Translate this page into:
Helicobacter pylori strains and their relationship with vacuolating cytotoxin A gene in the increased risk of gastric cancer
⁎Corresponding author. nicholaszam1990@gmail.com (Nicholas Daniel Amalorpavanaden) nicholasdaniel@mukuba.edu.zm (Nicholas Daniel Amalorpavanaden) anicholasdaniel@gmail.com (Nicholas Daniel Amalorpavanaden)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The main objective of this study is to analyze the relationship of H. pylori vacuolating gene in gastric cancer.
Methods
H. pyroli strains were isolated from the biopsy specimens collected from 50 patients suspected of gastritis, gastric carcinoma and peptic ulcer disease. H. pylori JK was identified and the virulence gene was determined. The presence of vacuolating cytotoxin A gene was amplified using a polymerase chain reaction. The growth kinetics of the strain JK were evaluated up to 96 h at 37 °C and the culture was used for the determination of VacA gene expression.
Results
The screened H. pylori JK was positive for vacA gene and showed heterogeneity among H. pylori. Bacterial growth increased up to 48 h incubation and declined after 48 h. The isolated strain showed maximum growth after 72 h incubation, whereas, the toxic protein reached maximum yield after 96 h incubation and was not associated with growth. It was resistant to antibiotics such as ceftriaxone, cefuroxime, cefalexin, and co-trimoxazole. The intracellular H. pylori culture extracts induced vacuolation in HeLa cell lines in vitro. The isolated H. pylori strain showed more than 43% vacuolating activity after 24 h incubation. Immunoblotting assay revealed the presence of an immuno-reactive protein band in the culture supernatant of H. pylori JK. These results indicated the pathogenic activity of the VacA gene and toxin production.
Conclusions
Generally, H. pylori persists for the lifetime of the individual, revealing the significance of eradication from the host.
Keywords
Gastric cancer
H. pylori
Drug resistance
vacA gene
Expression
Toxic protein
1 Introduction
Gastric Cancer (GC) is one of the most common cancers and causes mortality throughout the world (Ferlay et al., 2015). The number of GC cases increased in Eastern Europe, Latin America, and Eastern Asia (Ferlay et al., 2015). Environmental and genetic factors are significantly involved in GC. Salty foods, low intake of fresh fruits and vegetables, cigarette smoking and Helicobacter pylori infection are important risk factors of GC in most cases (Cheng et al., 2016). H. pylori is one of the common infections occurring in the stomach of humans. H. pylori is a Gram-negative bacterium, it affects gastric epithelial cells and involves an inflammatory response in mucosa cells. Studies revealed that the H. pylori gene was significantly associated with GC risk (Correa and Piazuelo, 2011). H. pylori has been considered a significant carcinogen. Moreover, only a certain fraction of H. pylori-infected patients develop symptoms. H. pylori is well known for genetic variations and adaptation to the human gastric epithelium. H. pylori strains showed various degrees of virulence and the strains harbouring the vacuolating toxin A (vacA) and cytotoxin-associated antigen (cagA) and these genes have been largely considered a disease risk (Costa et al., 2009).
In H. pylori, the porin protein, OipA is severely associated with neutrophil infiltration in gastric colonization and IL-8 induction was associated with GC risk among the human population (Su et al., 2016). Likewise, the blood-group antigen-binding adhesin (babA) which was coded by the gene, babA2 is one of the major adhesion and pathogenic factor among H. pylori strains. BabA2 is considered an active gene and showed the effective binding activity of Lewis-b blood group antigen on the host cell and gastric epithelium and determine the density of the H. pylori colony density. Many factors are seriously associated with H. pylori-induced inflammatory response and may lead to various oxidative stress, such as the production of superoxide by microbial activity, induction of reactive oxygen species (ROS) and depletion of the availability of vitamin C level by the pathogenic strains (Capurso et al., 2003). The main objective of the study was to analyze the vacuolating cytotoxin A gene from H. pyroli.
2 Materials and methods
2.1 Bacterial strain and culture
H. pyroli bacterial strains were obtained from the biopsy specimens collected from 50 patients suspected of gastritis, gastric carcinoma and peptic ulcer disease. A total of 10 strains were isolated, three from patients with duodenal ulcers, two from gastric ulcers, and four bacterial strains were characterized from the patients suspected of gastric carcinoma. This study has been approved by the Institutional Ethical Committee (IEC) and all works were performed according to the guidelines given by IEC. The collected samples were vortexed for 10 min. About 100 µL sample was spread onto Brain Heart Infusion agar plates (Himedia Laboratories, India) containing 6.5% sheep blood and antibiotic supplement. The culture plates were incubated for 3 days at 37 ͦC containing, 85% N2, 10% CO2 and 5% O2. Based on colony morphology, Gram-staining, biochemical properties, and 16S rDNA gene sequencing H. pyroli was identified. The bacterial strain was cultured in brain heart infusion for 18 h at 37 ͦC and stored with 20% glycerol and stored at − 80 °C.
2.2 H. pylori strain and culture conditions
H. pylori JK was cultured in sheep blood agar plates containing vancomycin (10 µg/mL). The agar plates were incubated for 48 h and it was inoculated in Brucella Broth medium containing fetal bovine serum (10%). It was further placed on an orbital shaker incubator at 150 rpm for 72 h at 37 °C.
2.3 Urease activity
H. pylori JK was incubated with a nutrient broth medium. After 96 h incubation, the culture medium was centrifuged (10,000 rpm × 10 min) and the cells were separated. The cells were lysed using lysis buffer with PMSF (protease inhibitor) and the protein content was estimated by the Bradford method. The total protein content was estimated using a spectrophotometer at 600 nm. To the protein suspension, 100 µL of 2% urea was added and incubated for 10 min at 37 °C. The amount of urease was determined by analyzing ammonia levels in the sample (Solomon et al., 2007).
2.4 Antibiotic susceptibility test
In this study in vitro antibiotic susceptibility testing of H. pylori strain was carried out. The bacterial suspension was inoculated by swabbing on MHA plates and the antibiotic discs were placed on the plates. The plates were further incubated for 24 h at 37 °C and the zone of inhibition was analyzed (Al-Dhabi et al., 2020).
2.5 Extraction of DNA from h. Pyroli bacterial strains
The genomic DNA of H. pyroli from the sample was extracted using a commercial DNA extraction kit according to the manufacturer’s instructions (Qiagen, Germany). The extracted DNA was further quantified using a nanodrop spectrophotometer and stored the DNA at −20 °C until further use.
2.6 Determination of vacA gene from h. Pylori
The presence of the vacA gene was determined using a polymerase chain reaction. Briefly, 1 µL of genomic DNA extracted from H. pyroli, 1 µM of forward and revised primers, 0.5 µL Taq DNA polymerase and 1 µL of 1 mM dNTPs mix were used to determine the available vacA gene from the genome of the pathogenic strains. PCR amplification was performed using a Thermal Cycler machine. The amplification conditions were 35 cycles (7 s at 98 °C), 56 °C for 30 s, and 72 °C for 30 s. Positive and negative controls were incorporated into each experimental trial. The amplified PCR product was analyzed and purified using 1.5% agarose gel electrophoresis. It was visualized using a gel documentation system (Syngene, UK).
2.7 Vacuolating cytotoxin assay
The bacterial strains were cultured in a BHI broth medium for 48 h at 37 °C. It was centrifuged at 5000 rpm for 10 min. Then the culture supernatant was precipitated with ammonium sulphate (40 – 50% ammonium sulphate). Then the precipitate was dissolved in phosphate-buffered saline and dialyzed. The dialyzed sample was concentrated against phosphate buffered saline. The concentrated protein sample was used for the vacuolating assay. To determine vacuolating assay, HeLa cells were cultured in Dulbecco’s culture medium containing 10% fetal bovine serum in microtiter plates. The sample was serially diluted in Dulbecco’s culture medium containing bovine serum (2%), ammonium chloride (10 mM), 100 µg/mL streptomycin, 100 U/mL penicillin and gentamicin (50 µg/mL). The sample was incubated with a tissue culture medium containing HeLa cells at 37 ͦC for 24 h. The HeLa cell morphology was analyzed and cell vacuolation was determined. The uninoculated sample was considered a negative control (Tummuru et al., 1993).
2.8 Western blotting
2.8.1 H. pylori culture and broth culture
The isolated bacterial strain was grown in a nutrient broth medium containing β-cyclodextrin (0.2%) and incubated for 24 h at 37 °C in an orbital shaker at 150 rpm. The cell density was monitored using a UV–visible spectrophotometer and incubated up to reach 0.8 OD. Then the sample was centrifuged for 15 min at 10,000 rpm for 4 °C. The culture supernatant was filtered using a 0.22 μm micron filter and stored at −20 °C.
2.8.2 Analysis of VacA gene by immuno-blotting
The H. pylori culture extract was loaded on sodium dodecyl sulphate gel electrophoresis (8%) and it was separated at constant power supply. The SDS-PAGE separated protein was transferred to nitrocellulose membrane and the membranes were blocked with protein (3% skim milk) in buffer (phosphate buffer saline) containing Tween (0.05%) for 2 h at 30 ± 2 ͦC. Cytotoxin activity was evaluated using rabbit anti-VacA antiserum and analyzed using peroxidase-conjugated anti-rabbit antibodies. Blot was analyzed using polyclonal antibodies by applying chemiluminescent detection reagents (Donati et al., 1997).
2.9 Statistical analysis
The results were analyzed using SPSS software. The “P” value lower than 0.05 was considered statistically significant.
3 Results
3.1 Growth and total protein content
The growth of H. pylori JK was determined and the result was described in Table 1. After 12 h incubation, the growth was 0.35 OD at 600 nm and it increased continuously up to 48 h incubation. After 12 h incubation, the intracellular protein content was 1.2 µg/mL and increased. The biosynthesis of protein was not growth associated. The isolated strain showed maximum growth after 48 h incubation, whereas, protein content reached a maximum after 96 h incubation.
Time (h)
Absorbance at 600 nm
Protein (µg/mL)
12
0.35
1.2
24
0.572
1.31
36
1.52
4.27
48
1.92
5.1
60
1.7
6.3
72
1.3
8.1
84
1.1
13.2
96
0.52
13.3
3.2 Determination of vacA gene from h. Pylori
H. pyroli strains were obtained from the biopsy specimens of patients suspected of gastritis, gastric carcinoma, dental plaque, and peptic ulcer disease. A total of 10 vaCA genes containing H. pyroli were isolated and the result was described in Table 2. A total of four vacA gene-positive strains were isolated from gastric carcinoma and three strains were isolated from duodenal ulcer. Two bacterial strains possessing the vaCA gene were isolated from gastric ulcers. Moreover, the detected H. pylori strains did not have the vacA gene isolated from the wound.
Clincal samples
vacA gene positive strain(No)
Duodenal ulcer
3
Gastric ulcer
2
Gastruc carcinoma
4
Oral cavity
1
Gastroduodenitis
0
Wound
0
3.3 Antibiotic susceptibility test
The antibacterial susceptibility result of the tested H. pylori strain JK was presented in Fig. 1. According to the results, the selected strain was resistant to ceftriaxone, cefuroxime, cefalexin, and co-trimoxazole. It was sensitive to antibiotics such as amikacin, gentamicin and chlorpromazine.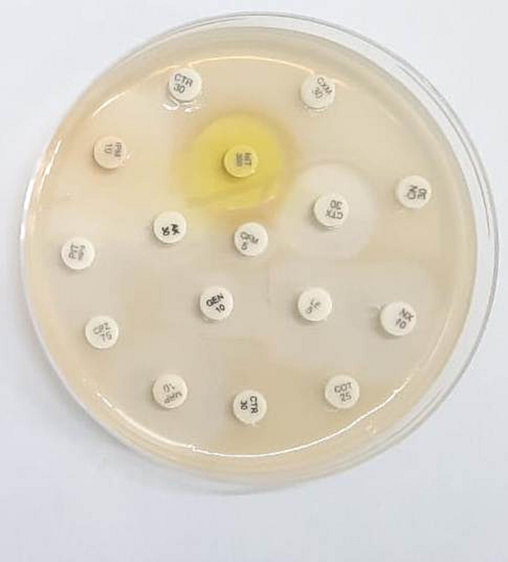
Antibiotic susceptibility of H. pylori isolated from the suspected gastritis sample.
3.4 Vacuolation of HeLa cells induced by the H. pylori extract
The cell-free extract of H. pylori culture induced vacuolation in HeLa cells. The isolated H. pylori strain showed vacuolating activity than the control strain. It showed more than 43% vacuolating activity after 24 h incubation. The development of vacuolation indicated more toxin production by the bacterial strain. H. pylori extract induced vacuolation after 24 h incubation and was photographed using an inverted microscope and the result was compared with uninoculated both cultures. The vacuolated cells comprised more than 35% of the total cells. This result indicated that the strain JK produced prominent vacuolation and than uninoculated culture Fig. 2.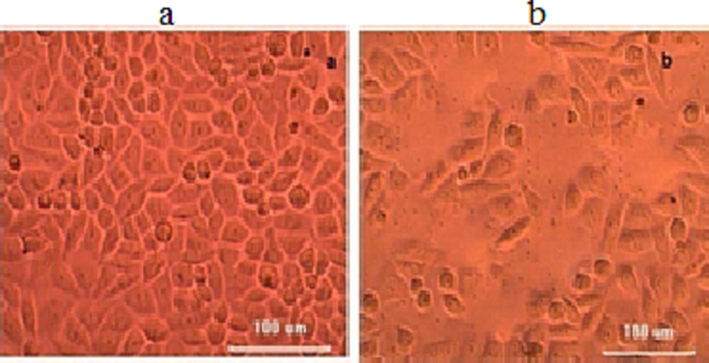
Effect of cell-free culture of H. pylori JK on HeLa cell lines. The cell-free extract (strain JK) was applied on the HeLa monolayer (a) and HeLa cells treated with unioculated strain (b).
3.5 Expression of VacA gene
The cell-free extract of H. pylori strain was used for the determination of VacA gene expression. Immunoblotting was performed using anti-VacA antibody in standard assay conditions. Immunoblotting assay revealed the presence of an immuno-reactive protein band in the culture supernatant of the H. pylori strain. The immuno-reactive protein band was thick in the H. pylori like positive control strain. These results revealed VacA gene expression and toxin production. The protein band was not established in the uninoculated control Fig. 3.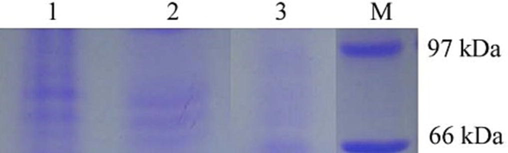
VacA gene detection using western blotting analysis (Lane 1 – strain JK, Lane 2 – positive control; Lane 2 – uninoculated control; Lane M – protein marker).
4 Discussion
The bacterium, H. pylori is one of the pathogenic agents that causes gastric infections and has been reported worldwide. The prevalence of this organism ranged between 25 and 80% in developing and developed countries. It is considered a carcinogenic bacterium and is associated with gastric cancer, peptic ulcers, and gastritis. This organism has been found in the oral cavity and transmitted among individuals (Yu et al., 2022). The mouth is considered the reservoir of H. pylori and is the source of gastric infection as has been demonstrated by various researchers. H. pylori caused peptic ulcer disease and it accounts for about 100% of duodenal ulcers and about 60% of gastric ulcers. Helicobacter pylori infection is mainly associated with gastrointestinal tract diseases such as gastric mucosa, peptic ulcer and chronic gastritis and lymphoid tissue lymphoma. Helicobacter pylori caused infection in the human population and successful eradication of this organism is mainly based on the choice of available antimicrobial agents (Malfertheiner et al., 2022). In this study, a total of four vacA gene-positive strains were isolated from gastric carcinoma and three strains were isolated from duodenal ulcers.
Antimicrobial resistance is a major concern and the unrestricted use of commercial antibiotics increased resistance among H. pylori strains. It has been previously reported that antibiotics induced chromosomal mutations in H. pylori strains (Mégraud, 2004; Ansari and Yamaoka, 2022). Moreover, various studies have revealed that the prevalence of drug resistance varies based on applications and that there is a various range of drug resistance based on the drug used. H. pylori isolated in this study showed resistance against antibiotics such as ceftriaxone, cefuroxime, cefalexin, and co-trimoxazole. Previous studies revealed that the drug resistance of Helicobacter pylori strains may be due to the unrestricted application of antibiotics to control H. pylori infections. The continuous applications of metronidazole for dental infections, pelvic infections and parasitic infections have increased metronidazole resistance in H. pylori (Li et al., 2021). Levofloxacin is frequently used to control respiratory tract and urinary tract infections. The continuous use of levofloxacin has been increased drug-resistance in H. pylori (Argueta et al., 2022).
In pathogenic H. pylori infection, the development of ailment mainly depends on environmental factors, host body and bacteria strain. The progression of the disease is determined by various virulence factors (Yamaoka, 2010). Among the virulence factor, vacuolating cytotoxin gene A (vacA), cytotoxin-associated gene A (cagA) and duodenal ulcer-promoting gene A (dupA) have been considered the virulence markers in H. pylori infection among hospitalized patients. Cytotoxin-associated gene A has been considered as the foremost oncoprotein produced by bacteria and probably the virulence gene with the significant potential of the pathogenic bacteria, H. pylori, moreover, the toxin associated with vacA gene mainly plays a potent role in the induction of gastric cancer and immune modulation in patients (Hatakeyama, 2014). Moreover, vacA and cagA genes have been diagnosed in patients associated with gastric diseases. In the duodenal ulcer population, the dupA gene has been determined in H. pylori infection (Alam et al., 2012). Moreover, dupA-negative H. pylori strains are involved in the development of stomach cancer and stomach ulcer (Abadi et al., 2012; Ahmad et al., 2009). In this study, vacA was used as the marker for diagnosis. The choice of the selected bacterial strain was mainly based on the availability of the bacterial strain and antibiotic sensitivity. PCR method was used for the determination of the vacA gene because of increased sensitivity. In this study, the vacA gene was detected in PCR results and the expressed protein was determined in blotting analysis. Antibiotic sensitivity tests and PCR based methods have been used previously for the determination of virulence because some of the bacterial strains of H. pylori did not show any growth on the agar medium (Willén et al., 2000; Hu et al., 2022). In this study, the isolated bacterial strains were subjected to PCR assay and determined vacA gene. The present outcome was highly correlated with clinical symptoms and pathogenesis. The vacA gene has been associated with peptic ulcer disease, mucosal-associated lymph tissue, and gastric adenocarcinoma in patients, whereas, the gene dupA has been determined from the patients associated with duodenal ulcer (Shiota et al., 2010). The present study revealed the presence of vacA genes among suspected cases. The presence of virulence gene vacA was determined from H. pylori isolated from various geographical areas, including, Thailand, South Africa and India (Tanih et al., 2010).
5 Conclusions
The pathogenic H. pylori JK was isolated from the clinical sample and the virulence gene was identified from the strain. The presence of the vacA gene in strain JK was detected after PCR amplification. The isolated strain showed toxic protein and reached the maximum amount after 96 h incubation. It was resistant to various second and third-generation antibiotics such as ceftriaxone, cefuroxime, cefalexin, and co-trimoxazole. The isolated H. pylori strain showed more than 43% vacuolating activity after 24 h incubation. Immunoblotting analysis revealed the presence of an immuno-reactive protein band in the culture supernatant of H. pylori JK revealing the expression of cytotoxin. These results indicated a chronic pathogenic profile of H. pylori JK and VacA gene for cytotoxin production.
Acknowledgement
The authors thank School of Natural Sciences, Mukuba University for the support. The authors extend their appreciation to the Researchers supporting project number (RSP2023R479) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Infection with Helicobacter pylori strains lacking dupA is associated with an increased risk of gastric ulcer and gastric cancer development. J. Med. Microbiol.. 2012;61(1):23-30.
- [Google Scholar]
- Prevalence of Helicobacter pylori pathogenicity-associated cagA and vacA genotypes among Pakistani dyspeptic patients. FEMS Immunol. Med. Microbiol.. 2009;55(1):34-38.
- [Google Scholar]
- Significant association of the dupA gene of Helicobacter pylori with duodenal ulcer development in a South-east Indian population. J. Med. Microbiol.. 2012;61(9):1295-1302.
- [Google Scholar]
- Probiotic and antioxidant potential of Lactobacillus reuteri LR12 and Lactobacillus lactis LL10 isolated from pineapple puree and quality analysis of pineapple-flavored goat milk yoghurt during storage. Microorganisms. 2020;8(10):1461.
- [Google Scholar]
- Helicobacter pylori infection, its laboratory diagnosis, and antimicrobial resistance: a perspective of clinical relevance. Clin. Microbiol. Rev.. 2022;35(3):e00258-e321.
- [Google Scholar]
- Clinical implication of drug resistance for H. pylori management. Antibiotics. 2022;11(12):1684.
- [Google Scholar]
- Intragastric ascorbic but not uric acid is depleted in relation with the increased pH in patients with atrophic body gastritis and H. pylori gastritis. Helicobacter. 2003;8(4):300-306.
- [Google Scholar]
- Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol Hepatol Rev.. 2011;7(1):59-64.
- [Google Scholar]
- Detection of serum antibodies to CagA and VacA and of serum neutralizing activity for vacuolating cytotoxin in patients with Helicobacter pylori-induced gastritis. Clinical Diagnostic Laboratory Immunology. 1997;4(4):478-482.
- [Google Scholar]
- Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136(5):E359-E386.
- [Google Scholar]
- Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15(3):306-316.
- [Google Scholar]
- Correlation analysis of gastric mucosal lesions with Helicobacter pylori infection and its virulence genotype in Guiyang, Guizhou province. China. Annals of Translational Medicine. 2022;10(24)
- [Google Scholar]
- Antibiotic resistance of Helicobacter pylori strains isolated from pediatric patients in southwest China. Front. Microbiol.. 2021;3647
- [Google Scholar]
- Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71(9):1724-1762.
- [Google Scholar]
- H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374-1384.
- [Google Scholar]
- Systematic review and meta-analysis: the relationship between the Helicobacter pylori dupA gene and clinical outcomes. Gut Pathog.. 2010;2(1):1-6.
- [Google Scholar]
- Measuring urease activity in aquatic environmental samples. Limnol. Oceanogr. Methods. 2007;5(9):280-288.
- [Google Scholar]
- Combination of OipA, BabA, and SabA as candidate biomarkers for predicting Helicobacter pylori-related gastric cancer. Sci. Rep.. 2016;6(1):1-12.
- [Google Scholar]
- Prevalence of Helicobacter pylori vacA, cagA and iceA genotypes in South African patients with upper gastrointestinal diseases. Acta Trop.. 2010;116(1):68-73.
- [Google Scholar]
- Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect. Immun.. 1993;61(5):1799-1809.
- [Google Scholar]
- Morphologic conversion of helicobacter pylori from spiral to coccoid form: scanning (SEM) and transmission electron microscopy (TEM) suggest viability. Ups. J. Med. Sci.. 2000;105(1):31-40.
- [Google Scholar]
- Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol.. 2010;7(11):629-641.
- [Google Scholar]
- Trends in Helicobacter pylori-related gastric ulcer research from 2012 to 2022: A bibliometric and visual analysis. Front. Med.. 2022;9:1027534.
- [Google Scholar]







