Translate this page into:
Hepato-protective effect of Pleurotus ostreatus extracts in cadmium- intoxicated rats
⁎Corresponding author. marwa.db@gmail.com (Marwa S.M. Diab)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The toxicity of cadmium in various animal models, in particular the liver, has been studied. The objective of this research was to determine the antioxidant and the hepatoprotective role of Pleurotus ostreatus (PE) extract in Cadmium-intoxicated rats.
Methods
Rats of the first group were used as control animals while rats of the second group were administered PE for 5 days. Animals of the third group were served as Cadmium intoxicated group and finally, rats of the fourth-group were treated orally with PE afterwards; cadmium was administered over 5 consecutive days. By using atomic absorption, the Cadmium concentration was measured in the liver. Hepatic histological changes were estimated through examination of stained tissue sections. The oxidative status in the liver was investigated through determining the concentration of glutathione (GSH), catalase (CAT), malondialdehyde (MDA) and the reactive oxygen species (ROS) in the liver homogenate. Finally the expression of Nrf2-mRNA was determined using real time PCR.
Results
Cadmium induced major hepatic histopathological damage, elevated levels of cadmium, and hepatic oxidative stress where the concentration GSH, CAT, MDA and ROS were affected in the liver. Additionally, cadmium administration resulted in substantial up-regulation of Nrf2-mRNA expression. Moreover, PE attenuated the hepatic cadmium intoxication where it improved the histological impairments and the level of accumulated cadmium in liver tissue was decreased. Likewise, the treatment attenuated the oxidative stress and down-regulated the expression of Nrf2.
Conclusions
Our results indicated that PE is associated with significant antioxidant and anti-inflammatory activity and alleviated the hepatotoxicity induced by cadmium chloride.
Keywords
Cadmium chloride
Mushroom
Hepatic histopathology
Oxidative stress
Nrf2
1 Introduction
One of the heavy metals most known to affect human health is the cadmium (Cd) (Schaefer et al., 2020). Due to its presence in cigarette smoke, fertilizers, pesticides and industrial waste galvanized steel pipes that supply drinking water, wood consumption and rubber tires, Cd may contaminate food, drinking water and air (Alkushi et al., 2018).
As Cd is not metabolized, it becomes toxic and accumulates mainly in the liver (Park et al., 2013). Moreover, Cd chronic intake in contaminated air or food induced hepatotoxicity, oxidative damage and apoptosis (Sumathi et al., 1996; Park et al., 2013).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that initiates an oxidative stress response and may have a major role against inflammation. Nrf2 regulates more than 90% of antioxidant genes related to glutathione, catalase and superoxide dismutase. In addition, inhibition of Nrf2 caused major enhancement of the ROS and by using compounds with stimulatory effect on Nrf2 signaling pathway can decrease oxidative stress induced injury (Ashrafizadeh et al., 2019; Liu et al., 2020).
Pleurotus ostreatus (Oyster mushroom) is regarded as one of the commercially important edible mushrooms throughout the world. It is commonly known as American oyster. P. ostreatus possess anti-inflammatory, anti-nociceptive activities (Jose et al., 2004; Sheena et al., 2003; Jayasuriya et al., 2020). Moreover, Bobek et al. (1993) reported that P. ostreatus inhibit the accumulation of cholesterol in the hepatic tissue. Wang et al. (2001) concluded that the medical effects of P. ostreatus due to the presence of several different bioactive compounds in it. Recently, we documented the existence of five phenolics and related compounds (gallic acid, chlorogenic acid, catechin, propyl gallate, and cinnamic acid) based on the phytochemical analysis of PE using HPLC (Dkhil et al., 2020). The aim of this study is therefore to investigate the antioxidant and the ameliorative effect of P. ostreatus extract on the toxicity caused by hepatic Cd in rats.
2 Materials and methods
2.1 Chemicals and kits
All chemicals were of analytical grade and obtained from Sigma-Aldrich except Qiazole reagent, it has been purchased from Qiagen. The kits for the biochemical investigations were purchased from Bio-diagnostics (Egypt). Kits for gene expression experiments were obtained from Qiagen.
2.2 Animals
Thirty six female albino rats weighing 210–260 g (10–12 weeks old) were kept in wire bottomed cages and they were supplied with balanced diet and water ad libitum. Animals were acclimatized to the environment for one week prior to experimental use. This study has been approved by state authorities in accordance with the National Organization for Drug Control and Research, Egypt, Ethical Committee for Animal Protection (approval no: NODCAR/ II/18/19).
2.3 Pleurotus ostreatus methanolic extract (PE)
The fruiting bodies of Pleurotus ostreatus were obtained from the Egyptian Agriculture Research Center during Summer. Fifty gram of dried, homogenized P. ostreatus fruiting bodies were used to prepare the methanolic extract. By using a ratio of 2:2:1 of methanol: chloroform: distilled water the metabolites were extracted according to (Kim et al., 2010). Finally the extract's upper layer was removed and vacuum-dried for 24 h to yield of 4.1 g% ww−1.
2.4 Experimental protocol
Animals were allocated into 4 groups with 9 rats per group. Rats of first group (control group) were intrapretoneally (i.p.) injected with saline for 5 consecutive days. PE was orally administered at a dose level 200 mgkg−1b.wt. for 5 days to rats of the second group (Nada et al., 2010). Rats of third group were orally gavaged with saline (using epigastric tube) and then, 1 h later, rats were i.p. injected with 1 mgkg−1 CdCl2 (Sigma-Aldrich, St. Louis, MO, USA) for 5 consecutive days (Kataranovski et al., 2009). Finally, rats of the fourth group were orally administered with PE (200 mgkg−1) (Nada et al., 2010), then, 1 h later Cd (1 mgkg−1b.wt.) was i.p. injected for 5 consecutive days. At the end of experimental intervals (6th day of treatment); rats of all groups were cervically decapitated after being anesthetized using ether and the liver was removed from each rat and washed with 0.9% NaCl. For the histological investigations; pieces of the liver were fixed in neutral buffered formalin while the rest of the liver was stored frozen at −80 °C for the biochemical and molecular investigations.
2.5 Histopathological investigations
Parts of livers were fixed in formalin (10%). Following the paraffin method routine procedure, hepatic tissues were processed up to blocks of paraffin. For routine histological analysis, the paraffin parts (5 μm thick) were stained with hematoxylin and eosin.
2.6 Cd concentration
Cd ion level in homogenate of the hepatic tissue was measured according to Murphy (1987). In brief, constant weight of the liver was left to be dried at 22 °C 24 h. To dried liver tissues, piperidine (2.5 mL; 1 mol L−1) was added; these tubes were incubated for 24 h at 60 °C till no tissue was visible in all tubes. Later on; all tubes were cooled at room temperature and 1 mL of perchloric acid was added. After; 10 min of deionized distilled water (3.5 mL) was added and mixed. After; 15 min all tubes were centrifuged (10 min at 1600 r.p.m.) finally; hepatic Cd ions were analyzed in the aliquots of supernatant by using Perkin-Elmer 2380 atomic absorption.
2.7 Biochemical investigations
In ice-cold Tris-HCl (50 mM) and 300 mM sucrose (pH 7.4), the liver was homogenized to produce 50% (w / v) homogeneous (Tsakiris et al., 2004). The homogenate was centrifuged for 10 min at 600 × g and then the supernatant was isolated.
The malondialdehyde (MDA) level in hepatic tissue was determined following Ohkawa et al. (1979) method. To determine the hepatic glutathione (GSH) level the Ellman’s method was used (Ellman, 1959). According to Aebi (1984), catalase (CAT) activity was determined. The Reactive Oxygen Species (ROS) was assayed according to Vrablic et al. (2001).
2.8 Gene expression
Total RNA was extracted from rat liver tissue using Qiazol reagent (Qiagen, Germantown, MD, USA) and then transformed into complementary DNA (cDNA) using the manufacturer's protocol cDNA Synthesis Kit (RevertAidTM H Minus Reverse Transcriptase, Fermentas, Thermo Fisher Science Inc., Canada). For gene expression analysis, the QuantiFast SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) was used where the quantitative real-time PCR was achieved as mentioned in Delić et al. (2010) using Applied Biosystems 7500 Instrument (Thermo Fisher Scientific, CA, USA). The primer set of Nrf2 was, forward, 5ʹ- CACATCCAGACAGACACCAGT -3ʹ and reverse, 5ʹ- CTACAAATGGGAATGTCTCTGC -3ʹ compared to the reference gene reference gene glyceraldehyde-3-phosphate dehydrogenase (gapdh). The fold changes in gene expression between treated and the control were analyzed using delta-delta cycle threshold (Ct) method (Livak and Schmittgen, 2001).
2.9 Statistical analysis
SPSS (Statistics Package for Social Sciences) software was used for the statistical analysis. The values were mean and standard error of the mean (SEM). Experimental groups were compared using one-way analysis of variance (ANOVA) and followed by Tukey’s post hoc comparison tests. A statistically significant p value ≤0.05 has been considered.
3 Results
The histological pictures revealed severe impairments in hepatic architecture as a result of Cd injection to rats. Microscopically structural alterations in hepatic sinusoids and increased number of Kupffer cells were appeared in Cd intoxicated liver tissue. In addition, abnormal nucleus ratio to cytoplasm, vacuolated cytoplasm and edema were observed (Fig. 1). Moreover, blood vessels cognition, inflammatory cells and necrosis were found. However; treatment of PE before Cd injection to rats caused improvements in hepatocytes architectures as compared to Cd group (Fig. 1).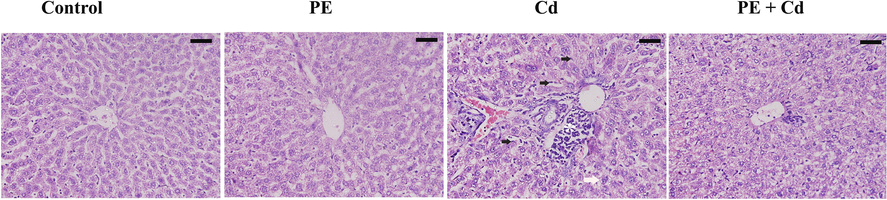
Histological picture of liver in cadmium (Cd) intoxicated rats; treated with the methanolic extract of P. ostreatus (PE + Cd). Control and PE groups appeared with normal architecture. Liver Inflammation (star) vacuolation (white arrow) and Kupffer cells hyperplasia (black arrow) were clearly appeared in Cd group. Livers of PE + Cd group were improved. Scale bar = 50 μm.
Cd concentration in hepatic tissue of rats (Fig. 2) showed nearly two fold increase in PE treated group and about 90 fold increase in rats intoxicated with CdCl2. However, PE administration before Cd intoxication to rats caused a reduction in hepatic Cd level as compared to Cd groups.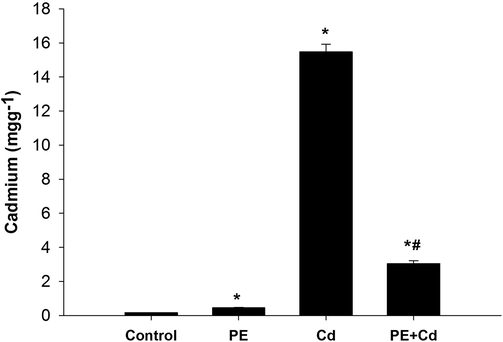
Effect of P. ostreatus extract treatment to cadmium (Cd) intoxicated rats on Cd level in hepatic tissue. Values are presented as mean ± SE. *: Significant against control group, #: significance against Cd group at P ≤ 0.05.
Cd caused a substantial decrease in hepatic GSH level relative to control group (Table 1). PE administration to Cd-intoxicated rats showed an increase in GSH at P ≤ 0.05. In the same manner, the CAT activity was decrease after Cd inoculation but after treatment with PE, the CAT activity was improved (Table 1). Values are expressed as means ± SE. *: Significance against control group at P ≤ 0.05, #: Significance against cadmium group at p < 0.01, n = 9.
Groups
GSH (mgg−1)
CAT (U g−1)
MDA (nmol g−1)
ROS (µmol g−1)
CNT
80.16 ± 2.30
1.73 ± 0.03
57.39 ± 2.62
1182 ± 45.17
PE
85.73 ± 2.82
1.66 ± 0.02
47.95 ± 2.14
1117 ± 60.98
Cd
51.97 ± 3.35 *
1.39 ± 0.06*
77.76 ± 2.06 *
1830 ± 41.73 *
PE + Cd
63.49 ± 1.68 *#
1.65 ± 0.03#
68.97 ± 2.57 *#
1315 ± 39.27#
As shown in table 1, Cd induced a significant increase in the level of MDA and ROS (P ≤ 0.05). Treatment of Cd-intoxicated rats with PE was able to ameliorate the increased level of both MDA and ROS to be 68.97 ± 2.57 and 1315 ± 39.27, respectively (Table 1).
There was an upregulation in Nrf2-mRNA expression (more than 2 fold) in rats group receiving cadmium (Fig. 3). However, treatment of animals with PE downregulated the gene expression of Nrf2 to nearly the control level (Fig. 3).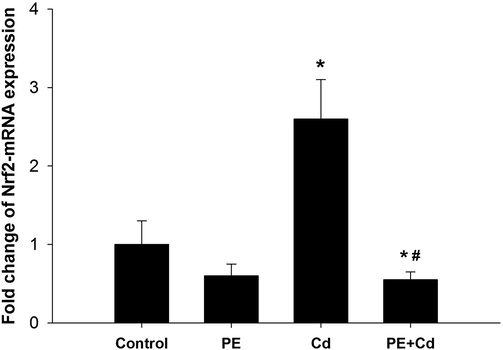
P. ostreatus extract treatment regulated the gene expression in liver tissue. Expression of hepatic Nrf2 was analyzed by quantitative Real-time PCR in all groups under investigation. The values represent means ± SE. *: Significantly different from control group (CONT) at P ≤ 0.05. # : Significantly different from Cd group at P ≤ 0.05, n = 6.
4 Discussion
In the present study, examination of the liver sections revealed that PE administration to Cd-intoxicated rats improved the structural alterations in hepatic sinusoids and the vacuolated cytoplasm. In addition, PE treatment decreased Kupffer cells, inflammatory cells, necrosis and blood vessels cognition significantly as compared to Cd group. Yamano et al. (2000) cleared that Cd treatment induced histopathological changes in hepatic tissue and the authors attributed the hepatopathological changes to the Kupffer cells activation that release a number of inflammatory mediators (e.g., cytokines, chemokines) and lead to inflammation and damage to the liver. In addition, inflammatory cells participate in the pathogenesis of hepatic injury by releasing cytotoxic inflammatory mediators. The Kupffer cells activation and induced inflammatory mediators in the liver tissue contribute to free radicals generation. MDA elevation increases the membrane stiffness so it is considered as an indicator of tissue damage. The generated ROS and marked depletion of hepatic GSH level were directly involved in apoptosis, necrosis and oxidative damage of cellular macromolecules such as nucleic acids in tissues (Thevenod, 2003; Jayakumar et al., 2006; Koyu et al., 2006). Moreover, PE treatment to rats revealed a hepatoprotective role preserving GSH level at normal values and reduces the free radicals generation (Jayakumar et al., 2006). Refaie et al., (2010) and Soares et al. (2013) found that the of insoluble non-starch polysaccharides from the P. ostreatus mycelium pre-treatment to rats prevented the carbon tetrachloride induced hepatocytes damage. Additionally, Soares et al. (2013) deduced that PE improved the antioxidant status and restored the hepatic damage.
In this study, Cd resulted in a substantial increase in hepatic Cd, while PE administration significantly decreased hepatic Cd levels relative to the Cd group. Cd has been documented to accumulate in hepatic tissue that induces toxicity by oxidative damage to the cell organelles of the ROS generation (Stohs and Bagchi, 1995; Renugadevi and Prabu, 2010). The important cellular target of Cd toxicity is the mitochondrion (Belyaeva et al., 2008). However, in the mitochondrial membrane Cd eventually binds to thiols that affects its transition to permeability, prevents the reaction of the respiratory chain and then produces ROS (Dorta et al., 2003; Liu et al., 2009). Moreover, Haouem et al. (2007) cleared that mainly Cd accumulation occur in the liver because it contains most of the metallothionein (a metal binding protein) with high affinity for Cd. After Cd absorption, most of the Cd is accumulated in the liver where it induces the production of metallothionein. When the synthesis of metallothionein becomes insufficient for binding all Cd ions in the liver, Cd not bound to metallothionein produce hepatocyte injury. So, antioxidants administration has been reported to prevent Cd hepatotoxicity by increasing metallothioneine, improving antioxidant enzyme activity and GSH levels (Karadeniz et al., 2009; Radosavljević et al., 2012).
As shown in the present findings, Cd injection into rats triggered a significant increase in hepatic MDA and ROS levels, whereas a significant reduction in GSH and CAT activity was observed in liver homogenate compared to control values. However, a significant decrease in the levels of hepatic MDA and ROS was revealed by the prospective effect of PE in Cd intoxicated rats. On contrary hepatic GSH level and CAT activity were increased significantly
According to Koyu et al. (2006), for 30 days Cd was administered to rats in drinking water caused a significant increase in the amount of hepatic MDA and a significant reduction in CAT and superoxide dismutase activities compared to control group. Similarly, Renugadevi and Prabu (2010) and Radosavljević et al. (2012) attributed the deterioration of pro-oxidants and antioxidants balance to the increased levels of lipid peroxidation and ROS production after Cd injection. Stohs et al. (2000) deduced that Cd does not generate free radicals directly; while, it generates radicals (superoxide, hydroxyl and nitric oxide), resulting in oxidative stress-consistent damage. Liu et al. (2009) further stated that inflammation induced by the hepatic is an important mechanism for oxidative stress induced by Cd. The treatment of PE protected the carbon tetrachloride induced injury in liver of rats where the level of hepatic MDA was decreased significantly. Moreover, hepatic GSH level and activities of CAT, glutathione peroxidase and superoxide dismutase were increased significantly wherefore the authors concluded that PE has ability to quench the generated free radicals (Jayakumar et al., 2006; Soares et al., 2013).
In addition; hepatic Cd intoxication induced a significant up-regulation in mRNA expression of Nrf2 in rats. While, Nrf2 expression revealed a down-regulation as a result of PE treatment to Cd-intoxicated rats, these results are in agreement with (Chen and Shaikh, 2009; Gong et al., 2019).
The transcription factor Nrf2 activated in response to disturbed antioxidant state (Nemmiche, 2017). Chen and Shaikh (2009) and Gong et al. (2019) reported that Cd-activated renal and hepatic Nrf2. Moreover, Gong et al. (2019) found that trehalose is a naturally occurring disaccharide acts as an antioxidant. The authors concluded that trehalose treatment significantly inhibited the Cd-activated hepatic Nrf2 pathway; this inhibition was attributed to trehalose antioxidant activity.
Nrf2 function provides oxidative stress with cell protection and acts an important role in the transcriptional activation of a range of antioxidant and detoxification genes (Gong et al., 2019). In the cytoplasm, the interaction between Nrf2 and Keap1 is disturbed during the oxidative stress (Gong et al., 2019). Into the nucleus, the Nrf2 is translocated and interacts with the antioxidant response element, which causes transcriptional induction of several cellular defense genes and direct ROS scavenging proteins (glutathione peroxidase, superoxide dismutase, and CAT) (Gong et al., 2019).
The activation of Nrf2 prevents Cd-induced oxidative stress and hepatic injury (Chen and Shaikh, 2009; Gong et al., 2019). In the same manner, Ge et al. (2019) reported that Cd induced nephrotoxicity in chickens which activated the signaling pathway of Nrf2 and induced the oxidative stress due to distortion of the mechanism of antioxidant defense. Moreover, Wu et al. (2012) reported that Cd activates the Keap1–Nrf2 pathway and ROS excessive generation was obtained after Cd exposure. Nrf2 suppression resulted in greater sensitivity, whereas over-expression of Nrf2 results in resistance to Cd induced apoptosis in kidney cells of rats. These results may explain Nrf2 up-regulation in the present study.
According to our recently published results Dkhil et al. (2020); Chromatographic analysis of the P. ostreatus confirmed the presence of some phenolics such as chlorogenic acid which has an antioxidant activity. Since, Gong et al. (2019) cleared that Cd induced apoptosis in liver tissue and Cheng et al. (2017) observed that in vivo treatment with chlorogenic acid significantly decreased apoptosis induced by aluminium toxicity, which prevents hepatocyte apoptosis in mice. Moreover; Gong et al. (2019) decided the close relationship between Cd-induced apoptosis and oxidative stress, it cleared that the trehalose anti-apoptotic effect against Cd toxicity due to its antioxidant activity. So, we attributed the ameliorative effect of PE to its antioxidant activity in hepatic tissue.
Collectively, we demonstrated that P. ostreatus extract attenuated the Cd-induced damage in the liver of rat by ameliorating the oxidative status, the inflammation in the liver tissue and the Nrf2 regulation. Additional studies are needed to know the signaling pathway and the mechanism of PE action.
Acknowledgments
This study was supported by Research Supporting Project (RSP-2020/23), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Structural changes in adult rat liver following cadmium treatment. Pak. J. Nut.. 2018;17(2):89-101.
- [Google Scholar]
- Ashrafizadeh, M., Ahmadi, Z., Farkhondeh, T., Samarghandian, S., 2019. Back to nucleus: combating with cadmium toxicity using Nrf2 signaling pathway as a promising therapeutic target. Biol. Trace Elem. Res. doi: 10.1007/s12011-019-01980-4.
- Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol. Appl. Pharmacol.. 2008;231:34-42.
- [Google Scholar]
- The mushroom Pleurotus ostreatus reduces secretion and accelerates the fractional turnover rate of very-low-density lipoproteins in rat. Ann. Nutr. Metab.. 1993;37:142-145.
- [Google Scholar]
- Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol. Appl. Pharmacol.. 2009;241(1):81-89.
- [Google Scholar]
- Protective and prophylactic effects of chlorogenic acid on aluminum induced acute hepatotoxicity and hematotoxicity in mice. Chem. Biol. Interact.. 2017;273:125-132.
- [Google Scholar]
- Testosterone-induced upregulation of miRNAs in the female mouse liver. Steroids. 2010;75(12):998-1004.
- [Google Scholar]
- Nephroprotective effect of Pleurotus ostreatus extract against cadmium chloride toxicity in rats. An. Acad. Bras. Cienc.. 2020;92(1):e20191121
- [Google Scholar]
- A proposed sequence of events for cadmium-induced mitochondrial impairment. J. Inorg. Biochem.. 2003;97:251-257.
- [Google Scholar]
- Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. STOTEN.. 2019;689:1160-1171.
- [Google Scholar]
- Trehalose prevents cadmium-induced hepatotoxicity by blocking Nrf2 pathway, restoring autophagy and inhibiting apoptosis. J. Inorg. Biochem.. 2019;192:62-71.
- [Google Scholar]
- Accumulation of cadmium and its effects on liver and kidney functions in rats given diet containing cadmium-polluted radish bulb. Exp. Toxicol. Pathol.. 2007;59:77-80.
- [Google Scholar]
- Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem. Toxicol.. 2006;44:1989-1996.
- [Google Scholar]
- Anti-Inflammatory Activity of Pleurotus ostreatus, a Culinary Medicinal Mushroom, in Wistar Rats. Evid Based Complement Alternat. Med.. 2020;2020:1-9.
- [Google Scholar]
- Methanol extract of the oyster mushroom, Pleurotus florida, inhibits inflammation and platelet aggregation. Phytother. Res.. 2004;18:43-46.
- [Google Scholar]
- The effects of Panax ginseng and Spirulina platensis on hepatotoxicity induced by cadmium in rats. Ecotoxicol. Environ. Saf.. 2009;72(1):231-235.
- [Google Scholar]
- Lungs: Remote inflammatory target of systemic cadmium administration in rats. Environ. Toxicol. Pharmacol.. 2009;28:225-231.
- [Google Scholar]
- Evaluation of the effects of cadmium on rat liver. Mol. Cell Biochem.. 2006;284:81-85.
- [Google Scholar]
- Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway. Int. J. Environ. Res. Public Health. 2020;17:138.
- [Google Scholar]
- Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol.. 2009;238(3):209-214.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402-408.
- [Google Scholar]
- Method for determination of sodium, potassium, calcium, magnesium, chloride and phosphate in the rat choroid plexus by flame atomic absorption and visible spectroscopy. Anal. Biochem.. 1987;161:144-151.
- [Google Scholar]
- Mushroom Insoluble Polysaccharides Prevent Carbon Tetrachloride-Induced Hepatotoxicity in Rat. Food Chem. Toxicol.. 2010;48(11):3184-3188.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Protective effects of Korean red ginseng extract on cadmium-induced hepatic toxicity in rats. J. Ginseng. Res.. 2013;37(1):37-44.
- [Google Scholar]
- Oxidative stress in rat liver during acute cadmium and ethanol intoxication. J. Serb. Chem. Soc.. 2012;77(2):159-176.
- [Google Scholar]
- Hepatoprotective activity of polysaccharopeptides from Pleurotus ostreatus mycelium on thioacetamide-intoxicated mice. Micol. Aplic Intern.. 2010;22:1-13.
- [Google Scholar]
- Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol.. 2010;62(2):171-181.
- [Google Scholar]
- Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci.. 2020;85(2):260-267.
- [Google Scholar]
- Antiinflammatory and anti-nociceptive activities of Ganoderma lucidum occurring in South India. Pharm. Biol.. 2003;41:301-304.
- [Google Scholar]
- Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med.. 1995;18(2):321-336.
- [Google Scholar]
- Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol. 2000;19(3):201-213.
- [Google Scholar]
- Relationship between Glutathione and DL Alpha-Lipoic Acid Against Cadmium-Induced Hepatotoxicity. Jpn. J. Med. Sci. Biol.. 1996;49(2):39-48.
- [Google Scholar]
- Nephrotoxicity and the proximal tubule. Insights from cadmium. Nephron Physiol.. 2003;93:87-93.
- [Google Scholar]
- Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+-ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol. Res.. 2004;49(5):475-479.
- [Google Scholar]
- Altered mitochondrial function and over generation of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-racglycero-3-phosphocholine in p53-defective hepatocytes. FASEB J.. 2001;15(10):1739-1744.
- [Google Scholar]
- Biological efficiency and nutritional values of Pleurotus ostreatus cultivated on spent beer grain. Biosoure. Technolo.. 2001;78:293-300.
- [Google Scholar]
- Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol. Appl. Pharmacol.. 2012;263(1):14-20.
- [Google Scholar]
- Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: role of Kupffer cells and inflammatory cytokines. Toxicol. Appl. Pharmacol.. 2000;162:68-75.
- [Google Scholar]







