Translate this page into:
Hepatoprotective potential of sciadopitysin against paraquat induced liver damage in rats
⁎Corresponding author at: Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad, Pakistan. rabianoorbwn@gmail.com (Rabia Azmat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Paraquat (PQ) is among the most widely used herbicides in the agriculture sector worldwide and is extremely noxious to both animals and humans. Paraquat exposure damages various organs in the body, particularly the liver. Sciadopitysin (SCD), a biflavonoid that is reported in Ginko biloba leaves, shows diverse biological potentials such as antioxidant and anti-inflammatory effects. The primary focus of this research was to estimate the effects of SCD against PQ-instigated hepatic toxicity in rats. Forty-eight male albino rats were randomly split into 4 groups: control group, PQ (5 mgkg−1) treated group, PQ (5 mgkg−1) + SCD (2 mgkg−1) co-treated group and SCD (2 mgkg−1) only treated group. After 30 days of experimentation, PQ exposure lowered the expression of Nrf-2 and antioxidants genes, while increasing the expression of Keap-1. The activities of antioxidants, including heme oxygenase-1 (HO-1), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GSR), glutathione peroxidase (GPx), and glutathione (GSH) as well as total protein levels, were reduced following PQ administration. Moreover, malondialdehyde (MDA) and reactive oxygen species (ROS) contents were elevated in PQ exposed rats. PQ intoxication also increased the expressions of Bax and Caspase-3, while down-regulating Bcl-2 expressions. Furthermore, PQ exposure induced morphological alterations in the liver. Nevertheless, SCD treatment reduced all PQ-elicited damages in the liver of rats owing to its free radical scavenging potential.
Keywords
Antioxidant
Apoptosis
Paraquat
Liver damage
Sciadopitysin
1 Introduction
Paraquat (PQ) is a potent synthetic herbicide that is extensively used in agricultural practices to prevent the growth of harmful weeds and undesirable vegetation (Peiró et al., 2007). PQ poses a substantial risk to human health due to its chemical characteristics such as high water solubility and volatility. It is reported that PQ is a hazardous toxicant due to lack of effective strategies against PQ induced toxicity (Ortiz-ortiz et al., 2011). PQ poisoning is a major concern in the developing countries, particularly in Asia. Human exposure to PQ occurs via skin contact, inhalation, and ingestion, while acute poisoning of PQ leads to death in three and a half hour. PQ exposure leads to mortality due to failure of multiple organs. PQ administration culminates in ROS synthesis, which impairs the normal functioning of biological system in the body (Liu et al., 2011).
According to previous study, PQ-induces pulmonary toxicity, reproductive toxicity, neurotoxicity and gastrointestinal toxicity, which are responsible for more than 50 % of pesticide-related fatality cases (Peiró et al., 2007). Liver diseases and environmental pollutants are strongly related, which is a grave concern related to human health. The liver is the primary organ for detoxification as it is involved in metabolism and is a major target of pesticides induced toxicity (Peiró et al., 2007). PQ induces hepatotoxicity due to over production of reactive species. PQ exposure induces oxidative stress (OS) in hepatic tissues, leading to a substantial rise in the levels of liver enzymes and up-regulation of genes expression that promote apoptosis. PQ exposure also leads to mitochondrial dysfunction, which causes mitochondrial membrane swelling in the liver of rats (Han et al., 2014). PQ-instigated liver damage results in centrilobular cholestasis, apoptosis in hepatocytes and macrophagic infiltration in the portal regions. The exposure to PQ leads to enlarged portal tracts due to an increase in collagen stroma and decreased lymphocyte and leukocyte infiltration (Bataller et al., 2000).
Flavonoids belong to the class of polyphenols that are widely used in nutraceutical, pharmacological and cosmetic industry. Biflavonoids are a type of flavonoid dimers, composed of two flavonoid units that are either similar or non-similar, connected symmetrically or asymmetrically by an alkyl-based linker (DeForest et al., 2014). Sciadopitysin (SCD) is a biflavonoid that found in the leaves of Ginko biloba and exhibits various therapeutic properties (Li et al., 2019). Hence, the current experiment was conducted to evaluate the therapeutic effects of SCD on PQ-instigated hepatotoxicity.
2 Materials and methods
2.1 Chemicals
PQ (CAS NO: 75365–73-0, purity: 98 %) and SCD (CAS NO: 521–34-6, purity ≥ 95 %) were bought from Sigma-Aldrich (Germany).
2.2 Experimental animals
The experiment was conducted using 48 male albino rats (weighing 200 ± 20 g). The rats were housed at the research center of University of Agriculture Faisalabad under standard conditions: temperature maintained at 23–26 °C, humidity at 45 ± 5 % and provided with unrestricted access to diet and water. Animals were managed according to the guidelines of European Union of Animals Care and Experimentation (14645–48/17–05-2022).
3 Research design
48 Rats were split into four different groups: Control group, PQ (5 mgkg−1) treated group, PQ (5 mgkg−1) + SCD (2 mgkg−1) co-treated groups and SCD (2 mgkg−1) only treated group. The doses of PQ and SCD were selected based on previous studies by Kheiripour et al. (2021) and El-Aarag et al. (2019), respectively. The doses were administered via oral gavage. After 30 days of experiment, rats were anesthetized with 60 mgkg−1 of ketamine and 6 mgkg−1 of xylazine before decapitation. Liver was removed and half lobe of liver was homogenized at 11,000 g for 20 min using cold phosphate-buffered saline (25 mM; pH: 7.4), for the estimation of different biomarkers, while the other lobe was fixed in CH2O (10 %) for histomorphological analysis
3.1 Evaluation of anti-oxidant enzymes
CAT activity was measured by using the protocol of Aebi (1984). SOD activity was measured using the approach demonstrated by Sun et al. (1988). GSH activity was measured using the methodology as stated by Sedlak and Lindsay (1968). In order to assess GPx activity the technique described by Lawrence and Burk (1976) was followed. GSR activity was calculated by using Factor et al. (1998) technique. GST activity was evaluated by using the method of Couri and Abdel-Rahman (1979) while the activity of HO-1 was appraised in compliance with Magee et al. (1999) technique. MDA level was determined using the procedure demonstrated by Ohkawa et al. (1979). The level of ROS was measured by using the protocol of Hayashi et al. (2007).
3.2 RNA isolation and real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The expressions of antioxidant genes, Nrf-2/Keap-1 along with apoptotic profile were assessed by qRT-PCR. RNA was isolated with the help of TRIzol reagent that was converted to cDNA following reverse transcription. Livak and Schmittgen (2001) strategy was employed to analyzed the alterations in expressions of these parameters through 2-ΔΔCT, using β-actin as an internal regulaor. The primer sequences of the target genes are shown in Table 1, as described earlier by Ijaz et al. (2022b) and Hamza et al. (2023).
Gene
Primers 5′— 3′
Accession number
Nrf2
Forward: ACCTTGAACACAGATTTCGGTG
NM_031789.1
Reverse: TGTGTTCAGTGAAATGCCGGA
Keap1
Forward: ACCGAACCTTCAGTTACACACT
NM_057152.1
Reverse: ACCACTTTGTGGGCCATGAA
CAT
Forward: TGCAGATGTGAAGCGCTTCAA
NM_012520.2
Reverse: TGGGAGTTGTACTGGTCCAGAA
SOD
Forward: AGGAGAAACTGACAGCTGTGTCT
NM_017051.2
Reverse: AAGATAGTAAGCGTGCTCCCAC
GPx
Forward: TGCTCATTGAGAATGTCGCGTC
NM_030826.4
Reverse: ACCATTCACCTCGCACTTCTCA
GSR
Forward: ACCAAGTCCCACATCGAAGTC
NM_053906.2
Reverse: ATCACTGGTTATCCCCAGGCT
GST
Forward: TCGACATGTATGCAGAAGGAGT
NM_031509.2
Reverse: CTAGGTAAACATCAGCCCTGCT
HO-1
Forward: AGGCTTTAAGCTGGTGATGGC
NM_012580.2
Reverse: ACGCTTTACGTAGTGCTGTGT
Bax
Forward: GCACTAAAGTGCCCGAGCTG
NM_017059.2
Reverse: CCAGATGGTGAGTGAGGCAG
Bcl-2
Forward: ACTGAGTACCTGAACCGGCA
NM_016993.1
Reverse: CCCAGGTATGCACCCAGAGT
Caspase-3
Forward: GTACAGAGCTGGACTGCGGT
NM_012922.2
Reverse: TCAGCATGGCGCAAAGTGAC
β-actin
Forward: AGGAGATTACTGCCCTGGCT
NM_031144
Reverse: CATTTGCGGTGCACGATGGA
3.3 Statistical analysis
Data were shown as Mean ± SE. One-way ANOVA and Tukey’s test was applied using Minitab software. Significance level was considered as p < 0.05.
4 Results
4.1 Impact of SCD on Nrf-2/Keap-1 pathway
PQ administration induced a significant (p < 0.05) reduction in the expressions of Nrf-2 and antioxidant genes, whereas elevating the Keap-1 expression as compared to the control group. However, co-treatment with SCD upregulated the expressions Nrf-2 and antioxidant genes, while downregulating the expression of Keap-1 as compared to PQ treated group. Furthermore, in rats treated with SCD only the expressions of these parameters were close to those of control group rats (Figs. 1, 2).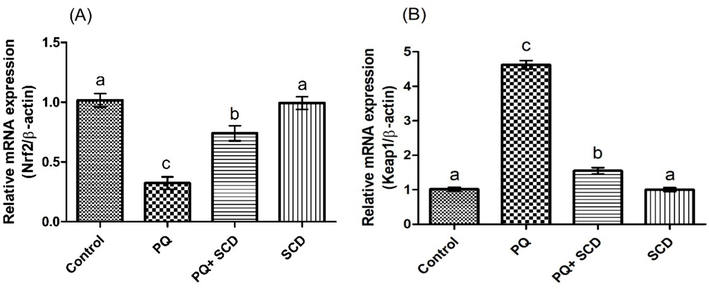
Protective effect of SCD on A) Nrf-2 and B) Keap-1 expression. Bars are shown on the basis of mean ± SEM. Different superscripts on bars presenting significant variation.
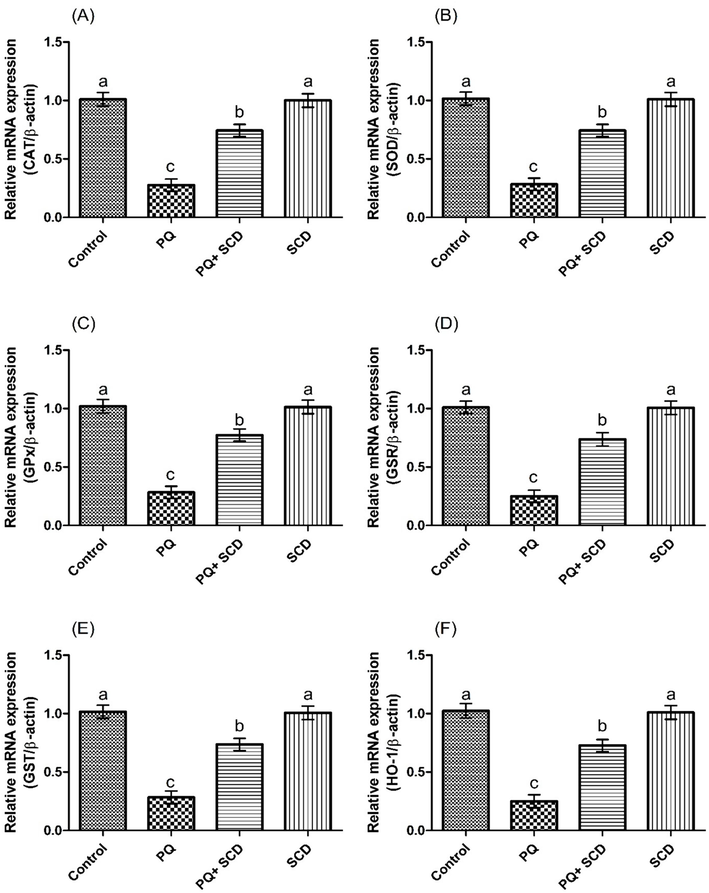
Protective effect of SCD on A) CAT, B) SOD, C) GPX, D) GSR, E) GST and F) HO-1 expression. Bars are shown on the basis of mean ± SEM. Different superscripts on bars presenting significant variation.
4.2 Impact of SCD on the activities of antioxidant enzymes
PQ inebriation prompted a substantial (p < 0.05) reduction in the activities of GST, CAT, GSR, SOD, GPx, OH-1 and GSH as well as total protein level in comparison to the control group. However, supplementation of SCD with PQ substantially improved antioxidant enzymes activities and total protein level in PQ + SCD administered group as compared to PQ-exposed rats. Moreover, no remarkable variation was noted in the values of these parameters in SCD only treated group and control group (Table 2). Values having different letters are significantly distinct from other groups.
PARAMETERS
GROUPS
Control
PQ
PQ + SCD
SCD
CAT (Umg−1 protein)
9.78 ± 0.19a
4.49 ± 0.15c
7.24 ± 0.13b
9.74 ± 0.21a
SOD (Umg−1 protein)
8.25 ± 0.08a
3.27 ± 0.09c
7.44 ± 0.27b
8.29 ± 0.11a
GPx (Umg−1 protein)
23.72 ± 1.86a
5.29 ± 0.32c
15.88 ± 1.16b
23.89 ± 2.27a
GSR (nM NADPH oxidized/min/mg tissue)
6.85 ± 0.13a
1.94 ± 0.27c
5.37 ± 0.17b
6.88 ± 0.14a
GST (nM/min/mg protein)
36.84 ± 1.02a
12.55 ± 1.58c
28.68 ± 0.90b
36.86 ± 1.18a
GSH (μM/g tissue)
19.38 ± 1.53a
4.26 ± 0.34c
15.78 ± 1.92b
19.45 ± 1.53a
HO-1(pmoles bilirubin/mg protein/h)
187.31 ± 6.35a
46.29 ± 2.92c
137.52 ± 4.55b
189.64 ± 6.68a
Total Protein (mg g−1 of tissue)
189.55 ± 3.27a
11.76 ± 3.14c
136.93 ± 2.44b
192.45 ± 3.10a
ROS (Umg−1 tissue)
1.44 ± 0.14a
9.56 ± 0.26c
2.60 ± 0.14b
1.43 ± 0.11a
MDA (nmol/mg protein)
0.83 ± 0.11a
7.98 ± 0.53c
1.77 ± 0.19b
0.81 ± 0.10a
4.3 Impact of SCD on levels of oxidative stress markers
PQ exposure considerably (p < 0.05) increased the levels of MDA and ROS as compared to control group. However, SCD and PQ treatment substantially reduced the levels of MDA and ROS in comparison to PQ-treated rats. Furthermore, the levels of aforementioned markers were similar in SCD only treated and control group (Table 2).
4.4 Impact of SCD on hepatic apoptotic markers
PQ poisoning led to a notable increase in the expressions of Caspase-3 and Bax, besides decreased the expression of Bcl-2 as compared to the control group. Nevertheless, SCD + PQ supplementation notably (p < 0.05) downregulated the expressions of Caspase-3 and Bax, while upregulating the expression of Bcl-2 as compared to the PQ administered group. Additionally, in SCD only administered group these expressions were comparable to control group (Fig. 3).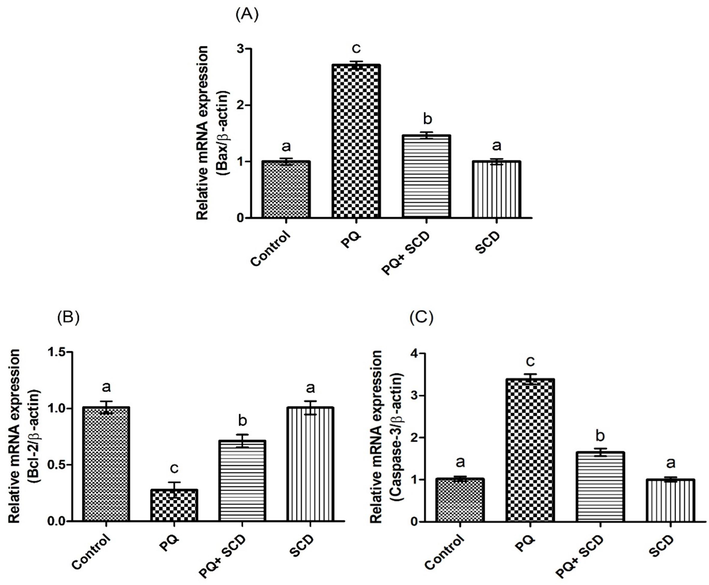
Protective effect of SCD on A) Bax, B) Bcl2, and C) Caspase-3, expressions. Bars are shown on the basis of mean ± SEM. Different superscripts on bars presenting significant variation.
5 Discussion
PQ is a prominent synthetic herbicide used in agriculture practices worldwide for many years. It is known for its ability to undergo redox cycling, acting quickly and increasing the synthesis of free radicals, leading to oxidative stress (OS) and cell damage (Asghari et al., 2017). PQ exposure can leads to dysfunction of vital organs such as kidney, brain, heart, and gastrointestinal tract. The liver, being the main site for xenobiotics transformation, has a higher potential for producing ROS, and is thus susceptible to toxins damage. Antioxidants can help prevent health risks resulting from the exposure of pesticides (Ahmadian et al., 2018). Biflavonoids have effective anti-oxidative properties and can be used to protect cells from detrimental impacts of these free radicals (Ye et al., 2012). Sciadopitysin (SCD) is a biflavonoid found in the leaves of Ginko biloba that displays ROS scavenging capabilities due to its structural configuration (Li et al., 2019). Therefore, this research was planned to determine the ameliorative potential of SCD against PQ induced hepatic impairment by evaluating antioxidants activity, levels of OS markers, liver serum markers, inflammatory markers, and apoptotic markers in albino rats.
PQ treatment reduced the expressions of antioxidant genes and Nrf-2, while increasing the expressions of Keap-1. Vomund et al. (2017) stated that Nrf-2 plays a key role in the regulation of OS, while Pintard et al. (2004) explained that Keap-1 acts as the inhibitor of Nrf-2 and controlling its stability. During ROS production, Nrf-2 separates from its negative inhibitor, Keap-1, through some physical modifications, moves into the nucleus, and stimulate the expression of various cellular proteins. Hawkes et al. (2014) reaffirmed that Nrf-2 plays an effective role in regulating the expression of antioxidant genes. Similarly, Yang et al. (2022) documented that high OS resulted in decreased Nrf-2 expressions, while increasing the expressions of Keap-1. Consequently, reduced Nrf-2 expressions leads to lower antioxidant genes expression. Plant-based flavonoid has potential to increase the activities of antioxidant enzymes (Ijaz et al., 2022a). However, SCD administration elevated the expressions of antioxidant genes and Nrf-2, while lowering Keap-1 expression. Therefore, it is assumed that SCD has the ability to regulate the Nrf-2 and Keap-1 expressions.
PQ administration resulted in a notable decrease in the activities of SOD, GPx, CAT, GSR, GST, OH-1, and GSH, while increasing the concentrations of MDA and ROS. These findings align with the study by Latif and Faheem (2020), who reported that PQ elevates ROS levels while reducing the activities of antioxidant enzymes. Antioxidant enzymes serve as a primary defense against harmful free radicals. SOD converts superoxide ions into H2O2, which is generated as a byproduct of oxidative stress (Kheradmand et al., 2010). CAT facilitates the transformation of H2O2 into H2O and O2 (Han et al., 2014). GPx helps in reducing H2O2 and lipid peroxide levels, working alongside CAT to mitigate the harmful effects of OH radicals by limiting free radical production (Gharu, 2022). GSH donates electrons in these reactions, playing the role of a donor, while GSR maintains GSH activity. HO-1 is involved in the breakdown of heme and plays a crucial role in cellular homeostasis (Bai et al., 2017). Reduced antioxidant activities lead to elevated ROS levels in the body, ultimately causing oxidative stress (Huang et al., 2016). The excessive production of radicals results in lipid peroxidation (LP), which disrupts macromolecules such as lipids, DNA, and proteins. MDA, a marker of LP, is associated with several negative outcomes, including increased membrane stiffness, osmotic fragility, and decreased mitochondrial longevity (Aydin et al., 2004). Moreover, excessive production of free radicals decreases the activities of antioxidant enzymes which ultimately impairs the endogenous cellular defence system (Ahmad et al., 2023). However, supplementation with SCD restored the biochemical profile and reduced the levels of oxidative stress indicators due to its antioxidant properties. Additionally, Liu et al. (2021) demonstrated that the three methoxy groups in the structure of SCD are responsible for its antioxidant activity.
PQ intoxication upregulated Bax and Caspase-3 while downregulating the Bcl-2 expression. Apoptotic indicators are the members of Bcl-2 family. Bax induces apoptosis, while Bcl-2 prevents apoptosis (Frenzel et al., 2009). An increase in the expressions of pro-apoptotic markers (Bax and Caspase-3), while reduction in anti-apoptotic protein (Bcl-2) results in an alteration in the selectivity of mitochondrial membrane, which increases cytochrome C liberation into cytosol (Gu et al., 2017). The increase in cytochrome C causes activation of Caspase-3, which triggers apoptosis (Cain et al., 2002). Grippa et al. (2015) reaffirmed that apoptosis can be averted by blocking the activation of Caspase-3, which is a key molecule in the apoptotic pathway. However, SCD treatment lowered the expressions of Bax and Caspase-3 whereas increased the Bcl-2 expression possibly due to its anti-apoptotic property.
6 Conclusion
The outcomes of this study revealed that PQ administration culminated in a reduction in the enzymatic activity of antioxidants, increased the oxidative stress markers and apoptotic markers in the hepatic tissues. Nevertheless, SCD treatment abrogated all these PQ induced adverse impairments in liver on account of its anti-apoptotic, antioxidant and hepatoprotective nature. So, it may be concluded that SCD can be used to treat liver damage in humans and animals. However, in this study, rats are used as animal model, so further clinical trials on humans are required in the future.
CRediT authorship contribution statement
Ansa Javed: Writing – original draft, Methodology, Investigation, Conceptualization. Rabia Azmat: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. Moazama Batool: Visualization, Validation, Formal analysis, Data curation. Amjad Islam Aqib: Writing – review & editing, Software, Formal analysis. Shaik Althaf Hussain: Writing – review & editing, Resources, Funding acquisition. Ayesha Ishtiaq: Writing – original draft, Validation, Software.
Acknowledgment
The authors would like to acknowledge the funding support by the Researchers Supporting Project number (RSP2024R371), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aebi, H., (1984). Catalase in vitro. In Methods in Enzymology. 105, 121–126. Academic press. https://doi.org/10.1016/S0076-6879(84)05016-3.
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-induced Nephrotoxicity in Rats. Pak. Vet. J.. 2023;43:623-627.
- [CrossRef] [Google Scholar]
- Betanin reduces organophosphate induced cytotoxicity in primary hepatocyte via an anti-oxidative and mitochondrial dependent pathway. Pestic. Bioch. Phy.. 2018;144:71-78.
- [CrossRef] [Google Scholar]
- A review of the protective effect of melatonin in pesticide-induced toxicity. Expert Opin. Drug Metab. Toxicol.. 2017;13:545-554.
- [CrossRef] [Google Scholar]
- The level of antioxidant enzymes, plasma vitamins C and E in cement plant workers. Clin. Chim. Acta. 2004;341:193-198.
- [CrossRef] [Google Scholar]
- Supplemental effects of probiotic Bacillus subtilisfmb on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci.. 2017;96:74-82.
- [CrossRef] [Google Scholar]
- Prolonged cholestasis after acute paraquat poisoning through skin absorption. Am. J. Gastroenterol. Suppl.. 2000;95:1340-1343.
- [CrossRef] [Google Scholar]
- The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie.. 2002;84:203-214.
- [CrossRef] [Google Scholar]
- Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol.. 1979;3:451-460.
- [Google Scholar]
- 4′, 4‴, 7, 7 ″-Tetra-O-methylcupressuflavone Inhibits Seed Germination of Lactuca sativa. J. Nat. Prod.. 2014;77:1093-1096.
- [CrossRef] [Google Scholar]
- Melittin exerts beneficial effects on paraquat-induced lung injuries in mice by modifying oxidative stress and apoptosis. Molecules. 2019;24:1498.
- [CrossRef] [Google Scholar]
- Disruption of redox homeostasis in the transforming growth factor-α/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J. Biol. Chem.. 1998;273:15846-15853.
- [CrossRef] [Google Scholar]
- Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584-596.
- [CrossRef] [Google Scholar]
- Defense mechanism of natural antioxidants against free radicals. CAJMS.. 2022;3:163-170.
- [Google Scholar]
- The seipin complex Fld1/Ldb16 stabilizes ER–lipid droplet contact sites. J. Cell Biol.. 2015;211:829-844.
- [CrossRef] [Google Scholar]
- Protective effects of paeoniflorin on TNBS-induced ulcerative colitis through inhibiting NF-kappa B pathway and apoptosis in mice. Int. Immunopharmacol.. 2017;50:152-160.
- [CrossRef] [Google Scholar]
- Hepatoprotective effects of astragalin against polystyrene microplastics induced hepatic damage in male albino rats by modulating Nrf-2/Keap-1 pathway. J. Funct. Foods.. 2023;108:105771
- [CrossRef] [Google Scholar]
- Betanin attenuates paraquat-induced liver toxicity through a mitochondrial pathway. Food Chem. Toxicol.. 2014;70:100-106.
- [CrossRef] [Google Scholar]
- Regulation of the human thioredoxin gene promoter and its key substrates: a study of functional and putative regulatory elements. Biochim. Biophys. Acta Gen. Subj.. 2014;1840:303-314.
- [CrossRef] [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ.. 2007;631:55-61.
- [CrossRef] [Google Scholar]
- Nonylphenol induced apoptosis and autophagy involving the Akt/mTOR pathway in prepubertal Sprague-Dawley male rats in vivo and in vitro. Toxicology. 2016;373:41-53.
- [CrossRef] [Google Scholar]
- Hepatoprotective potential of Genkwanin against aflatoxin B1-induced biochemical, inflammatory and histopathological toxicity in rats. Pak. Vet. J.. 2022;42:499-504.
- [CrossRef] [Google Scholar]
- Chemoprotective effect of vitexin against cisplatin-induced biochemical, spermatological, steroidogenic, hormonal, apoptotic and histopathological damages in the testes of Sprague-Dawley rats. Pharm. J.. 2022;30:519-526.
- [CrossRef] [Google Scholar]
- Evaluation of the hepatoprotective effects of curcumin and nanocurcumin against paraquat-induced liver injury in rats: modulation of oxidative stress and Nrf2 pathway. J. Biochem. Mol. Toxicol.. 2021;35:22739.
- [CrossRef] [Google Scholar]
- Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul. Pept.. 2010;162:84-89.
- [CrossRef] [Google Scholar]
- Study of oxidative stress and histo-biochemical biomarkers of diethyl phthalate induced toxicity in a cultureable fish. Labeo Rohita. Pak. Vet. J.. 2020;40
- [Google Scholar]
- Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun.. 1976;71:952-958.
- [CrossRef] [Google Scholar]
- Anticancer effects of five biflavonoids from ginkgo biloba l. Male Flowers in Vitro. Molecules.. 2019;24:1496.
- [CrossRef] [Google Scholar]
- Consumption of hydrogen water reduces paraquat-induced acute lung injury in rats. Biomed. Res. Int. 2011
- [CrossRef] [Google Scholar]
- Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharm. Biomed. Anal.. 2021;193:113704
- [CrossRef] [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402-408.
- [CrossRef] [Google Scholar]
- In Vitro and in Vivo Immunomodulatory Effects of RDP1258, a Novel Synthetic Peptide. J. Am. Soc. Nephrol.. 1999;10:1997-2005.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [CrossRef] [Google Scholar]
- Protective effect of the glial cell line-derived neurotrophic factor (GDNF) on human mesencephalic neuron-derived cells against neurotoxicity induced by paraquat. Environ. Toxicol. Pharmacol.. 2011;31:129-136.
- [CrossRef] [Google Scholar]
- Hepatotoxicity related to paraquat and diquat absorption through intact skin. Dig. Dis. Sci.. 2007;52:3282-3284.
- [CrossRef] [Google Scholar]
- Cullin-based ubiquitin ligases: Cul3–BTB complexes join the family. EMBO J.. 2004;23:1681-1687.
- [CrossRef] [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- A simple method for clinical assay of superoxide dismutase. Clin. Chem.. 1988;34:497-500.
- [CrossRef] [Google Scholar]
- Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci.. 2017;18:2772.
- [CrossRef] [Google Scholar]
- Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol.. 2022;283:114739
- [CrossRef] [Google Scholar]
- Isolation and free radical scavenging activities of a novel biflavonoid from the shells of Camellia oleifera Abel. Fitoterapia.. 2012;83:1585-1589.
- [CrossRef] [Google Scholar]







