Translate this page into:
High plasma levels of the TSLP cytokine in Saudi patients with chronic stable asthma
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Allergic asthma is an inflammatory bronchial disease associated with the IgE response, allergic inflammation of the airways, recruitment of infiltrated inflammatory cells, increased mucus production, constriction of airways, and difficulty breathing. TSLP cytokine is derived mainly from airway epithelial cells and have been shown to play an essential role in the pathogenesis of asthma. This study was designed to determine the plasma protein concentrations of total IgE and TSLP cytokines in patients with chronic stable asthma. Furthermore, we examined TSLP-r in B cells and determined whether the expression of TSLP is correlated with increased total IgE. Thirty-one patients with chronic stable asthma and nineteen normal controls were enrolled in the study. Blood samples were separated using the Ficoll gradient method, and plasma and lymphocyte cells were collected. Plasma levels of total IgE and TSLP were quantified in patients with asthma and normal controls by specific ELISAs. Moreover, the surface expression of TSLP-r on B cells was analyzed using flow cytometry. Total IgE protein concentrations in the plasma of patients with chronic stable asthma (344 IU/ml, P < 0.002) were considerably higher than those in normal controls (mean 130 IU/ml). TSLP levels were significantly higher in the asthma group (78 Pg/ml, P < 0.02) than those in the normal control group (40 pg/ml). Additionally, the expression of TSLP-r was markedly higher in asthma patients (430 × 103 MFI, P < 0.0065) than in normal controls (280 × 103). Collectively, the findings indicate that measuring plasma levels of TSLP in patients with stable asthma can be used to assess asthma symptoms and possibly evaluate the efficacy of corticosteroid therapy.
Keywords
TSLP
TSLP-r
Asthma
IgE
B cells
1 Introduction
Allergic asthma is an airway disorder that mainly affects the bronchial airways of the lungs (Alturaiki et al., 2021). The incidence rate of asthma is increasing each year, and approximately 400 million people have asthma worldwide, creating an increased economic burden (Baos et al., 2017). Recently, asthma prevalence has increased in Saudi Arabia (Musharrafieh et al., 2020). This disease is categorized by allergic inflammation of the lungs due to a robust response to specific allergens, leading to hyperresponsiveness and increased airway remodelling (AWR), alterations in the structure of the bronchial epithelial cells, and increased production of mucus. These changes can cause narrowing of the airways and result in difficulty breathing (Fehrenbach et al., 2017). The exact cause of asthma is still unknown. However, multiple factors and varieties of cells and molecules contribute to asthma pathogenicity (Alturaiki, 2020; Alturaiki, 2020).
Thymic stromal lymphopoietin cytokine (TSLP) is primarily expressed by airway epithelial cells and can also be released from other cells, including dendritic cells, granulocytes, macrophages, airway smooth muscle cells, mast cells, stromal cells, fibroblasts, Dendritic cells (DCs), and monocytes (Mitchell and O'Byrne, 2017; Verstraete et al., 2017).
Furthermore, it has been shown that cultured primary bronchial epithelial cells isolated from patients with severe asthma can secrete TSLP spontaneously (Semlali et al., 2010), which suggests that TSLP plays a role in human asthma etiology (Mitchell and O'Byrne, 2017). In addition, aberrant TSLP expression has been shown to correlate with other allergic diseases, such as atopic dermatitis, pulmonary fibrosis, eosinophilic esophagitis and chronic obstructive pulmonary disease (Mitchell and O'Byrne, 2017; Noti et al., 2013; Redhu and Gounni, 2012; Datta et al., 2013). The expression of TSLP is induced by viral, bacterial, and parasitic infections as well as allergen agents (Mitchell and O'Byrne, 2017; Datta et al., 2013; Glück et al., 2016). Additionally, stimulation of airway epithelial cells isolated from asthmatic patients with viral double-stranded RNA responded strongly and produced higher levels of TSLP relative to normal controls (Brandelius et al., 2011). TSLP has been shown to activate naïve T cells to differentiate into Th2 cells, which can produce higher levels of IL-4, IL-5, and IL-13 that enhance the production of immunoglobulin (Ig)E, activation of mast cells, and mucus production and promote airway hyperresponsiveness (So et al., 2006; Dorman et al., 2004; Borowski et al., 2013). Furthermore, a mouse model of asthma revealed that TSLP overexpression in the lungs resulted in asthma-like symptoms (Headley et al., 2009). This study aimed to indirectly determine pulmonary inflammation in allergic asthma by measuring the plasma protein concentrations of total IgE, TSLP, and TSLP-r in patients with chronic stable asthma as potential markers for lung inflammation.
2 Materials and methods
2.1 Subjects
The study comprised thirty-one Saudi patients who were clinically diagnosed and examined for chronic stable asthma according to Saudi Initiative for Asthma standards and nineteen normal controls (Al-Moamary et al., 2021). Patients were chosen based on their symptoms and responses to an asthma control test questionnaire, with a score of 20 or higher classified as chronic stable asthma (Table 1). The study excluded other respiratory or chronic disorders, smoking, obesity, pregnancy, diabetes mellitus, hypertension, and recent vaccination. Healthy volunteers were chosen from the blood bank as normal controls. The normal controls had no recent respiratory infections, chronic conditions, smoking, recent vaccinations or treatments, and no history of other allergic diseases. The subjects of the study completed a consent form, and Majmmah University's ethics committee permitted the study (MURECApril. 01/COM-206). Budesonide (200 μg/day) Beclomethasone dipropionate (100 μg) ± Salbutamol inhaler
Category
Normal control
Asthma patients
Subjects number
19
31
Sex (male, female)
11/8
17/14
Age (mean years)
33.07
35.04
Duration of asthma (mean years)
–
8.5
Treatment types
–
Inhaled corticosteroids (ICS), including:
2.2 Isolation of blood samples
All of the study participants had peripheral blood samples (5 ml) collected in an EDTA tube. Then, using the Ficoll gradient method, plasma and lymphocytes were separated as follows. Blood was gently mixed with an equal volume of Ficoll before centrifugation at 1800 rpm for 30 min at 25 °C, and two layers were formed. Plasma (top layer) was aspirated and aliquoted into Eppendorf tubes. After that, lymphocytes were collected from the interface layer and counted using a hemocytometer after being washed in PBS. The lymphocytes were then resuspended in RPMI 1640 media supplemented with 12.5 % FCS and 20 % DMSO and frozen at −20 °C.
2.3 Quantification of total IgE and TSLP levels
Specific ELISAs were used to measure the levels of total IgE (cat: ab108650; Abcam, UK) and TSLP (cat: DTSLP0; R&D Systems, USA) in the plasma according to the manufacturer’s datasheet. An ELISA plate reader was used to measure the optical density at a wavelength of 450 nm. Additionally, KC Junior software was used to determine the final protein concentration for each protein.
2.4 Flow cytometric analysis of TSLP receptor (TSLP-r)
Human lymphocytes (2 × 105) were thawed, washed and blocked with 0.02 % BSA for 30 min at room temperature (25 °C). After washing the cells, specific conjugated antibodies for B cells (PerCP anti-human CD19, cat. no. 363014; BioLegend, USA) and PE anti-human TSLP-r (cat. no. 322906; BioLegend) were then incubated in BSA for 30 min at 4 °C in cool and dark conditions. Negative control cells (unstained cells) were applied in each experiment. Subsequently, after incubation, the stained cells were rinsed and resuspended in 0.5 ml PBS and flow cytometrically analyzed with a FACSCanto II (BD Biosciences). BD FACSDivaTM software was used to analyze the data and determine the fluorescence intensity (BD Biosciences).
2.5 Statistical analysis
The results of the experiments are expressed as the mean and standard deviation (SD). The Mann–Whitney U test was used to examine the data using statistical software (GraphPad Prism 6.0 Software Inc., La Jolla, CA, USA). The association between total IgE and TSLP levels was analyzed using Pearson's test. A* P value<0.05 indicated a statistically significant value.
3 Results
3.1 Patients with stable asthma produced significantly high levels of total IgE
Total IgE concentrations were markedly increased in patients with stable asthma (mean = 344 IU/ml, P < 0.002) compared with those in normal controls (mean 130 IU/ml) (Fig. 1).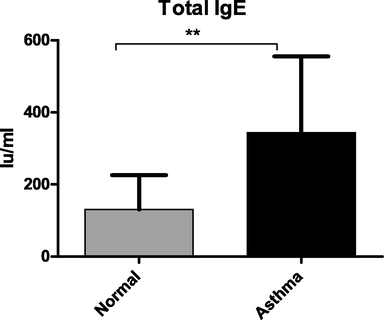
Quantification of total IgE levels concentrations in plasma. A specific ELISA was used to measure total IgE protein concentrations. Stable chronic asthma produced significantly higher levels of total IgE than normal controls. (** P < 0.002).
3.2 Increased TSLP protein levels in patients with chronic stable asthma
Levels of TSLP protein were significantly increased in the chronic stable asthma group (mean = 78 Pg/ml, P < 0.02) compared with the normal controls (40 pg/ml) (Fig. 2a). Additionally, no association was noticed between increased expression of TSLP and total IgE production (Fig. 2b).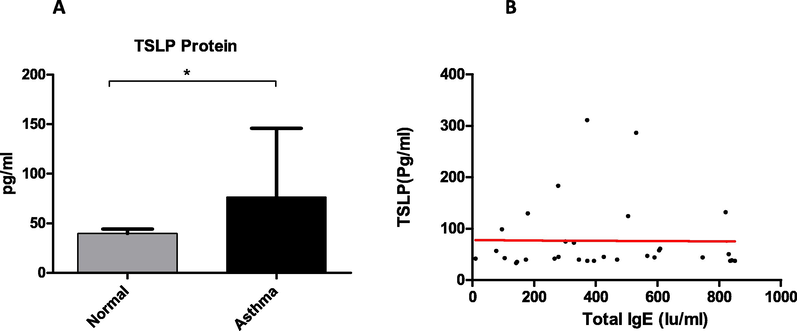
Quantification of TSLP and its correlation with total IgE in plasma. A specific ELISA was used to measure TSLP protein concentrations. Patients with stable asthma and normal controls have significantly different amounts of TSLP protein (a). The mean and standard deviation (SD) are used to represent the data. According to Pearson's test (r = 6.272e-006), there is no relationship between TSLP and total IgE levels in the plasma of asthmatic patients (B).
3.3 TSLP-r expression increased significantly in patients with chronic stable asthma
Surface expression of TSLP-r was examined by flow cytometric analysis in gated peripheral blood B cells (CD19 + ). TSLP-r levels were markedly increased in patients with asthma (mean 430 × 103, P < 0.0065) compared to normal controls (280 × 103 MFI) (Fig. 3).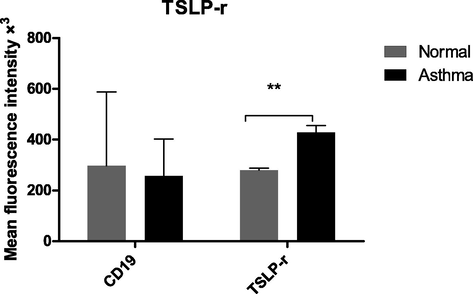
Surface expression of TSLP-r in gated B cells. TSLP-r levels in patients with chronic stable asthma and normal controls were investigated using flow cytometric techniques. The data are shown as the mean and standard deviation of four different experiments. For each experiment, negative controls were used. **p = 0.006.
4 Discussion
Bronchial allergic asthma is an airway allergy distinguished by elevated total IgE levels (Crespo-Lessmann et al., 2020). Similarly, in the present study, patients with chronic stable asthma expressed significantly higher concentrations of total IgE in the plasma than normal controls. Uncontrolled cytokine expression is one of the other possible causes of asthma pathogenicity (Pandey et al., 2021).
Furthermore, the role of TSLP cytokines in asthma has been explored in various studies. It has been shown that TSLP levels were increased significantly in the exhaled breath condensate, sera, sputum, and bronchoalveolar lavage fluid (BALF) of asthmatic patients compared with normal controls (Glück et al., 2016; Chai et al., 2017; Berraïes et al., 2016; Li et al., 2018). In addition, TSLP levels are associated with asthma severity (Shikotra et al., 2012; Barnes, 2010). In agreement with these studies, patients with stable asthma had considerably higher TSLP protein plasma levels than normal controls.
Although inhaled corticosteroids (ICS) have been reported to inhibit the expression of inflammatory cytokines and chemokines in asthma (Kabata et al., 2013), TSLP plasma levels were markedly higher in patients with stable asthma who used ICS. This finding is consistent with another study that found that TSLP levels were increased significantly in severe asthmatic patients regardless of the use of high-dose corticosteroid treatments (Shikotra et al., 2012).
Additionally, it has been shown that TSLP mediates an essential role during airway inflammation and enhances the induction of corticosteroid resistance in vitro and in vivo via modulation of the phosphorylation process of STAT5 and expression of Bcl-xL in NH cells, and inhibition or blockade of the TSLP/STAT5 pathway with a neutralizing antibody improves the sensitivity to corticosteroids (Szefler et al., 2002). Collectively, these outcomes may suggest that measuring TSLP levels in patients with chronic stable asthma may be used as an additional marker to monitor asthma symptoms and possibly to evaluate the response to ICS therapy, as it has been reported that patients with asthma do not respond correspondingly to corticosteroid therapy (Pelaia et al., 2021).
Furthermore, TSLP can indirectly induce IgE production by influencing the Th2 response and cytokine production, including IL-4, IL-5 and IL-13, which promote IgE synthesis. However, there was no association between increased TSLP expression and total IgE (Cianferoni and Spergel, 2014).
TSLP exerts its biological function after interaction with its receptor TSLP-r. TSLP-r is expressed by several immune cells and tissues (Zhou et al., 2005). Flow cytometric analysis of TSLP-r in B cells (CD19) from patients with asthma showed that the surface expression of TSLP-r was significantly higher than that in normal controls. Similarly, increased expression of TSLP protein corresponds with increased expression of TSLP-r receptor. This result shows the possible elevated B-cell response to TSLP cytokines. Moreover, it has been shown that mice lacking TSLP-r or treated with anti-TSLP-r fusion antibody failed to develop allergic inflammation compared to control animals (Al-Shami et al., 2005); suggesting the importance of TSLP and TSLP-r in maintaining airway inflammation.
The current study had some limitations, one of which was the limited sample size. Therefore, a larger sample size is required to examine whether expression of TSLP and its ligand (TSLP-r) is associated with asthma symptoms, such as mild, moderate, and severe symptoms. Furthermore, the analysis of TSLP expression and total IgE levels in lung secretions or tissue sections was out of the study's scope.
Finally, this study showed that TSLP protein levels in patients with chronic stable asthma are considerably greater than those in normal controls. TSLP-r levels were also significantly higher in the asthma group than in the normal control group. Further studies are required to identify the precise role of TSLP in enhancing IgE production in the lungs and how TSLP might drive the Th2 cell response in the airways that can activate allergic inflammation in the lungs. Additionally, measuring TSLP protein levels in the plasma of asthmatic patients could be a useful method for determining asthma symptoms and monitoring treatment response.
Acknowledgements
I would like to thank the administration of Alzulfi General Hospital represented by Mr. Abdulaziz Alshalani and the head of the central laboratory and blood bank, Mr. Khaled Aldurymeh, for their kind help and continuing support. Extended thanks to Dr. Sajad Mir for tremendous help in patient characterization. Additionally, I am thankful to Dr. Adnan Afridi for his help in sample collection. Thanks to Dr. Suresh Mickymaray for his extraordinary efforts in the processes and isolation of samples during the study.
Funding
I would like to thank the Deanship of Scientific Research at Majmaah University for funding the study under project number [R-2022-211].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The Saudi Initiative for Asthma - 2021 Update: Guidelines for the diagnosis and management of asthma in adults and children. Ann. Thorac. Med.. 2021;16(1):4.
- [Google Scholar]
- A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med.. 2005;202(6):829-839.
- [Google Scholar]
- Evaluation of C-C Chemokine Ligand 5 (CCL5) Chemokine, Interleukin 5 (IL-5) Cytokine, and Eosinophil Counts as Potential Biomarkers in Saudi Patients with Chronic Asthma During Sandstorms. Cureus.. 2020;12(4) e7809-e
- [Google Scholar]
- The roles of B cell activation factor (BAFF) and a proliferation-inducing ligand (APRIL) in allergic asthma. Immunol. Lett.. 2020;225:25-30.
- [Google Scholar]
- Plasma levels of BAFF and APRIL are elevated in patients with asthma in Saudi Arabia. Saudi J. Biol. Sci.. 2021;28(12):7455-7459.
- [Google Scholar]
- Biomarkers associated with disease severity in allergic and nonallergic asthma. Mol. Immunol.. 2017;82:34-45.
- [Google Scholar]
- Increased expression of thymic stromal lymphopoietin in induced sputum from asthmatic children. Immunol. Lett.. 2016;178:85-91.
- [Google Scholar]
- Expression analysis and specific blockade of the receptor for human thymic stromal lymphopoietin (TSLP) by novel antibodies to the human TSLPRα receptor chain. Cytokine. 2013;61(2):546-555.
- [Google Scholar]
- dsRNA-induced expression of thymic stromal lymphopoietin (TSLP) in asthmatic epithelial cells is inhibited by a small airway relaxant. Pulm. Pharmacol. Ther.. 2011;24(1):59-66.
- [Google Scholar]
- The significance of the levels of IL-4, IL-31 and TLSP in patients with asthma and/or rhinitis. Immunotherapy.. 2017;9(4):331-337.
- [Google Scholar]
- The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol.. 2014;10(11):1463-1474.
- [Google Scholar]
- Total and specific immunoglobulin E in induced sputum in allergic and non-allergic asthma. PLoS ONE. 2020;15(1) e0228045
- [Google Scholar]
- Evidence for a functional thymic stromal lymphopoietin signaling axis in fibrotic lung disease. J. Immunol.. 2013;191(9):4867-4879.
- [Google Scholar]
- Sputum CD34+ IL-5Rα+ cells increase after allergen: evidence for in situ eosinophilopoiesis. Am. J. Respir. Crit. Care Med.. 2004;169(5):573-577.
- [Google Scholar]
- Airway remodeling in asthma: what really matters. Cell Tissue Res.. 2017;367(3):551-569.
- [Google Scholar]
- Increased levels of interleukin-33 and thymic stromal lymphopoietin in exhaled breath condensate in chronic bronchial asthma. Int. Arch. Allergy Immunol.. 2016;169(1):51-56.
- [Google Scholar]
- TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J. Immunol.. 2009;182(3):1641-1647.
- [Google Scholar]
- Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat. Commun.. 2013;4(1)
- [Google Scholar]
- Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J. Immunol.. 2018;200(7):2253-2262.
- [Google Scholar]
- Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma. Pharmacol. Ther.. 2017;169:104-112.
- [Google Scholar]
- A nationwide study of asthma correlates among adolescents in Saudi Arabia. Asthma Res. Pract.. 2020;6:1-8.
- [Google Scholar]
- Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat. Med.. 2013;19(8):1005-1013.
- [Google Scholar]
- An update on the diagnostic biomarkers for asthma. J Family Med Prim Care.. 2021;10(3):1139.
- [Google Scholar]
- Tezepelumab: A Potential New Biological Therapy for Severe Refractory Asthma. Int. J. Mol. Sci.. 2021;22(9):4369.
- [Google Scholar]
- Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin. Exp. Allergy: J. Br. Soc. Allergy Clin. Immunol.. 2012;42(7):994-1005.
- [Google Scholar]
- Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J. Allergy Clin. Immunol.. 2010;125(4):844-850.
- [Google Scholar]
- Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immun.. 2012;129(1):104-111.e9.
- [Google Scholar]
- Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc. Natl. Acad. Sci.. 2006;103(10):3740-3745.
- [Google Scholar]
- Significant variability in response to inhaled corticosteroids for persistent asthma. J. Allergy Clin. Immunol.. 2002;109(3):410-418.
- [Google Scholar]
- Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun.. 2017;8(1)
- [Google Scholar]
- Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol.. 2005;6(10):1047-1053.
- [Google Scholar]







