Translate this page into:
Hepatoprotective role of swimming against arsenic induced oxidative stress in mice
⁎Corresponding author. zaigham.mmg@pu.edu.pk (Zaigham Abbas)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Arsenic is the most prevalent and common environmental contaminant, found in drinking water with varying concentrations. Inorganic arsenic is involved in the generation of reactive oxygen species (ROS). The aim of present study was to determine the activities of antioxidant enzymes in liver of mice, changes with arsenic exposure and to determine whether it is associated with the induction of oxidative stress. Furthermore, we intended to determine whether swimming exercise can modulate the activities of antioxidant enzymes in vivo rendering protection to the liver of mice during arsenic exposure.
Methods
Mouse model was established, mice were administrated with two different doses (10 and 20 mg/kg/day) of sodium arsenite. These mice underwent swimming exercise for about 8 weeks regularly. First group was negative control which received distilled water and no exercise. Group 2 and 4 were positive controls in which 10 mg/kg/day and 20 mg/kg/day of sodium arsenite were administered respectively, for 8 weeks. Group 3 and 5 underwent swimming exercise for 60 min daily along with 10 mg/kg/day and 20 mg/kg/day of sodium arsenite for same duration and act as treatment group.
Results
Our results revealed that arsenic level was higher (0.13 mg/L) in the liver of mice lacking exercise in comparison to the exercise group (0.04 mg/L). Similarly, Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were found higher in mice lacking swimming exercises showing sodium arsenite had more severe pathological damages in liver as compared to the exercise groups. On the other hand, catalase (2.61 nmol/mg) and glutathione levels (1.25 nmol/mg) and expression of two antioxidant pathway genes i.e. NQO-1 and HO-1 were found elevated in exercise group.
Conclusion
This study reveals that swimming alleviate the arsenic induced liver damages in mice through induction of Nrf2 antioxidant pathways. All these findings allowed us to speculate the involvement of swimming exercise which is responsible for hepato-protective activity.
Keywords
Sodium arsenite
Swimming exercise
Antioxidants
Oxidative stress
Signaling pathway
Pathological damages
- Nrf2
-
Nuclear factor erythroid 2-related factor 2
- NQO-1
-
NAD (P) H: Quinone oxidoreductase 1
- HO-1
-
Heme oxygenase 1
- ALT
-
Alanine Aminotransferase
- AST
-
Aspartate Aminotransferase
- MMA
-
Monomethylarsonic acid
- DMA
-
Dimethylarsonic acid
- UNICEF
-
United Nations Children's Fund
- PCRWR
-
Pakistan Council of Research in Water Resources
- WHO
-
World Health organization
- As
-
Arsenic
- ROS
-
Reactive Oxygen Species
- GSH
-
Glutathione
- NADPH
-
Nicotinamide adenine dinucleotide phosphate
- SOD
-
Superoxide Dismutase
- Keap-1
-
Kelch like ECH associating protein 1
- ARE
-
Antioxidant response element
- ICP-AES
-
Inductively coupled plasma atomic emission spectroscopy
- NHMRC
-
National Health and Medical Research Council
Abbreviations
1 Introduction
Natural processes as well as anthropogenic activities intrude the natural environment by the introduction of toxic ingredients (Ahsan et al., 2006). In nature, arsenic mostly present in two forms, organic and inorganic. Inorganic forms of the arsenic are much toxic and prevalent (Alam et al., 2016). The World Health Organization (WHO) and National Health and Medical Research Council set the threshold limit of 10 µg/L of Arsenic in drinking water in 2008 and 2011, respectively. According to approximate calculations by WHO more than 200 million people may be exposed to > 10 µg/L concentration of arsenic in drinking water, either as (As III) or arsenate (As V) (Amat et al., 2010). Large scale arsenic-related drinking water contaminants have been reported in different countries including Bangladesh, Taiwan, Chile, Argentina & India (Amer et al., 2016; Argos et al., 2012; Argos et al., 2010; Bashir et al., 2006; Bryan et al., 2013; Calatayud et al., 2014; Carp et al., 2016). Recently, United Nations Children's Fund (UNICEF) and PCRWR (Pakistan Council of Research in Water Resources) reported that groundwater arsenic ranges between 10 and 200 µg/L in Pakistan (Chen et al., 2010). Exposure of humans to arsenic is mainly by the drinking water, industrial activities, cosmetics, smoking, and through air sources (Chen et al., 2015; Dewanjee et al., 2015). Exposure of arsenic is associated with various cancers like bladder cancer (Farooqi et al., 2007), lung cancer & liver cancer, high concentration of arsenic about 0.64 mg/L increases the incidence of liver cancer (Fitzgerald et al., 2014).
Arsenic is involved in the generation of reactive oxygen species (ROS) and the exact mechanism for the production of ROS is not clear. But at a point when cell is exposed to the arsenic it induces the oxidative stress conditions in the cells. Free radicals like singlet oxygen (1O2), superoxide anion radical (O2−•), hydrogen peroxide (H2O2), nitric oxide (NO), dimethylarsonic radical [(CH3)2As•], peroxyl radical (ROO·), and dimethylarsonic peroxyl radicals ([(CH3)2AsOO•]) can cause harm to lipids, proteins, and DNA. Arsenic is capable of generating NO by the assistance of nitrogen synthase and may cause genotoxicity and mutagenicity (Fischer et al., 2008).
The antioxidants play a very substantive and significant role in the elimination of ROS. Oxidative stress, which is caused by metals (arsenic) simply overcome by the nuclear factor (erythroid derived-2) related factor-2 (Nrf2). Nrf2 maintains the redox homeostasis by the regulation of detoxification and antioxidant enzymes (Flora et al., 2011). Keap1, a suppressor protein is present in the cytoplasm that binds with the Nrf2 and prevents the translocation of Nrf2 into the nucleus. Under oxidative Nrf2 gets phosphorylated and easily translocated into the nucleus, binds with the antioxidant response element [ARE]-containing promoter (Flora et al., 2011) and upregulates the expression of antioxidant enzymes like NQO-1 (NAD (P) H: Quinone oxidoreductase 1), catalase, SOD, HO1 (heme oxygenase 1), GSH (glutathione) and many other enzymes (Frasier et al., 2013). For example during exercise free radicals, which are produced during the contraction of skeletal muscles act as signaling molecules resulting in the increased expression of genes involved in antioxidant pathways (Nrf2) and decrease the level of oxidative stress (Gao et al., 2013). Therefore, present study aimed at determining the hepato-protective role of swimming on arsenic induced liver injuries. However, hepato protective role of physical activities, for instance, swimming in arsenic-mediated hepatic injuries was determined in the present study.
2 Material and methods
2.1 Experimental design
For this study a prior approval was obtained by Institutional Biosafety and Ethical Committee of MMG, University of the Punjab, Lahore. In total 30 Male BALB/c mice (30 g ± 4.3 wt and 8–10 weeks of age) were kept in laboratory animal house of Department of Microbiology and Molecular Genetics (MMG), University of the Punjab Lahore Pakistan at constant temperature (24 ± 2) in natural light–dark cycle (12–12 hrs.). All animals were fed with standard diet and water ad libitum. The animals were randomly divided into 5 groups each group containing 6 animals (n = 6).
The first group was negative control which received distilled water and no exercise. Group 2 and 4 were positive controls in which 10 mg/kg/day and 20 mg/kg/day of sodium arsenite were administered respectively, for a period of 8 weeks. Group 3 and 5 underwent swimming exercise for 60 min daily along with 10 mg/kg/day and 20 mg/kg/day of sodium arsenite (Sigma Aldrich, Germany), for same number of days and act as treatment group. During swimming exercise water temperature was maintained at 32 ± 2 °C. The dose of sodium arsenite (10 mg/kg/day and 20 mg/kg/day) and swimming conditions were selected, based on already reported studies (Ghatak et al., 2011). Sodium arsenite was administered in the drinking water and freshly prepared every day.
2.2 Arsenic level estimation in mice liver
In order to determine arsenic levels 100 mg of liver tissue of each mouse was taken and treated with 1 ml of concentrated nitric acid followed by 1 ml of per chloric acid. Tissue samples were placed in the sand bath until their color turned yellow. 5 ml deionized water was added to each digest and arsenic level was estimated by using ICP atomic absorption spectrometer.
2.3 Gene expression analysis
100 mg of liver was used to extract RNA by using Trizol reagent (Invitrogen, USA). The extracted RNA quantity and quality was measured by nano drop. Equal amount (1 μg) of RNA from each sample was converted into complementary DNA (cDNA) by using Thermo scientific RevertAid First strand cDNA synthesis Kit (Thermo Scientific, USA), according to the manufacturer’s protocol. Quantitative real time PCR (Qpcr) was performed on synthesized cDNA to evaluate the expression NQO-1 and HO-1. The qPCR was performed by using Maxima SYBER Green/ROX qPCR Master Mix 2x (Thermo Scientific, USA) in piko real detection system (Thermo Fisher Scientific). The were reaction performed was, initial denaturation at 95 °C for 10 min, 40 cycles of amplification at 95 °C for 10 sec, 60 °C for 20 sec respectively, followed by a cooling period of 5 sec at 50 °C. The primer sequences are listed in the Table1. The change in expression of mentioned genes were calculated by delta Ct methods.
Gene
Forward Primer
Reverse Primer
NQO1
agggttcggtattacgatcc
agtacaatcagggctcttctcg
HO-1
ctgctagcctggtgcaaga
ccaacaggaagctgagagtga
Β-actin
aaggccaaccgtgaaaagat
gtggtacgaccagaggcatac
2.4 Estimation of antioxidant enzymes
Level of antioxidant enzymes; Glutathione and Catalase, were estimated in the liver of mice. For this, 0.65 ml of distilled water was added into the tube and mixed well. Then 0.1 of the solution was added into 0.9 ml of distilled water and mixed well. It was then stored at 4 °C. After that, 0.1 g of liver tissue was excised and processed by using micro plate kit method (Cohesion Biosciences, UK) according to the manufacturer’s protocol.
2.5 ALT & AST estimation
Blood was collected in the yellow capped gel vacutainers. ALT (GPT) & AST (GOT) detection kits were purchased from Roche, Switzerland. Tubes were centrifuged for 10 min at 2500 rpm and transferred to storage tubes for ALT & AST analysis. Blood was centrifuged for 10 min at 15,000 rpm to isolate serum. 50ul of serum was mixed with 500ul of the working reagent and incubated at 37 °C in the incubator. Then, 50ul of analyzing sample was pipette into working reagent tube and read the absorbance within one minute at 340 nm wavelength.
2.6 Histological assessment
Liver were carefully harvested in PBS. After that collected tissues were fixed in Bouin’s fixative for 24 h and vials were kept on shaker during the fixation. Then fixed tissues were washed with 70% (every 30 min until the yellow colour diminished), 90% and 100% ethanol respectively, each for one day (Gomes et al., 2012). Tissues were shifted in half xylene and half paraffin wax and were placed in an oven set at 66 °C for 24 h. Sections of 3um were cut using microtome. Sections were picked up on a clean glass slide. Haematoxylin and eosin staining was performed according to protocol described by Ho et al. (2004).
2.7 Statistical analysis
Standard error means and arithmetic mean on all observations were calculated by using this Graph Pad Prism version 5software. Comparison between mean differences between groups were analyzed by applying ANOVA and t-test through Graph Pad Prism software at minimum population significance difference p < 0.05 level.
3 Results
3.1 Swimming alleviates arsenic accumulation in mice liver
After 8 weeks, mice exposed to sodium arsenite in water showed significant accumulation of arsenic in their liver. However, Arsenic accumulation was higher (0.131 mg/L) in 20 mg/kg/day [denoted by As(20) group] exposed group animals as compared to those administered with the low dose 10 mg/kg/day [marked by As(10) group] in which arsenic level was 0.016 mg/L. Our results revealed that arsenic accumulation was reduced in both groups when underwent swimming exercise [E + As(10) & E + As(20)]. However, the difference in the arsenic alleviation was statistically significant only in high dose group [E + As(20)] (0.042 mg/L) from their liver (Fig. 1).![Arsenic accumulation in mice liver. Accumulation of arsenic in mice liver after Sodium arsenite administration As(10) and 20 mg/kg/day. The histogram shows that arsenic level increases in dose dependent manner. The arsenic accumulation significantly reduces in exercise groups [E + As(10) & E + As(20)]. The data was expressed as mean ± SEM (n = 5). This data was analyzed by applying One Way ANOVA and t test. #p < .005 vs. control group, ###p < .001 vs. control group, *p < .005 vs. As(20).](/content/185/2020/32/1/img/10.1016_j.jksus.2019.02.011-fig1.png)
Arsenic accumulation in mice liver. Accumulation of arsenic in mice liver after Sodium arsenite administration As(10) and 20 mg/kg/day. The histogram shows that arsenic level increases in dose dependent manner. The arsenic accumulation significantly reduces in exercise groups [E + As(10) & E + As(20)]. The data was expressed as mean ± SEM (n = 5). This data was analyzed by applying One Way ANOVA and t test. #p < .005 vs. control group, ###p < .001 vs. control group, *p < .005 vs. As(20).
3.2 Expression level of NQO-1 and HO-1 gene enhanced by exercise
qPCR was performed to analyze the expression profiles of genes which are regulated through Nrf2 signaling pathway, in mice liver following the sodium arsenite treatment. The expression of both NQO-1 and HO-1 genes increased with increasing dose of arsenite. Interestingly, swimming exercise positively affected on the expression since the expression of both genes was significantly higher, after swimming [E + As(10) and E + As(20)] groups as compared to non-swimming groups, respectively. Overall, swimming induced higher expression of HO-1 as compared to the NQO-1 (Fig. 2).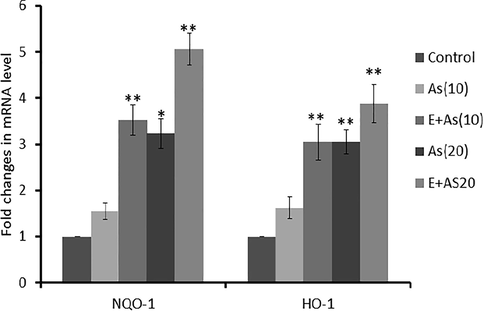
Relative expression of Nrf2 genes. The expression of NQO-1 and HO-1 genes in liver of control treated and exercise groups. Increase in the expression of NQO-1 and HO-1 in exercise groups significant difference was observed in Exercise dose group as compared to the dose group. p < .05 marked as * while p < .005 as **.
3.3 Exercise enhanced the antioxidant enzyme level (glutathione & catalase)
After the arsenic exposure, the levels of oxidative stress regulating enzymes; glutathione & catalase) was measured. Significant decrease in the glutathione (1.6901–1.2 nmol/mg) and catalase level (0.732–0.321 nmol/mg) were observed after arsenic treatment [As(10) and As(20)] group. However, the levels of glutathione (2.653–3.214 nmol/mg) and catalase (1.464–2.582 nmol/mg) significantly increased after swimming exercise [E + As(10) and (E + As(20) groups] (Fig. 3).![Exercise enhanced the antioxidant enzyme level (Glutathione & Catalase). III(a)This graph reveals that Glutathione level is significantly higher in Exercise + dose groups [E + As(10) & E + As(20)] as compared to the Dose group As(10) and As(20). The data was expressed as mean + SEM (n = 5per group). This data was analyzed by applying One Way ANOVA and t-test. ##P < .005 vs. control group, ## p < .005 vs. control group *p < .005 vs. As(10),** p < .005 vs. As(20). III(b) Catalase level is significantly higher in Exercise + dose groups [E + As(10) & E + As(20)] as compared to the Dose group As(10) and As(20).#p < .005 vs. control group, #p < .005 vs. control group *p < .005 vs. As(10).](/content/185/2020/32/1/img/10.1016_j.jksus.2019.02.011-fig3.png)
Exercise enhanced the antioxidant enzyme level (Glutathione & Catalase). III(a)This graph reveals that Glutathione level is significantly higher in Exercise + dose groups [E + As(10) & E + As(20)] as compared to the Dose group As(10) and As(20). The data was expressed as mean + SEM (n = 5per group). This data was analyzed by applying One Way ANOVA and t-test. ##P < .005 vs. control group, ## p < .005 vs. control group *p < .005 vs. As(10),** p < .005 vs. As(20). III(b) Catalase level is significantly higher in Exercise + dose groups [E + As(10) & E + As(20)] as compared to the Dose group As(10) and As(20).#p < .005 vs. control group, #p < .005 vs. control group *p < .005 vs. As(10).
3.4 The effect of exercise and arsenic on hepatic enzymes (ALT &AST) activity
Clinical indications of hepatic damage were observed by measuring AST and ALT levels in serum of mice after arsenic exposure. Positive co-relation was observed between the arsenic exposure and the hepatic enzyme alanine amino transferase (ALT) aspartate amino transferase (AST) as ALT & AST activities increased from (130–170 U/L) to (150–210 U/L) in [As 10 mg/kg/day] and [As 20 mg/kg/day] age groups respectively (Fig. 4). However, [E + As 10 mg/kg/day] group showed low level of ALT & AST enzymes (40–130 U/L) as compared to the counterpart [E + As 20 mg/kg/day] group (55–170 U/L). Therefore, these results indicated that liver damage occurred due to the chronic arsenic exposure. On the other hand, swimming exercise has provided hepatoprotection against the arsenic damages.![The effect of exercise and arsenic on hepatic enzymes (ALT &AST) activity. IV(a) Level of alanine aminotransferase is higher in dose groups As(10) and As(20) as compared to the Exercise + dose groups [E + As(10) & E + As(20)]. The data was expressed as mean + SEM (n = 3per group). The data was analyzed by applying One Way ANOVA and t-test. # p < .001 vs. control group,* p < .001 vs. As(20). IV(b). Level of aspartate aminotransferase is significantly higher in dose groups As(10) and As(20) as compared to the Exercise + dose groups [E + As(10) & E + As(20)].#p < .001 vs. control group, ##p < .005 vs. control group.](/content/185/2020/32/1/img/10.1016_j.jksus.2019.02.011-fig4.png)
The effect of exercise and arsenic on hepatic enzymes (ALT &AST) activity. IV(a) Level of alanine aminotransferase is higher in dose groups As(10) and As(20) as compared to the Exercise + dose groups [E + As(10) & E + As(20)]. The data was expressed as mean + SEM (n = 3per group). The data was analyzed by applying One Way ANOVA and t-test. # p < .001 vs. control group,* p < .001 vs. As(20). IV(b). Level of aspartate aminotransferase is significantly higher in dose groups As(10) and As(20) as compared to the Exercise + dose groups [E + As(10) & E + As(20)].#p < .001 vs. control group, ##p < .005 vs. control group.
3.5 Hepatoprotective effect of swimming exercise against arsenic induced liver injury
Histological examination of liver samples of healthy control and arsenic treated and swimming mice groups was performed in order to observe the pathological changes in the liver samples. Histological analysis of the healthy control group showed normal structure of hepatocytes in mice. On the other hand, the liver of arsenic treated groups manifested sinusoidal spaces, fibrosis, severe necrosis and binucleated cells showing that arsenic badly damage the liver (Fig. 5). However, swimming exercise reduced the adverse effects as in the exercise groups (E + As(10) and E + As(20) groups) minor defects as compared to [As(10) and As(20)] groups were observed (Fig. 5). Based on these finding, it can be inferred that swimming exercise reduces the liver damages caused by the sodium arsenite.![Hepatoprotective effect of swimming exercise against arsenic induced liver injury Stained portion of mouse liver shows marked hepatotoxicity as compared to the control group. Increased sinusoidal spaces (ISS), mitosis (M) and necrosis (N) are visible in sections treated. On the other hand, restoration of damages like fibrosis and sinusoidal spaces are visible in Exercise groups + dose groups [E + As(10) & E + As(20)]. (A&B) Control group. (C&D) Mice group administrated with 20 mg/kg/day sodium arsenite. (E&F) Mice group administrated with 20 mg/kg/day sodium arsenite dose along with swimming exercise. (G&H)) Mice group administrated with 10 mg/kg/day sodium arsenite dose (I&J) Mice group administrated with 10 mg/kg sodium arsenite dose along with swimming. Note: Increased sinusoidal spaces (ISS), normal hepatocytes (HC), necrosis (N), mitosis (M) and Pyknosis (p).](/content/185/2020/32/1/img/10.1016_j.jksus.2019.02.011-fig5.png)
Hepatoprotective effect of swimming exercise against arsenic induced liver injury Stained portion of mouse liver shows marked hepatotoxicity as compared to the control group. Increased sinusoidal spaces (ISS), mitosis (M) and necrosis (N) are visible in sections treated. On the other hand, restoration of damages like fibrosis and sinusoidal spaces are visible in Exercise groups + dose groups [E + As(10) & E + As(20)]. (A&B) Control group. (C&D) Mice group administrated with 20 mg/kg/day sodium arsenite. (E&F) Mice group administrated with 20 mg/kg/day sodium arsenite dose along with swimming exercise. (G&H)) Mice group administrated with 10 mg/kg/day sodium arsenite dose (I&J) Mice group administrated with 10 mg/kg sodium arsenite dose along with swimming. Note: Increased sinusoidal spaces (ISS), normal hepatocytes (HC), necrosis (N), mitosis (M) and Pyknosis (p).
4 Discussion
Arsenic is a toxic metal and carcinogenic to human. It enters into the environment through the anthropogenic activities (Hybertson et al., 2011). Millions of people are daily being exposed to the arsenic through drinking arsenic contaminated water, food via agricultural and industrial contamination of our environment (Argos et al., 2012). Low level of Arsenic increase the transcription rate of genes, increase the protein levels, and repair the DNA damages. But higher concentration of arsenic (>10 μg/L) is involves in apoptosis, oxidative DNA damage and necrosis (Jiang et al., 2009). Incidences of liver damage due to acute arsenic exposure has been reported (Jomova et al., 2011).
In the present study it was revealed that exercise play very important role in the hepatoprotection and removal of arsenic from liver. In addition, it activates Nrf2 pathway to overcome oxidative stress. Previously it has been documented that higher concentration of arsenic (>10 μg/L) has been involved in apoptosis, oxidative DNA damage and necrosis. Incidences of liver damage due to acute arsenic exposure have also been reported (Jomova et al., 2011). Present findings demonstrated that arsenic concentrations were higher in the As (10) and As (20) groups as compared to the [E + As (10)] and [E + As (20)] group (Fig. 1). These results clearly demonstrated that exercise has an important role in the elimination of arsenic from the mice body. In a previous study, mice exposed to different concentrations of sodium arsenite doses (0, 5, 10, 20 mg/kg/day) showed high level of accumulation in their livers. Several other studies have indicated that exposure of body to the different concentrations of arsenic probably results in the accumulation in different body organs like kidney, eyes, blood and most importantly in liver (Maiti and Chatterjee, 2001). Furthermore, (Mazumder, 2005) suggested the greater susceptibility of arsenic in liver as compared to the brain due to blood brain barrier.
We have hypothesized that moderate exercise upregulates the Nrf2/ARE signaling pathway thereby increase the expression of antioxidant enzymes. Our experimental results have confirmed this hypothesis that exercise has up regulated the Nrf2 signaling pathway. In the current study results, [E + As(10)] and [E + As(20)] groups indicated the up regulation in the transcription of NQO-1 & HO-1 genes as compared to the dose groups (Fig. 2). Importantly, these antioxidant genes are essential to cope with the arsenic induced oxidative stress in mice. Previous studies have also confirmed that, in the oxidative stress condition when ROS generation was excessive, Nrf2 transcription factor dissociate from the Keap-1 protein and enter into the cell activates the transcription rate of genes which play a significant role in the up regulation of antioxidant enzyme (Flora et al., 2011; Minelli et al., 2012). Moreover, in another study, it has been reported that arsenic administration in mouse disturbed the balance between the ROS and antioxidant enzymes. Increased level of ROS caused liver damages (Muthusamy et al., 2012). ROS production due to arsenic exposure can be controlled by the swimming exercise in mouse. Actually, during exercise consumption of oxygen become increased by mitochondria and it led towards the generation of free radicals. ROS generation during acute exercise promoted the dissociation of Nrf2 and keap1 complex & up regulated the transcription of ARE dependent antioxidant mechanism (Naujokas et al., 2013).
In 2011, it was reported that level of glutathione was significantly decreased in liver due to the administration of sodium arsenite dose in mice (Oberoi et al., 2014). This reduction in the level of glutathione was a protective effect against the oxidative stress, which was generated by the sodium arsenite. Glutathione played a very essential role in maintaining the redox status of the cell. It protects the body against the arsenic toxicity (Shi et al., 2004). In our study it could be depicted that glutathione levels were much higher in [E + As(10)] and [E + As(20)] group as compared to the As(10) and As(20) dose groups (Fig. 3(a)). Activity of glutathione in 20 mg/kg/day dose group was decreased in liver because of its utilization by glutathione peroxidase in order to combat the free radial generation. According to a study (Shi et al., 2005) Glutathione-S-transferase used the glutathione as a co factor it may led towards the depletion of glutathione activity after the exposure of different doses of arsenic. Exercise led to sustainable hepatoprotection through the glutathione replenishment. Similarly, Catalase is very important in providing cellular defense by the decomposition of highly reactive oxygen species (hydrogen peroxide) (Smith and Harris, 2007). Overexpression of catalase rendered cells impervious to the toxicity of hydrogen peroxide (Smith, 2010). From the Fig. 3(b) it was inferred that levels of catalase were higher in [E + As(10)] and [E + As(20)] group as compared to the counterpart As(10) and As(20) dose group.
Liver is considered as a vital organ for the detoxification and metabolism. Liver damages were detected by hepatic enzymes, like alanine amino transferase and aspartate aminotransferase. Serum levels of ALT and AST increase due to the exposure of different arsenic concentrations (Snow et al., 2005; Steinmaus et al., 2014). In recent study, from the Figs. 4(a) and 4(b) it could be depicted that serum ALT and AST activities were markedly increased by 10 mg/kg/day, 20 mg/kg/day arsenic exposure. However, swimming showed hepato-protection by reduced level of both ALT and AST.
Previous studies reported that hepatic enzymes (ALT & AST) were increased after the chemical or immunological intoxication as compared to the control mice (Weydert and Cullen, 2010). In another study, it has been reported that ALT and AST enzyme levels were increased in NaAsO2 group and long term intake of NaAsO2 caused oxidative damages in rat liver (Wu et al., 2015). To a certain degree exercise could relive the arsenic induced liver injuries (Shi et al., 2005). It was observed that, size of liver in experimental animals (administrated with sodium arsenite) was larger than their swimming and healthy control group counterparts.
Arsenic related liver injury in animal model is confirmed by the pathological changes in liver associated with increased arsenic content in the liver. In early studies arsenic was reported in causing liver, bladder damages and DNA damages (Young-Seoub et al., 2014; Yuan et al., 2010). In our study arsenic caused detectable physiological changes in liver (Fig. 5). Mice administrated with sodium arsenite showed higher level of liver damages as compared to that exercise group. In [E + As(10)] and [E + As(20)] groups, Nrf2 signaling pathway has helped to alleviate the liver damages caused by arsenic. On the other hand, in arsenic group 20 mg/kg/day liver damages were specifically higher as compared to their exercise counterpart group. Previous studies demonstrated that increase in the fibrosis on liver histology along with the profibrogenic cytokines in mice, after the high dose exposure of arsenic (Yu et al., 2015). Another histological study conducted in 2015 reported that mice’s liver stained with H&E revealed extensive liver damages in NaAsO2 treated mice (Zheng et al., 2013). The mechanism involved in liver injury due to arsenic exposure may be due to the development of oxidative stress and the generation of ROS.
5 Conclusion
The results of present study revealed that exposure of sodium arsenite for a long period of time can cause liver damages & kidney dysfunction. Sodium arsenite increase these production of reactive oxygen species, these ROS disturbs the balance of oxidative stress and antioxidants. These findings allowed us to speculate the involvement of swimming exercise which is likely responsible for hepato-protective activity. Exercise activates the Nrf2 dependent signaling pathway. Nrf2 transcription factor up regulates the expression of NQO-1 & HO-1 genes, which enhance the production of antioxidant enzymes. These antioxidant enzymes might have a hepato-protective role to cope with the arsenic induced oxidative stress in mice.
Acknowledgements
We acknowledge University of the Punjab, Lahore for providing financial support for this research study.
Declaration of interest
No potential conflict of interest was reported by authors.
References
- Health effects of arsenic longitudinal study (HEALS): description of a multidisciplinary epidemiologic investigation. J. Eposure Sci. Environ. Epidemiol.. 2006;16(2):191.
- [Google Scholar]
- Inflammatory process in Alzheimer’s and Parkinson's diseases: central role of cytokines. Curr. Pharm. Des.. 2016;22(5):541-548.
- [Google Scholar]
- In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium L. against chemically and immunologically induced liver injuries in mice. J. Ethnopharmacol.. 2010;131(2):478-484.
- [Google Scholar]
- Protective role of some antioxidants on arsenic toxicity in male mice: physiological and histopathological perspectives. Biol. Med.. 2016;8(1):1.
- [Google Scholar]
- Arsenic and human health: epidemiologic progress and public health implications. Rev. Environ. Health 2012
- [Google Scholar]
- Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376(9737):252-258.
- [Google Scholar]
- Arsenic-induced cell death in liver and brain of experimental rats. Basic Clin. Pharmacol. Toxicol.. 2006;98(1):38-43.
- [Google Scholar]
- The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol.. 2013;85(6):705-717.
- [Google Scholar]
- Trivalent arsenic species induce changes in expression and levels of proinflammatory cytokines in intestinal epithelial cells. Toxicol. Lett.. 2014;224(1):40-46.
- [Google Scholar]
- Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan. Environ. Res.. 2010;110(5):455-462.
- [Google Scholar]
- Water spinach, Ipomoea aquatica (Convolvulaceae), ameliorates lead toxicity by inhibiting oxidative stress and apoptosis. PLoS One. 2015;10(10):e0139831
- [Google Scholar]
- Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ. Pollut.. 2007;145(3):839-849.
- [Google Scholar]
- British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63(1):7-42.
- [Google Scholar]
- Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. 2008;2008(5) p. pdb. prot4986
- [Google Scholar]
- Interactive effect of arsenic and fluoride on cardio-respiratory disorders in male rats: possible role of reactive oxygen species. Biometals. 2011;24(4):615-628.
- [Google Scholar]
- Arsenic-induced oxidative stress and its reversibility. Free Radical Biol. Med.. 2011;51(2):257-281.
- [Google Scholar]
- Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc. Res.. 2013;98(1):47-55.
- [Google Scholar]
- Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem. Toxicol.. 2013;59:739-747.
- [Google Scholar]
- Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol. Appl. Pharmacol.. 2011;251(1):59-69.
- [Google Scholar]
- Oxidants, antioxidants, and, the beneficial roles of exercise-induced production of reactive species. Oxidative medicine and cellular. Longevity 2012
- [Google Scholar]
- Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J. Biol. Chem.. 2004;279(31):32804-32812.
- [Google Scholar]
- Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med.. 2011;32(4–6):234-246.
- [Google Scholar]
- Nrf2 protects against As (III)-induced damage in mouse liver and bladder. Toxicol. Appl. Pharmacol.. 2009;240(1):8-14.
- [Google Scholar]
- Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol.. 2011;31(2):95-107.
- [Google Scholar]
- Effects on levels of glutathione and some related enzymes in tissues after an acute arsenic exposure in rats and their relationship to dietary protein deficiency. Arch. Toxicol.. 2001;75(9):531-537.
- [Google Scholar]
- Effect of chronic intake of arsenic-contaminated water on liver. Toxicol. Appl. Pharmacol.. 2005;206(2):169-175.
- [Google Scholar]
- Cyclo (His-Pro) exerts anti-inflammatory effects by modulating NF-κB and Nrf2 signalling. Int. J. Biochem. Cell Biol.. 2012;44(3):525-535.
- [Google Scholar]
- Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radical Biol. Med.. 2012;52(2):366-376.
- [Google Scholar]
- The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ. Health Perspect. (Online). 2013;121(3):295.
- [Google Scholar]
- The global burden of disease for skin, lung and bladder cancer caused by arsenic in food. Cancer Epidemiol. Prevent. Biomarkers 2014
- [Google Scholar]
- Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem.. 2004;255(1–2):67-78.
- [Google Scholar]
- Regulation of LSD1 histone demethylase activity by its associated factors. Mol. Cell. 2005;19(6):857-864.
- [Google Scholar]
- Determining the terminal electron-accepting reaction in the saturated subsurface. In: Manual of Environmental Microbiology (third ed.). American Society of Microbiology; 2007. p. :860-871.
- [Google Scholar]
- Evidence from Chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am. J. Epidemiol. 2010:kwq383.
- [Google Scholar]
- Arsenic, mode of action at biologically plausible low doses: what are the implications for low dose cancer risk? Toxicol. Appl. Pharmacol.. 2005;207(2):557-564.
- [Google Scholar]
- Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am. J. Epidemiol.. 2014;180(11):1082-1087.
- [Google Scholar]
- Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. NatureProtocols. 2010;5(1):51.
- [Google Scholar]
- Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Scientific Reports. 2015;5:12291.
- [Google Scholar]
- Kidney cancer mortality: fifty-year latency patterns related to arsenic exposure. Epidemiology. 2010;21(1):103-108.
- [Google Scholar]
- Fibroblast growth factor (FGF21) protects mouse liver against D-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol. Cell. Biochem.. 2015;403(1–2):287-299.
- [Google Scholar]
- ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49(1):18-29.
- [Google Scholar]







