the effect of Tinopal LPW on the most active variant. Int. J. Pest Manage.. 2003;49(2):147-153.
- [Google Scholar]
- Comparative bio-efficacy of nuclear polyhedrosis virus (NPV) and Spinosad against American bollwormm, Helicoverpa armigera (Hubner) Revista Brasileira de Entomologia. 2019;63(4):277-282.
- [Google Scholar]
- A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) J. Appl. Entomol.. 2021;145(6):467-498.
- [Google Scholar]
- Baculoviruses: Members of integrated pest management strategies. Integrated Pest Management and Pest Control-Current and Future Tactics 2012:463-480.
- [Google Scholar]
- Sajid, Z., Ramzan, M., Shafiq, M.M., Usman, M., Murtaza, G. and Pareek, V., 2021. A Review on Nucleopolyhydroviruses (NPV) as Biological Control of Army Worm, Spodoptera litura.
- Lacanobia oleracea nucleopolyhedrovirus (LaolNPV): a new European species of alphabaculovirus with a narrow host range. PLoS ONE. 2017;12(4):e0176171.
- [Google Scholar]
- Characterization and field evaluation of tetrahedral and triangular nucleopolyhedrovirus of Spilosoma obliqua (SpobNPV) strain NBAIR1 against jute hairy caterpillar. Egypt J. Biol. Pest Control. 2020;30:82.
- [CrossRef] [Google Scholar]
- Biological activity of Spodoptera exigua nuclear polyhedrosis virus against S. exigua larvae. J. Invertebr. Pathol.. 1988;51(2):107-114.
- [Google Scholar]
- Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol.. 2013;31(6):510-521.
- [Google Scholar]
- Isolation and characterization of Ha NPV associated with Helicoverpa armigera insect pest. J. Pharmacognosy Phytochem.. 2019;8(4):3044-3046.
- [Google Scholar]
- Vanderzant, E.S., Richardson, C.D., Fort Jr, S.W., 1962. Rearing of the bollworm on artificial diet. J. Econ. Entomol., 55(1), pp.140-140.
- Generating a host range-expanded recombinant baculovirus. Sci. Rep.. 2016;6(1)
- [CrossRef] [Google Scholar]
- Evaluation of Nuclear Polyhedrosis Virus (NPV) and Emamectin Benzoate against Spodoptera litura (F.) (Lepidoptera: Noctuidae) Egypt. J. Biol. Pest Control. 2020;30(1):1-6.
- [Google Scholar]







