Histopathology unveiling the structural damage in gonads of Catla catla due to freshwater contamination
⁎Corresponding author. arif143@yahoo.com (Tayyaba Sultana)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Potentially harmful materials, e.g., heavy metals, pesticides, dyes, and hydrocarbons, are often released untreated into freshwater bodies. When such pollutants are released in large quantities, there may be large-scale, sudden mortalities of aquatic organisms. This study summarizes experimental evidence about the disruption of gonadal development due to freshwater contamination. Adult fish specimens of Catla catla were collected from the River Chenab downstream of the Chakbandi Main Drain (CMD) entrance into the Chenab River. CMD collects domestic and industrial sewage wastes from Faisalabad city and disposes them into River Chenab, polluting this water body. Water samples were analyzed from each site of fish harvest for water quality parameters. Collected fish specimens were subjected to histopathological evaluation to detect the impact of pollution on the gonads of this fish species. The male Catla catla gonads showed marked histopathological alterations such as testes degeneration, generalized tissue degeneration produced by fragmentation and detachment of basement membrane, necrosis, and fibrosis in the testis. A loss of tubular arrangement, rupturing of the blood vessels, delimiting seminiferous lobules, vascular hypertrophy of the interstitial compartment, and appearance of a clustered organization were observed. The follicular epithelium in vitellogenic oocytes, rupturing and detachment of follicular basement membrane tissue with cytoplasmic vacuolization, immature oocytes with scattered yolk droplets, hypertrophied follicular epithelium, interstitial proteinaceous fluid deposition, and structural deterioration of follicles with early oocytes were observed in the ovary.
Keywords
Toxicity
Industrial Pollutants
Histopathology
Catla catla
Gonads
1 Introduction
Industrial and domestic sewage wastes generally contain a wide variety of inorganic and organic pollutants, such as heavy metals, suspended solids, solvents, oils, fertilizers, and pesticides (Aldemir et al., 2014). Such toxicants reduce water quality and may cause severe threats to living organisms, such as diseases and histological alterations (EI-Ghamdi et al., 2014; Caglar et al., 2019; Soler et al., 2020). Freshwater contamination due to industrial effluents has become one of the most concerning environmental issues (Blanco et al., 2019). Various toxicity endpoints have evaluated the harmful effects of effluents on fish, such as oxidative stress, reducing the detoxification ability of the liver, reduction in digestion in the stomach, impairing the reproductive performance, inducing microbiota dysbiosis in the gut, affecting the genetic coding to offspring and the gene expression (He et al., 2021).
A contaminated environment may also result in histological alteration of fish gonads (Zulfahmi et al., 2018; Caglar et al., 2019). Histopathological alterations have been widely used as biomarkers in evaluating the health of fish exposed to toxicants in field and laboratory studies. One of the substantial advantages of histopathological biomarkers in environmental assessment studies is target organ examination (EI-Ghamdi et al., 2014). An organism's reproductive health is a crucial indicator of its ability to sustain itself. One study by Lemly (2004) reported that about 10 mg/L of selenium in aquatic habitats could lead to 90 % reproductive failure in Lepomis macrochirus (Bluegill sunfish). Xenobiotics such as polybrominated diphenyl ether, polychlorinated biphenyl, and dioxins have been reported to damage the development of gonads and fish embryos (Domínguez-Castanedo et al., 2022).
There are different industrial sectors in Faisalabad city. Among all these sectors, textile dying industries are flourishing very fast. These industries are of great environmental importance because of the consumption of large quantities of water and produce highly polluted water into the water bodies housing diverse faunae (Mehmood et al., 2014). It is essential to perform histological analysis as physiological studies alone cannot comprehensively determine the harmful effects of toxicants (Hussain et al., 2022). The present study aimed to assess the impact of these toxicants on tissues of commercially important fish species Catla catla in aquaria-controlled conditions.
2 Material and methods
2.1 Experimental design
Adult fish specimens of Catla catla were collected from the River Chenab downstream to the Chakbandi Main Drain (CMD) entrance into the Chenab River (31°34′14.0″N 72°32′03.0″E). CMD collects sewage and industrial wastes from Faisalabad city and disposes them into River Chenab. A sampling site Thali, upstream of CMD entrance into the Chenab River, was selected as a control for comparison. Fish was harvested with drag nets and gill nets. Fifteen fish specimens were collected from different experimental sites ranging from 2200 to 2750 g in weight. Water samples were also organized with the help of a water sampler from each site and analyzed for water quality parameters according to the protocols defined by Boyd (1981), while metals were detected through atomic absorption spectrophotometry.
2.2 Histopathological analysis
The fish were carried to the general research laboratory at Government College University Faisalabad in oxygen-filled bags. Fish specimens were anesthetized with phenoxyethanol (Sigma Aldrich) and dissected to remove the gonads (Korkmaz et al., 2022).
2.2.1 Fixation
Fresh gonadal tissues were fixed in sera for 4–6 h. The sera comprised 60 mL of 100 % alcohol (ethanol), 15 mL of glacial acetic acid, and 35 mL of formaldehyde. Then dehydration was performed in ascending grades of ethanol, 70 % (12 h at 20 °C), 80 %, 90 %, and 100 % (2 h at 20 °C each). Fixed tissues were poured into cedar wood oil after dehydration unless they became clear and transparent at 20 °C (Johnson et al., 2009).
2.2.2 Embedding
The tissue specimens were infiltrated with wax. Tissues were fixed into a block of paraffin wax, having the ability to be clinched into a microtome. Tissue specimens are carefully orientated in the mold into the melted wax by considering the plane of the section. After removing, the bubbles, the paraffin wax was allowed to cool down and solidify. Solid wax blocks containing tissues were trimmed with a razor blade and fixed on microtome for sectioning (Johnson et al., 2009).
2.2.3 Microtomy
Paraffin wax-embedded tissue blocks were mounted on wooden blocks for section cutting. Sectioning was performed at 5 µm thickness. The ribbons of sections with tissues were stretched on water (if necessary) and fixed to previously clean albumenized glass slides on a slide warmer at 60 °C. The glass slides were incubated for 12 h to complete stretching.
2.2.4 Staining of tissues
Hematoxylin and eosin staining was performed on the sections. Firstly, the hematoxylin stain was prepared by the following procedure. Hematoxylin (2 g) was added to 100 mL ethanol. Ammonium alum solution was prepared by adding 3 g of alum in H2O and then boiled this solution. The hematoxylin solution was mixed with ammonium alums solution. Glycerol and sodium iodate was added carefully, and then acetic acid was added to this solution and mixed thoroughly. Eosin was prepared by adding the salt of eosin (1 g) in 100 mL of 70 % ethanol. For applying stain to the tissues, paraffin was removed by placing slides in Xylene overnight. Tissue sections on the slides were hydrated in different grading solutions of alcohol in descending order, 100 %, 90 %, 80 %, 70 %, 60 %, 50 %, 40 %, and 30 % ethanol (2 min at 20 °C each). Add hematoxylin and wash in tap water; repeat dipping three times and washing in tap water until the tissues are blue. Sections were dehydrated in ascending grades of alcohol, 30 %, 50 %, 70 %, and 90 % ethanol for 5 min at 20 °C each (Johnson et al., 2009). Slides were then dipped twice in eosin, in 90 % ethanol, and once in Xylene for 10 min. Slides were fixed in Canada balsam (Hussain et al., 2022). Slides were incubated for 12 h, and the coverslipped. Xylene removed the extra Canada balsam.
2.2.5 Light microscopy
Tissue sections were observed under a light microscope at 40x and 100x magnifications. All the slides were photomicrographed and analyzed by comparing them to recent literature.
2.3 Statistical analysis
Acquired data regarding freshwater contamination, the means, standard error, and analysis of variance were calculated using the SPSS-9 program. Means of water quality parameters were compared through DMR tests at a 5 % significance level.
3 Results
Physicochemical parameters analyzed to detect the water quality of River Chenab were found to be higher than the permissible limits indicating the acute level of aquatic toxicity (Table 1). Testis of the control specimen of Catla catla showed everyday arrangements of seminiferous tubules and other testicular anatomical features and showed no abnormalities within the gonadal tissues (Fig. 1). While fish collected from the polluted locale of the River Chenab showed considerable tissue alterations. Photomicrographs of testicular histopathology showed tissue fragmentation and fibrosis in the tissues of the testis. A normal blood flow was seen in some areas of the tissues along with a typical structures of blood vessels (Fig. 2). Photomicrographs indicated a loss of tubular arrangement, degeneration of basement membrane, necrosis, vacuolization in some regions of the tissues (Fig. 3) and rupturing of the blood vessels was also seen in some fish specimens resulting in the release of blood in the tissues (Fig. 4). Gonadal tissues from fish collected from a polluted area of the river also showed testis degeneration, generalized tissue degeneration produced by fragmentation and detachment of basement membrane (Fig. 5).
| Water Quality Parameters (mg/L) | Downstream to entrance of CMD | Upstream to entrance of CMD | Permissible limits |
|---|---|---|---|
| Sulphates | 441 ± 5.71 a | 347 ± 6.94c | D: <400 mg/L, P: 400 mg/L |
| Salinity | 1908 ± 7.69 a | 4123 ± 3.03b | P: <100 mg/L |
| Phenols | 2.32 ± 0.03b | 0.69 ± 0.05c | D: 0.001 mg/L, P: 0.002 mg/L |
| Conductivity (µS/cm) | 3.32 ± 0.03b | 1.3 ± 0.07 a | D: 2.1 µS/cm, P: 2.1 µS/cm |
| Hardness | 577.19 ± 8.13c | 208.42 ± 4.52b | P: 120–170 mg/L P: ** |
| BOD | 77.94 ± 0.39c | 44.7 ± 0.08 a | †D: 30 mg/L, P: ** |
| COD | 189.95 ± 1.94c | 71.4 ± 0.24 a | †D: 250 mg/L, P: ** |
| TSS | 310 ± 2.46 a | 207 ± 5.22c | D: 100 mg/L, P: ** |
| TDS | 2417 ± 6.88 a | 1319 ± 8.01b | D: 500 mg/L, P: 2000 mg/L |
| pH | 11.9 ± 0.25 a | 8.1 ± 0.08b | D: 6.5–8.5, P: ** |
| Mercury | 1.446 ± 0.04b | 0.012 ± 0.0c | D: 0.001 mg/L, P: ** |
| Cobalt | 0.98 ± 0.006b | 0.834 ± 0.06 a | D: 0.05 mg/L |
| Manganese | 2.74 ± 0.2b | 1.83 ± 0.07 a | D: 0.1 mg/L, P: 0.3 mg/L |
| Lead | 2.89 ± 0.047c | 0.15 ± 0.03 a | D: 0.05 mg/L, P: ** |
| Copper | 2.0 ± 0.07 a | 0.85 ± 0.08b | D: 0.05 mg/L, P: 1.5 mg/L |
| Chromium | 0.679 ± 0.07 a | 0.29 ± 0.04b | D: 0.05 mg/L, P: ** |
| Zinc | 0.6 ± 0.04c | 0.2 ± 0.04b | D: 5 mg/L, P: 15 mg/L |
| Tin | 0.49 ± 0.02 a | 0.02 ± 0.0b | D: 0.01 mg/L, P: ** |
| Cadmium | 0.27 ± 0.03 a | 0.021 ± 0.0b | D: 0.01 mg/L, P: ** |
Values (Mean ± SE) are the average of five samples analyzed in triplicate. CMD: Chakbandi Main Drain, BOD: Biological oxygen demand, COD: Chemical oxygen demand. TDS: Total Dissolved solids, TSS: Total suspended solids. Different letters in the same row represent significant (P < 0.05) differences. D; Desirable limits. P; Permissible limits. †; Effluent inland surface water quality standards. ** No relaxation.
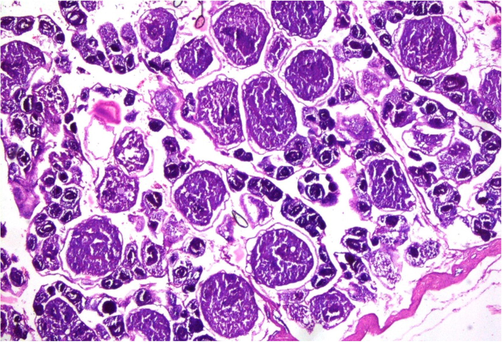
- Photomicrograph of testicular histopathology, illustrating the normal structural pattern of tissue in the testis of fish (control fish specimen from upstream areas).
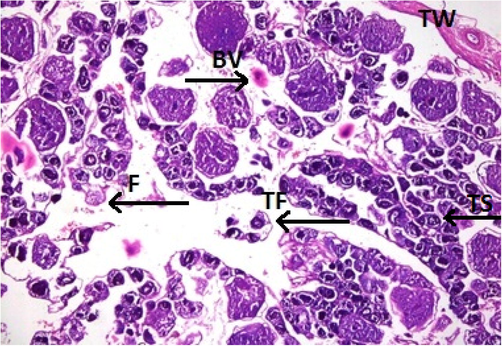
- Photomicrograph of testicular histopathology, illustrating the fibrosis (F) and tissue fragmentation (TF). BV; blood vessel, TW; testicular wall (fish specimen from downstream areas).
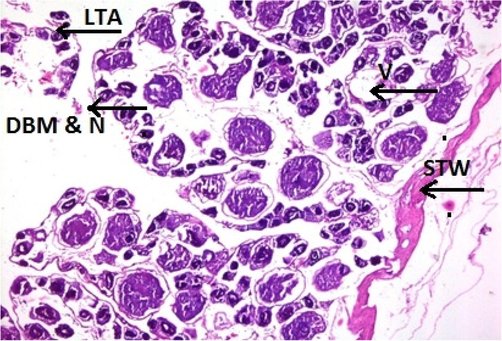
- Photomicrograph of testicular histopathology, illustrating the loss of tubular arrangement (LTA), degeneration of basement membrane (DBM), necrosis (N), vacuolization (V). STW; the surface of the testicular wall (fish specimen from downstream areas).
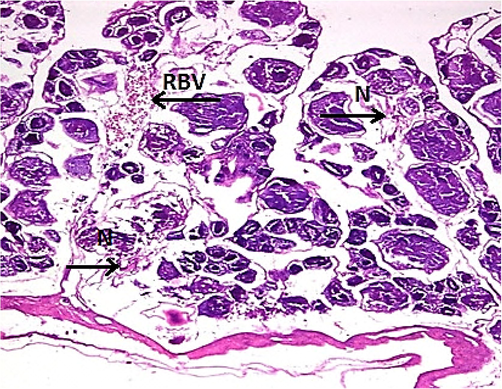
- Photomicrograph of testicular histopathology, illustrating the necrosis (N) and rupturing of the blood vessels (RBV) (fish specimen from downstream areas).
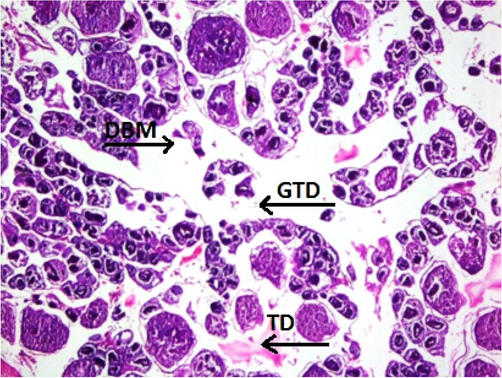
- Photomicrograph of testicular histopathology, illustrating the testis degeneration (TD), generalized tissue degeneration (GTD) produced by fragmentation, and detachment of basement membrane (DBM) (fish specimen from downstream areas).
Fish specimens harvested from the contaminated area of the river downstream CMD were also analyzed for ovarian histopathology. Ovaries of the control group showed the standard architecture in which follicular cells and tunica albuginea were found to be normal (Fig. 6). Photomicrograph of ovarian tissue sections of fish collected from contaminated areas illustrated expansion of the follicular epithelium in vitellogenic oocytes, cyst formation and rupturing of basement membrane leading to necrosis (Fig. 7). Other specimens from this area of acute pollution also showed ovarian tissue alterations such as previtellogenic oocyte, interstitial proteinaceous fluid surrounding previtellogenic oocytes, vitellogenic oocytes with ooplasmic retraction and detachment of follicular epithelium (Fig. 8). Photomicrograph of ovarian histopathology of such fish specimens also illustrated structural deterioration of follicles, oocytes were found with cytoplasmic vacuolization, cyst formation among the tissues, follicular cyst and vacuolization, interstitial proteinaceous fluid deposition and structural decline of follicles (Fig. 9).
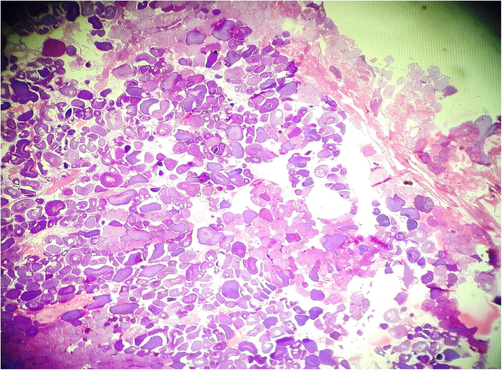
- Photomicrograph of ovarian histopathology, illustrating the normal structure of ovary and development of oocytes (control fish specimen from upstream areas).
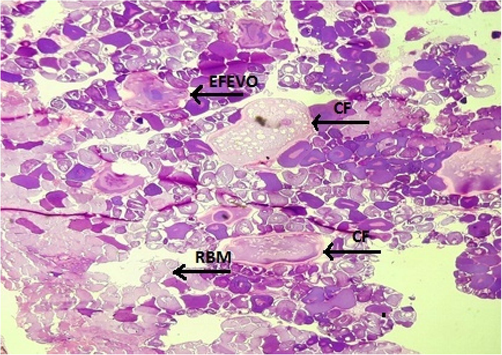
- Photomicrograph of ovarian histopathology, illustrating the expansion of the follicular epithelium in vitellogenic oocytes (EFEVO), cyst formation (CF) and rupturing of basement membrane (RBM) leading to necrosis (fish specimen from downstream areas).
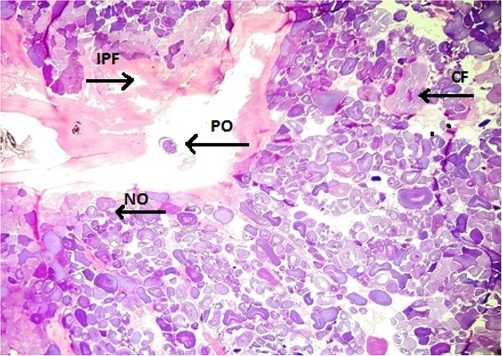
- Photomicrograph of ovarian histopathology, illustrating the previtellogenic oocyte (PO), Interstitial proteinaceous fluid (IPF) surrounding previtellogenic oocytes, vitellogenic oocytes with ooplasmic retraction, and detachment of follicular epithelium, normal oocytes (NO), and cyst formation (CF) (fish specimen from downstream areas).
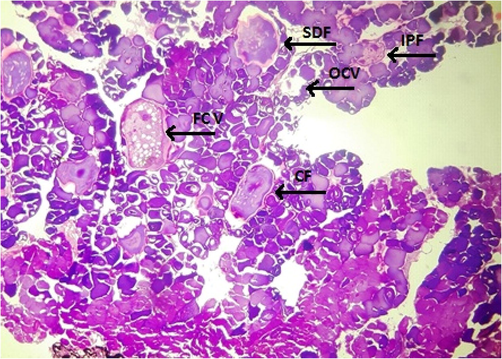
- Photomicrograph of ovarian histopathology, illustrating the structural deterioration of follicles (SDF), oocytes with cytoplasmic vacuolization (OCV), cyst formation (CF), follicular cyst and vacuolization (FCV), interstitial proteinaceous fluid deposition and structural deterioration of follicles (IPF) (fish specimen from downstream areas).
4 Discussion
Literature has sufficient information about the tissue health status of different fish species inhabiting the contaminated water of rivers around the world. However, the impact of this freshwater contamination on the gonadal status of this native species of Indian major carp is scanty in literature. An elevated level of freshwater contamination intensifies stress in fish and induces tissue alterations (Hussain et al., 2021). Catla catla showed dysgenesis, severe hypergonadism, hypogonadism, focal necrosis and changes, and degeneration of gonad cells. The study also indicated that polluted water induces histopathological variations in fish (EI-Ghamdi et al., 2014). Blanco et al. (2019) reported that higher levels of xenobiotic compounds in bile resulted in delayed testes' maturation and increased ovarian aromatase, corroborating this project's findings. In conclusion, they described the impact of pollutants and endocrine disrupters in fish from contaminated water using a combination of histological, chemical, and biochemical biomarkers. Water quality parameters and feeding rate are significant factors in fish growth (Desai and Singh, 2009; Perrier et al., 2012), as the case in this study.
Another study by Hayati et al. (2022) also demonstrated that environmental pollutants affect estradiol and testosterone levels in fish. These hormones regulate the development of male tilapia reproductive organs (Panti et al., 2017). They also demonstrated that pollutants also affect the cell and tissue structure of fish testes resulting in changes in the size of the seminiferous tubules. Hayati et al. (2022) mentioned that exposure to plastic particle pollutants affected spermatogenic development and metabolic disorder that could inhibit the mitotic process, resulting in a lower number of spermatocytes fish compared to the control. They also demonstrated that such types of pollutants could also carry other contaminants, including heavy metals and hydrophobic organic pollutants.
Deng et al. (2020) indicated that contaminants cause serious health risks to aquatic organisms by altering endocrine functions (Abdollahpour et al., 2022; Galloway et al., 2019). Based on these assumptions, a study by Yin et al. (2021) declared that it poses a high risk to humans to consume aquatic organisms (e.g., fish and shrimp) exposed to such contaminants. Another study by Vajda et al. (2008) and Niemuth and Klaper (2015) reported that variation in sex ratio was observed in polluted sites when compared to control areas (Zhu et al., 2022). The latter study also found changes and improper responses in gonadal development (Du et al., 2022), alterations in tissue histology (Talas et al., 2014), and steroid hormone production (Sutha et al., 2022). Blanco et al. (2019) also demonstrated that biochemical alterations in the fish's body might be of low ecological importance, but they always precede the changes at the organism and population level. So it is essential to consider them as early warnings of ecosystem stress. The current study's findings show that the River Chenab cannot cope with contamination and highlight the need to improve water quality to protect this native fish species.
5 Conclusion
It has been that male Catla catla collected from polluted locations showed marked histopathological alterations in the testis. Ovaries also showed tissue alterations as the expansion of the follicular epithelium in vitellogenic oocytes, rupturing and detachment of follicular basement membrane, tissue with cytoplasmic vacuolization, immature oocytes with scattered yolk droplets, hypertrophied follicular epithelium, interstitial proteinaceus fluid deposition and structural deterioration of follicles with early oocytes. It hast been observed that River Chenab has an acute level of contamination and highlighted the need to recover water quality to protect this freshwater body and its inhabitants.
Acknowledgments
“The authors (FAM) express their sincere appreciation to the Researchers Supporting Project Number (2021/24) King Saud University, Riyadh, Saudi Arabia”.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of water temperature and food availability on growth performance, sex ratio and gonadal development in juvenile convict cichlid (Amatitlania nigrofasciata) J. Therm. Biol. 2022:103255.
- [Google Scholar]
- Role of propolis on oxidative stress in various tissues of fish. Fresenius Environ. Bull.. 2014;23(12):3546-3550.
- [Google Scholar]
- Assessing the impact of wastewater effluents on native fish species from a semi-arid region. NE Spain. Sci. Total Environ.. 2019;654:218-225.
- [Google Scholar]
- Water quality in warm water fish ponds. Craftmaster Auburn: Alabama, USA, Printers Inc; 1981.
- Determination of some heavy metal levels in three freshwater fish in Keban Dam Lake (Turkey) for public consumption. Iran. J. Fish. Sci.. 2019;18(1):188-198.
- [Google Scholar]
- Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int.. 2020;143(105916):1-10.
- [Google Scholar]
- The effects of water temperature and ration size on growth and body composition of fry of common carp, Cyprinus carpio. J. Therm. Biol.. 2009;34:276-280.
- [Google Scholar]
- Protogynous functional hermaphroditism in the North American annual killifish. Millerichthys robustus. Sci. Rep.. 2022;12(1):1-14.
- [Google Scholar]
- Timing of early gonadal differentiation and effects of estradiol-17β treatments on the sex differentiation in Largemouth bass (Micropterus salmoides) Fish Physiol. Biochem.. 2022;48(3):805-815.
- [Google Scholar]
- Structural alterations in gills, liver and ovaries of tilapia fish (Saratherodon galilaeus) as a Biomarker for Environmental Pollution in Ismalia Canal. Catrina. Int. J. Environ. Sci.. 2014;9(1):7-14.
- [Google Scholar]
- Plastics additivesand human health: A case study of Bisphenol A (Bpa) Environ. Sci. Technol.. 2019;47(1):131-155.
- [Google Scholar]
- Assessing the recovery of steroid levels and gonadal histopathology of tilapia exposed to polystyrene particle pollution by supplementary feed. Vet. World.. 2022;15(2):517-523.
- [Google Scholar]
- Enhanced toxicity of triphenyl phosphate to zebrafish in the presence of micro-and nano-plastics. Sci. Total Environ.. 2021;756:143986
- [Google Scholar]
- Assessment of cytonephrotoxicity and growth performance in Labeo rohita induced by fluorescein dye Y and B. J. King Saud Univ. Sci.. 2021;33(8):101672
- [Google Scholar]
- Assessment of hepatotoxicity and nephrotoxicity in Cirrhinus mrigala induced by trypan blue - An azo dye. Environ. Res.. 2022;215(1):114120
- [Google Scholar]
- OECD guidance document for the diagnosis of endocrine-related histopathology of fish gonads. Organization for Economic Co-operation and Development; 2009. p. :15.
- Effects of Lead on Reproduction Physiology and Liver and Gonad Histology of Male Cyprinus carpio. Bull. Environ. Contam. Toxicol.. 2022;108(4):685-693.
- [Google Scholar]
- Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol. Environ. Saf.. 2004;59(1):44-56.
- [Google Scholar]
- Mehmood., A.S.I.F., 2014. Urban Pakistan. State of Pakistani Cities Report. UN Habitat, Pakistan. Pp. 52.
- Emerging wastewater contaminant metformin causes intersex and reduced fecundity in fish. Chemosphere. 2015;135:38-45.
- [Google Scholar]
- Panti, C., Rysky, E., Pedà, C., Maricchiolo, G., 2017. Gene expression in liver of European Sea Bass Dicentrarchus labrax experimentally exposed to PVC microplastics. Conference: SETAC Europe 2017. 27th Annual Meeting. DOI: 10.13140/RG.2.2.12883.68640.
- Effects of temperature and food supply on the growth of whitefish Coregonus lavaretus larvae in an oligotrophic peri-alpine lake. J. Fish Biol.. 2012;81:1501-1513.
- [Google Scholar]
- Effects of industrial pollution on the reproductive biology of Squalius laietanus (Actinopterygii, Cyprinidae) in a Mediterranean stream (NE Iberian Peninsula) Fish Physiol. Biochem.. 2020;46(1):247-264.
- [Google Scholar]
- Long term exposure to tris (2-chloroethyl) phosphate (TCEP) causes alterations in reproductive hormones, vitellogenin, antioxidant enzymes, and histology of gonads in zebrafish (Danio rerio): In vivo and computational analysis. Comp. Biochem. Physiol. Part - C: Toxicol. Pharmacol.. 2022;254:109263
- [Google Scholar]
- Antioxidant effects of propolis on carp Cyprinus carpio exposed to arsenic: biochemical and histopathologic findings. Iran J. Fish. Sci.. 2014;108(3):241-249.
- [Google Scholar]
- Reproductive disruption in fish downstream from an estrogenic wastewater effluent. Environ. Sci. Technol.. 2008;42(9):3407-3414.
- [Google Scholar]
- A comparative review of microplastics and nanoplastics: Toxicity hazards on the digestive, reproductive and nervous system. Sci. Total Environ.. 2021;774(145758):1-14.
- [Google Scholar]
- Development and gene expression analysis of gonad during 17α-methyltestosterone-induced sex reversal in mandarin fish (Siniperca chuatsi) Aquac. Rep.. 2022;23:101049
- [Google Scholar]
- Reproductive performance and gonad histopathology of female Nile tilapia (Oreochromis niloticus Linnaeus 1758) exposed to palm oil mill effluent. Egypt. J. Aquat. Res.. 2018;44(4):327-332.
- [Google Scholar]







