Translate this page into:
Heavy Metals Accumulation Effects on The Photosynthetic Performance of Geophytes in Mediterranean Reserve
⁎Corresponding author. t.houri@bau.edu.lb (Tarek Houri),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In Bentael natural reserve, Byblos-Lebanon, a recently constructed road, adjacent to its Southside, is threatening the wildlife of the reserve. The first source of this pollution is a vehicle derived contamination of soil, air and plants. This study determines heavy metals contamination levels evaluated by a rare plant species “Urginea maritima”, which is used as a biomonitor of airborne pollution. Four heavy metals were chosen for their toxic effects on the floral species. Lead, cadmium, aluminium and chromium were studied in plant leaves. The analysis of heavy metals is completed by a measurement of the photosynthetic activity of the plant. Two years period study showed lead upgrade in leaves versus a chromium decrease of plant uptake from the first year of road inauguration. Aluminium and cadmium levels increase for the first months after road works beginning then decreased sharply. As for photosynthetic pigments, the study showed an adverse effect of heavy metals on ordinary photosynthetic activities of the plants. Chlorophyll revealed primarily a decline in activities the first year but then amplification, in contrast of pheophytin which concentrations displayed a sharp rise especially in the most closely site to the road. These results indicated that landscape activities near natural reserves initiate heavy metals pollution for plants with a high risk of accumulation.
Keywords
Heavy metals
Photosynthetic activity
Contamination
Accumulation
Biomonitor
- ROS
-
reactive oxygen species
- LARI
-
Lebanese Agricultural Research Institute
- ICARDA
-
International Center for Agricultural Research in the Dry Areas
- Car
-
carotenoids
- Chl
-
chlorophyll
- Pheo
-
pheophytin
- P1
-
very polluted area
- P2
-
medium polluted area
- Ctrl
-
control area
- DNA
-
deoxyribonucleic acid
- Cr
-
chromium
- Pb
-
lead
- Cd
-
cadmium
- Al
-
aluminium
- Co
-
cobalt
Abbreviations
1 Introduction
Bentael Natural reserve – Lebanon- was founded in 1981 by the motivation of the inhabitants and the determination to protect this zone against urbanization projects, mission that must be strongly maintained nowadays with the inauguration of a new road in the south side of the reserve that menace its biodiversity.
The impact that can be generated from this vehicle pollution is converted into plant contaminations. “Urginea maritima”, a species declared rare by the United Nations Environment Program, was chosen to evaluate the heavy metals depositions on its photosynthetic performance and thus elucidate the danger of these metals and their transmissions to the plant.
One of the negative outcomes that can affect plant’s leaves is the establishment of leaf senescence. Leaf senescence is a natural developmental process that leads to cell death through highly regulated genetically controlled processes in plants. Leaf senescence may not be the only result of plant growth cycle, but it can be induced by environmental stresses (Sedigheh et al., 2011). This feature enables the plant to reorganize the nutrients distribution in the plant organs.
Generally, plants remobilize nutrients from old, senescent leaves to reproductive and young ones. One of the principal causes of leaf senescence is heavy metals pollution. Heavy metal is a term used to cover a wide range class of chemical elements that have specific weights higher than 5 g/cm3 (Appenroth, 2010). In plant nutrition, some metals are essential nutrients needed for the development of the plant, but others can be potentially toxics if they are excessively uptake by the plant, and which can be translated into alteration of different biochemical and physiological activities (Tchounwou et al., 2012). They may inhibit photosynthesis, cellular elongation, respiration and many others physiological processes. The toxicity of heavy metals reside in their direct generation of ROS by oxidation and by Fenton reaction, or by blocking the essential functional groups of biomolecules, or by substituting essential metal ions by other ones(Küpper et al., 2002). Transition metals (Cu, Fe, Mn…) have an unpaired electron in their orbitals which enables them to accept or donate single electron. This electron can be transferred to ground state oxygen O2 and thus generate ROS. Another mechanism of attack of heavy metals is the inactivation of antioxidant enzymes responsible of the scavenging of ROS. In general, heavy metals toxicity is induced when they reach a critical concentration value and especially when they are at their available form (Nagajyoti et al., 2010). The most generalized effect of heavy metals in plants is their attack to the photosynthetic apparatus. This properties is common to all heavy metals and isn’t specific to a particular metal, which make measuring the photosynthetic activities a good screening method for detecting heavy metal stress (Appenroth, 2010).
2 Material and methods
2.1 Plant sampling and preparation
The zone of study is “Bentael” natural reserve, (34°8'26“N 35°42'14”E): surface of 110 ha and an altitude between 300 m and 850 m above sea level (INMA et al., 2005). This study was performed in two years (2015 and 2016) on the months May, June and December, when the species “Urginea maritima” finishes the germination stage of the growth cycle and begins the budding phase(Al-Tardeh et al., 2008). The sampling areas were divided to three zones: roadside labeled as P1 which stands for “Very Polluted”, 200 m zone far from P1 and labeled P2 for “Medium polluted zone” and Ctrl for “Control area” (Fig. 1). Once the samples collected, there were placed in carton bags to avoid discoloration, and transported them directly to the laboratory of the “Lebanese Agricultural Research Institute” – LARI – Fanar, for analysis. Fresh leaves analysis was processed and the rest of the leaves were oven dried at 70 °C then reduced to powder (Hui et al., 2011).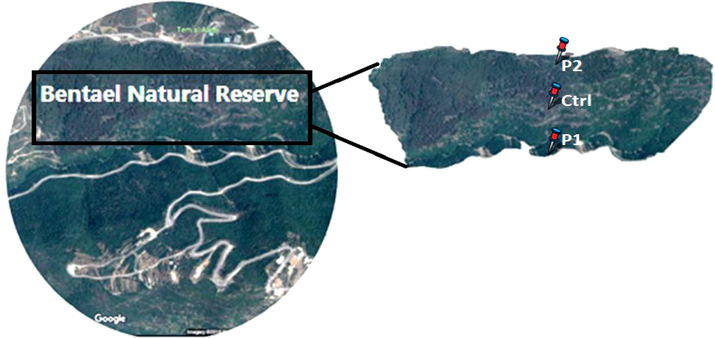
Sampling areas in Bentael natural reserve. Labeled sites are presented as P1-P2 and Ctrl for respectively: Very polluted, Medium polluted and Control.
2.2 Soil sampling
ICARDA’s manual for sampling procedure was followed: with the use of an auger, three subsamples were collected from each site at a depth of 20 cm (Estefan et al., 2013).
After sampling, soils were air dried in the laboratory then crushed and sieved at 2 mm diameter with the use of soil grinder (ELE international Fb523-100-01). After soil preparation, the analysis of five toxic heavy metals were realized: chromium, aluminium, cobalt, lead and cadmium (Ouelhadj et al., 2006).
2.3 Soil’s and plants heavy metals
Following the tri acid wet digestion method according to the ICARDA’s manual of soil, water and plants methods of analysis, the determination of heavy metals in soil and leaves was proceeded (Estefan et al., 2013). Briefly, to 0.5 g of dry plant material were added 30 mL of tri acid mixture (nitric, sulfuric, perchloric acids – 10:1:4 ratio). Digestion is maintained under 250 °C during 120 min. Then the solutions filtrates were used for determination of heavy metals by Atomic Absorption using iCE 3000 series AA spectrometers (Thermo Fisher Scientific).
2.4 Photosynthetic pigments
According to the method mentioned by Arnon and McKinney (Hu et al., 2013), the photosynthetic pigments were assessed. 0.5 g of fresh leaves were immersed into 30 mL of methanol (100%), and the submersion was conserved in the dark until total discoloration. Spectrophotometric readings were performed at wavelengths of 665.2 nm for “chlorophyll a”, 652.4 nm for “chlorophyll b”, 654.2 nm for “pheophytin a”, 647.6 nm for “pheophytin b” and 470 nm for “carotenoids”. Turbidity check was inspected at 750 nm (absorbance zero) and 520 nm (absorbance less than 10%). These analyses were performed using Thermo/Evolution600/160908 UV/VIS Spectrophotometer (Thermo Fisher Scientific Inc Middle East).
2.5 Statistical analysis
Experiments were carried in three replicates. Data were analyzed using Minitab 18 statistical software. The mean ± SD is the data presentation mode. T-test was used to assess significant differences between means, and which was believed significant when p < 0.05.
3 Results
Above, the charts (Figs. 2 and 3) of the four hazardous heavy metals studied: chromium, aluminium, lead and cadmium found in “Urginea maritima” leaves between years 2015–2016 (Fig. 4).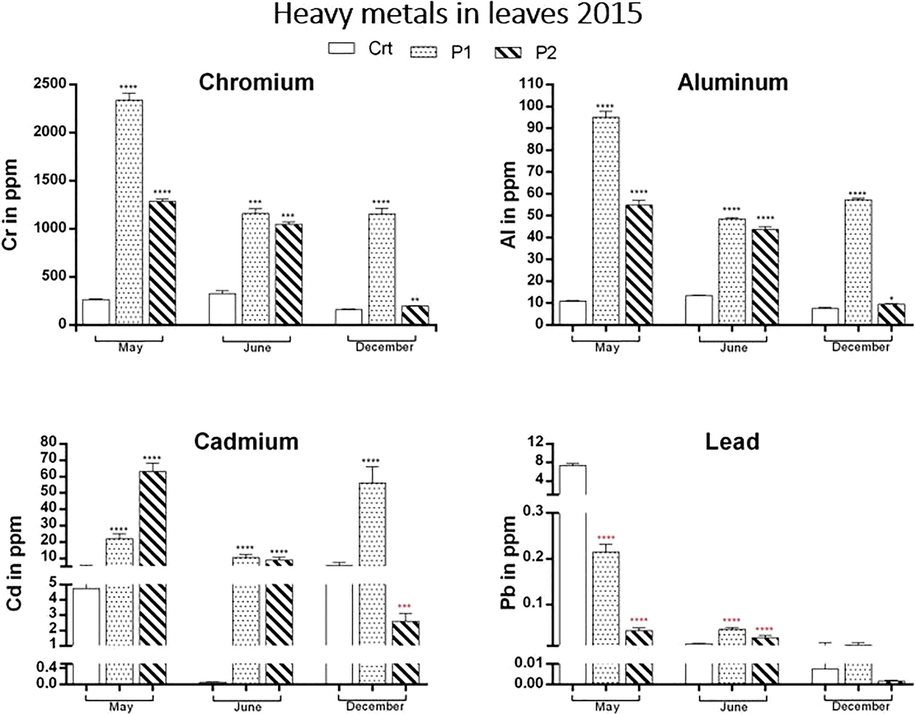
Variation of heavy metals concentration in leaves in 2015 and between the three studied sites Ctrl, P1 and P2. Chromium and Aluminium graphs show the highest levels of these elements in P1 all over year 2015. Cadmium concentration is highest in P2 in May 2015 and in P1 the other months. As for Lead, in May 2015 it recorded the highest concentration in Ctrl site and in P1 the other month.
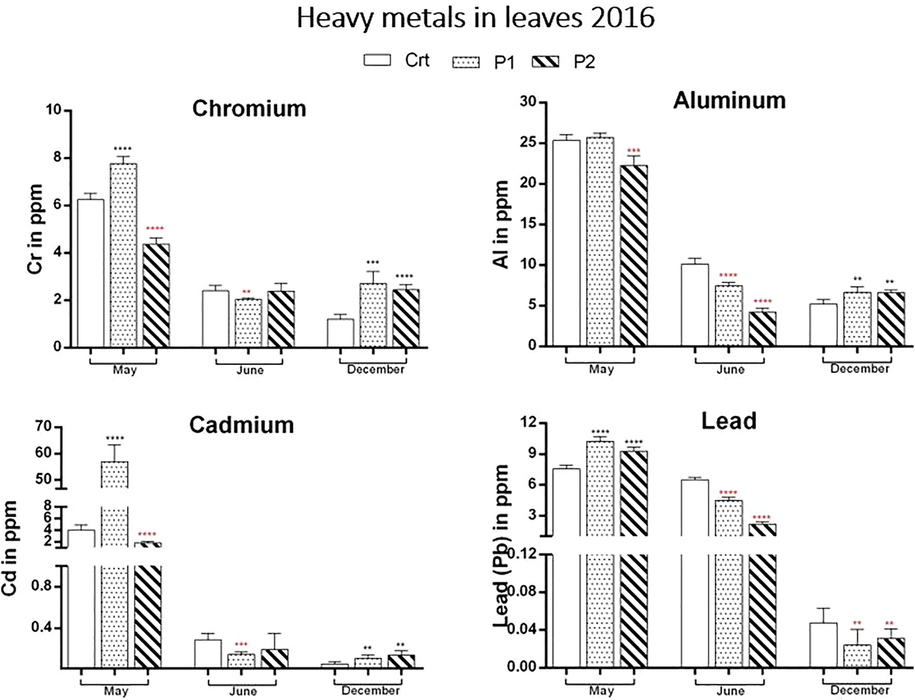
Heavy metals in 2016: recorded differences between sites are shown in the graphs. P1 is recording the highest level in May for all heavy metals. In June Ctrl site is recording the highest value and in December P1 is taking over chromium and aluminium concentration. P2 and Ctrl recorded the highest value in December for Cadmium and Lead respectively.
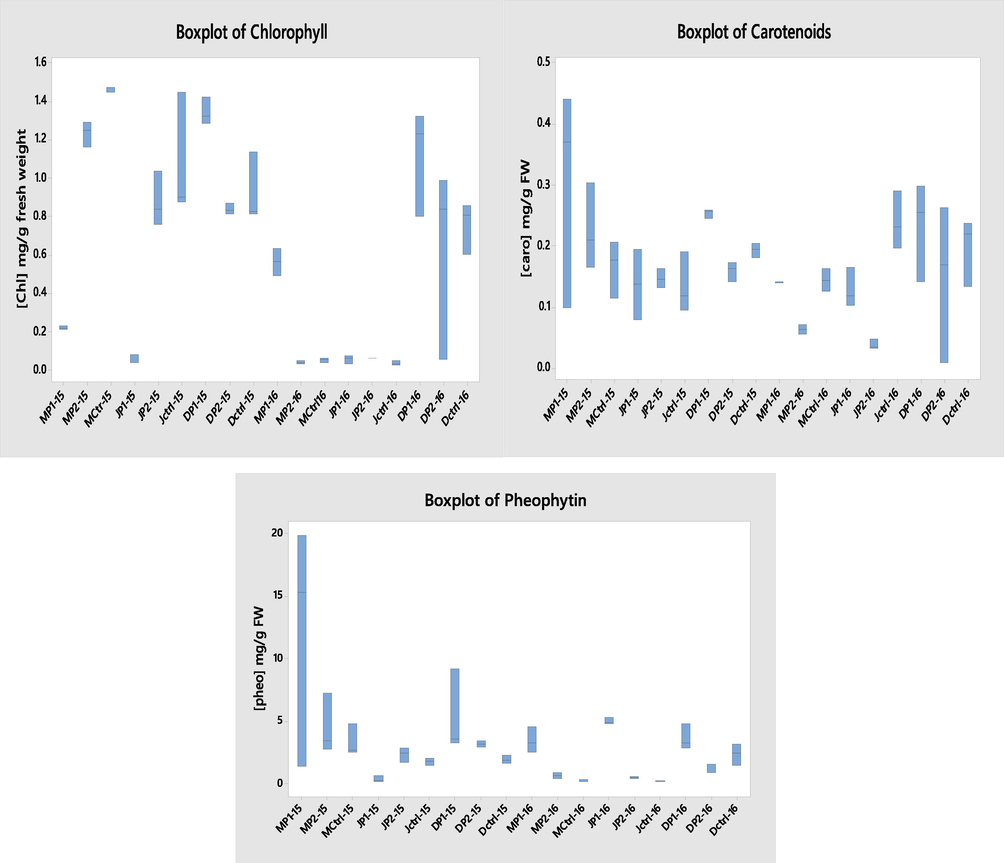
Boxplots showing chlorophyll, pheophytin and carotenoids variations between years 2015 and 2016 within the studied months.
After measuring heavy metals concentrations in plant’s leaves, the photosynthetic pigments levels were recorded in Table 1.
May-15
Jun-15
Dec-15
May-16
Jun-16
Dec-16
Site
Concentration
Chl
Ctrl
161.715 ± 8.4
158.308 ± 22.9
333.983 ± 22.5
285.074 ± 16.2
148.565 ± 23.4
118.774 ± 9.9
mg/g
P1
0.221 ± 0.009
0.53 ± 0.81
1.343 ± 0.07
0.564 ± 0.07
0.057 ± 0.02
1.117 ± 0.27
F.W.
P2
1.235 ± 0.06
0.876 ± 0.14
0.839 ± 0.03
0.041 ± 0.007
0.063 ± 0.002
0.629 ± 0.5
Pheo
P1
10.884 ± 9.64
0.333 ± 0.24
1.901 ± 3.32
3.434 ± 1.04
4.987 ± 0.24
3.622 ± 1.05
mg/g
P2
4.463 ± 2.4
1.765 ± 0.59
3.154 ± 0.24
0.666 ± 0.23
0.487 ± 0.08
1.088 ± 0.38
F.W.
Ctrl
3.340 ± 1.28
2.322 ± 0.32
5.329 ± 0.35
0.260 ± 0.08
0.199 ± 0.04
2.334 ± 0.83
Caro
P1
0.304 ± 0.18
0.027 ± 0.05
0.255 ± 0.006
0.141 ± 0.001
0.129 ± 0.03
0.233 ± 0.08
mg/g
P2
0.227 ± 0.07
0.135 ± 0.05
0.159 ± 0.01
0.064 ± 0.007
0.0369 ± 0.008
0.147 ± 0.12
F.W.
Ctrl
0.166 ± 0.04
0.148 ± 0.01
0.193 ± 0.01
0.145 ± 0.01
0.24 ± 0.04
0.197 ± 0.05
In May–June 2015, total chlorophyll concentrations in Ctrl were higher than in the other sites. Then, in December 2015, P1 recorded the greatest level. In May 2016, P1 noted a considerable raise, also in June, P2 scored the higher level and in December, P1 overtook the other sites. As for pheophytin, their amounts reach their highest levels in P1 followed by P2 and finally Ctrl. Similarly, carotenoids quantities were higher in P1 and Ctrl sites alternatively. These levels are relative to pheophytin’s concentrations in almost all sites (Tables 2 and 3).
F-value
Compound
Site
Month
Year
Site × year
Site × month × year
Chlorophyll
0.41 (a a ab)
6.82a *** (a a b)
18.48*** (a b)
7.25*** (a b c)
26.65*** (b a c)
Carotenoids
3.55* (a a b)
2.41 (a a a)
3.78 (a a)
3.92** (a a b)
3.15** (a b c)
Pheophytin
5.87* (a b b)
2.18 (a a a)
4.59* (a b)
3.6* (a a b)
3.79*** (a b b)
Cr
15.93*** (a b c)
5.35** (a b b)
133.19*** (a b)
130.27*** (a b c)
541064*** (a b c)
Al
22.88*** (a b c)
16.22** (a b b)
58.48*** (a b)
15.07*** (a b c)
5102.37*** (a b c)
Cd
16.88*** (a b c)
18.2** (a b b)
14.3*** (a b)
13.59** (a a b)
395.25*** (a b c)
Pb
3.77* (b b a)
33.92** (a b b)
119.06*** (b a)
30.05*** (c a b)
1629.91*** (a b c)
May-15
Jun-15
Dec-15
May-16
Jun-16
Dec-16
Site
Car/Chl (a + b)
P1
1.374
1.000
0.190
0.251
2.257
0.208
P2
0.184
0.126
0.267
1.563
0.618
0.234
Ctrl
0.114
0.168
0.209
2.740
6.289
0.261
Chl a/Chl b
P1
1.361
0.858
2.147
2.467
6.003
2.627
P2
2.458
0.278
2.189
1.120
5.218
3.561
Ctrl
2.017
1.363
1.881
0.087
1.355
2.683
An analysis of variance (One way ANOVA) with Tukey comparisons showed a significant difference of chlorophyll, carotenoids, and pheophytin when comparisons were done between sites versus month and year. As for the heavy metals, they all showed a significant difference between year, site, months, site versus year and particularly between site versus month versus year.
4 Discussion
Metals are essential for plants to complete their functional processes (Peralta-Videa et al., 2009). Some elements are required in minor quantity and others in higher concentrations. The problem appears when unessential elements are absorbed in large proportions; these transition metals then become toxic, especially that their stable character make them persistent and non-degradable so, they accumulate in other bodies (Srivastava et al., 2017). Chromium toxicity is related to its oxidation status. Cr3+ is the most stable form but Cr6+ is the highest noxious state for plants. Cr6+ enters cells under physiological conditions and may be reduced to reactive intermediated, such as Cr5+, Cr4+, thiylradicals, hydroxyl radicals, and ultimately, Cr3+. These species attacks DNA, proteins, membrane lipids and consequently disrupt cellular integrity (Tchounwou et al., 2012). Moreover, highly Cr uptake by plants can reduce concentration of major macronutrients such as potassium, phosphorus, iron and magnesium (Kabata-Pendias and Pendias, 2001).
Cadmium is known as one of the most toxic metals because it reveals contrary effects on plant metabolism. Cd is a nonessential element for metabolic processes in plants and can become toxic if absorbed in high amounts. It is well absorbed by both root and leaf system, is very mobile in plant and its danger reside in disrupting many enzymes activities. Cadmium ions have strong affinity for sulfhydryl groups, phosphate groups and side chains proteins, which will lead, once it interacts with them, to the inhibition of their functions (Kabata-Pendias and Pendias, 2001).
Lead is a metal of very low mobility; most of its portion is retained in soil at the root level (Oropeza-Garcia et al., 2014). Pb uptake by plants is passive and conducted by the hairy root system and can penetrate leaves by their waxy cuticles. Once transported into the plant, it can cause damages to membranes, enzymes and various protein components. Small proportions of Pb may inhibit respiration and photosynthesis due to the disturbance of electron transfer chain reaction (Doǧanlar and Atmaca, 2011). However, the most deteriorating effect of Pb in the plants is the destruction of the plasma lemma, which will disturb the water membrane permeability and also mimicking the physiological behavior of calcium and thus restraining the reaction of some enzymes.
As for Aluminium, its functions in plants are not elucidated clearly but it seems to have a beneficial effect on plant growth in small quantities that can activates some enzymes. Nonetheless, Al toxicity is often reported in plants grown in acid soils because of its capacity to induce negative interaction with essential plant nutrients (Nitrogen, phosphorus, potassium, calcium and magnesium) and limit their uptake. In addition, Al was related to plant chlorosis, shallow rooting and drought susceptibility (Kabata-Pendias and Pendias, 2001).
The most significant consequence of heavy metal excess is the damage of photosynthetic apparatus that is starting to combat the pollution by its raise in pheophytin and the launching of the adaptation mode revealed by chlorophyll increase.
The considerable degradation of chlorophyll, supports the argument that the chloroplast is the primary site of attack by air pollutants (Sewelam et al., 2016). The decreasing order of chlorophyll in Ctrl over the years indicates the pollution effects on the plants. Barakat 2011 demonstrated that an increase in chlorophyll content in air polluted sites is a way of fighting oxidative stress generated from pollution conditions.
Chlorophyll, the active molecule of the chloroplast, plays a critical role in plant's photosynthesis. The amount of chlorophyll is significantly affected by environmental conditions. Significant reduction in P1 occurred in 2015 in comparison to Ctrl site. As a result of anthropogenic actions, the content of chlorophyll decreased. Furthermore, depending on the distance between the plant and the anthropogenic sources of pollution, the rates of chlorophyll varies: the minimum chlorophyll is recorded in P1, which is the nearest site to the road, followed by P2 and finally by Ctrl. The diminution in chlorophyll content may be due to an increase in chlorophyll degradation or a decrease in chlorophyll synthesis. The dilemma is resolved by calculating the ratio Chlorophyll a/chlorophyll b (Singh and Pandey, 2011).
Chlorophyll degradation occurs from chlorophyll a because chlorophyll a is more sensitive to air pollutant as it is easily converted to pheophytin a (Conti and Cecchetti, 2001). So, a reduction in the latter molecule is consequently a result of chlorophyll breakdown.
In addition, the ratio of carotenoids to total chlorophyll declines from P1 to Ctrl in 2015 which indicates higher carotenoids and thus a superior necessity of photoprotection by this non enzymatic antioxidant. In 2016, chlorophyll levels are higher which demonstrates that the scavenging system in the plant is appropriately working. Additionally, inflation in chlorophyll concentrations indicates that plants are adapting to pollution’s situation. These fluctuations in chlorophyll concentrations can be identified as markers of atmospheric pollution. As for pheophytin results, their high level is mainly due to the replacement of magnesium atom in chlorophyll by two hydrogen atoms (İnanç, 2011).
It is demonstrated that, under stress conditions, chlorophyll undergoes several photochemical reactions such as oxidation, reduction and pheophytinisation (Govindaraju et al., 2010). Schelbert et al. (2009) demonstrated that, under stressful conditions, pheophytin accumulated in huge quantities, which explain the results of high levels of pheophytin in P1.
The final role is for carotenoids that protect plant cell. Among the antioxidant molecules in the plant defense system against oxidative damage, carotenoids are very efficient in scavenging ROS. They take a vital part in channeling harvested light energy to the plant's photosystem and on using non phytochemical quenching for dissipation of excess light (McElroy and Kopsell, 2009). Beside their structural function in the photosynthetic antenna and reaction center, carotenoids play a crucial role in protecting the photosynthetic system from oxidative damage by scavenging reactive oxygen species (You and Chan, 2015).
5 Conclusion
Air quality is highly affected by human activities. Bentael nature reserve – Lebanon – is facing a huge threat after inauguration of a paved road on its south side, but not even a valuable research has been done in this area. Vehicles transportations are a major source of heavy metals emissions. Aluminium is a catalyst; diesel engines emit primarily Cadmium. In addition, lubricants produce Cadmium and Lead. The measurements of photosynthetic pigments in “Urginea maritima” demonstrated its fight against stress. Photosynthetic pigment contents are one of the most frequently used techniques to measure the degree of several abiotic stresses and follow the time-course evolution of leaf senescence. It has been demonstrated that the plant is fighting stress by its acclimation with the environmental conditions, regenerating of chlorophyll after its decline along with a constant level of pheophytin and a higher level of carotenoids. Useful actions can be executed as perspectives of prevention of airborne pollution. The implementation of weather stations that include pollution detectors for that climate conditions, physico-chemicals properties of air pollutants and their residence time in the atmosphere have impact on surrounding fauna and flora. The establishment of biomonitoring programs is an urgent need. The use of phytoremediation of soils from heavy metals depositions is of great interest with a low cost. The most important proceedings that can be executed are the organization of Green belts around cities.
6 Disclosure of funding
The studies reported in this publication were supported by Beirut Arab University and the Lebanese Agricultural Research Institute. The terms of this arrangement have been reviewed and approved by Beirut Arab University and the Lebanese Agricultural Research Institute in accordance with their policies on objectivity in research.
7 Disclosure of conflict of interest
The authors whose names are listed in the title page certify that they have NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Nectary structure and nectar presentation in the Mediterranean geophyte, Urginea maritima (Hyacinthaceae) Botany. 2008;86:1194-1204.
- [CrossRef] [Google Scholar]
- Appenroth, K.-J., 2010. Definition of “Heavy Metals” and Their Role in Biological Systems. pp. 19–29. https://doi.org/10.1007/978-3-642-02436-8_2.
- Biological monitoring: lichens as bioindicators of air pollution assessment — a review. Environ. Pollut.. 2001;114:471-492.
- [CrossRef] [Google Scholar]
- Influence of airborne pollution on Cd, Zn, Pb, Cu, and Al accumulation and physiological parameters of plant leaves in Antakya (Turkey) Water Air Soil Pollut.. 2011;214:509-523.
- [CrossRef] [Google Scholar]
- Estefan, G., Sommer, R., Ryan, J., 2013. Methods of Soil, Plant, and Water Analysis: A manual for the West Asia and North Methods of Soil, Plant, and Water Analysis: A manual for the West Asia and North Africa region 143.
- Impact assessment of air pollution stress on plant species through biochemical estimations. J. Environ. Monit.. 2010;4:935-938.
- [Google Scholar]
- Simple extraction methods that prevent the artifactual conversion of chlorophyll to chlorophyllide during pigment isolation from leaf samples. Plant Methods. 2013;9:19.
- [CrossRef] [Google Scholar]
- Defining optimal sampling effort for large-scale monitoring of invasive alien plants: a Bayesian method for estimating abundance and distribution. J. Appl. Ecol.. 2011;48:768-776.
- [CrossRef] [Google Scholar]
- Chlorophyll: structural properties, health benefits and its occurrence in virgin olive oils. Akad. Gıdatr (A.L. İnanç). 2011;9:90-344.
- [Google Scholar]
- INMA, SRI, U., Ministry of Environment, Ministry of Tourism, 2005. Lebanon’S Nature Reserves Bentael 26.
- Kabata-Pendias, A., Pendias, H., 2001. Trace elements in soils and plants. New York 2nd, 331. https://doi.org/10.1201/b10158-25.
- Kinetics and efficiency of excitation energy transfer from chlorophylls, their heavy metal-substituted derivatives, and pheophytins to singlet oxygen. Biochim. Biophys. Acta – Gen. Subj.. 2002;1572:107-113.
- [CrossRef] [Google Scholar]
- Physiological role of carotenoids and other antioxidants in plants and application to turfgrass stress management. New Zeal. J. Crop Hortic. Sci.. 2009;37:327-333.
- [CrossRef] [Google Scholar]
- Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett.. 2010;8:199-216.
- [CrossRef] [Google Scholar]
- Transport of heavy metals in materials with diameter analogous to xylem vessels. Int. J. Environ. Res.. 2014;8:123-132.
- [Google Scholar]
- Heavy metal stress and leaf senescence induce the barley gene HvC2d1 encoding a calcium-dependent novel C2 domain-like protein. New Phytol.. 2006;170:261-273.
- [CrossRef] [Google Scholar]
- The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int. J. Biochem. Cell Biol.. 2009;41:1665-1677.
- [CrossRef] [Google Scholar]
- Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in arabidopsis. Plant Cell. 2009;21
- [CrossRef] [Google Scholar]
- Global plant stress signaling: reactive oxygen species at the cross-road. Front. Plant Sci.. 2016;7:1-21.
- [CrossRef] [Google Scholar]
- Effect of nickel-stresses on uptake, pigments and antioxidative responses of water lettuce, Pistia stratiotes L. J. Environ. Biol.. 2011;32:391-394.
- [Google Scholar]
- Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front. Environ. Sci.. 2017;5:64.
- [CrossRef] [Google Scholar]
- ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci.. 2015;6:1-15.
- [CrossRef] [Google Scholar]







