Translate this page into:
Health and environmental effects of silent killers Organochlorine pesticides and polychlorinated biphenyl
⁎Corresponding author. szu_sfli@163.com (Shuangfei Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Persistent organic pollutants (POPs) are known to be silent killers due to their bioaccumulative and long-lasting existence. These pollutants are present everywhere in our environment, including plants, animals, and humans. POPs can be stored in several aquatic environmental matrices and biomagnified by the food web, thus presenting a danger to aquatic habitats and human health. During recent decades, they have gained substantial attention considering their possible persistent threats. In the aquatic environment, legacy POPs, such as organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs), were widely found and recorded. A complete description of OCPs and PCBs amounts and their distribution in the aquatic environment is necessary for a detailed understanding of the activities and threats of POPs in aquatic ecosystems.
Keywords
Aquatic
Bioaccumulation
Bioindicators
Ecosystem
Food chain
Remediations
1 Introduction
The ecosystem is still constantly polluted by numerous contaminants. There are multiple forms of pollution in various locations. Some are persistent in degrading the environment (photolytic, chemical, and biochemical reactions) and have been present for long periods. The contaminants in our ecosystem that last long are considered persistent organic pollutants (POPs) are the contaminants in the environment for a long time (Bhatt et al., 2020). There are many POPs contaminants, polychlorinated biphenyls and pesticides are also part of these POPs contaminants. These pollutants are transferred from one area to another, also over borders. These have been identified in areas where never been used. These contaminants accumulate in the food chain and contribute to numerous legendary health risks and environmental consequences (Bhatt et al., 2020; Yaghmour et al., 2020).
It is not easy to trace the occurrence, delivery, and destiny of POPs in the atmosphere since they can occur in various processes. Sediments may be viewed as a drain with multiple POPs. POPs can often come into contact with solid particulate matter (SPM) after release in water bodies or maybe bioaccumulated in aquatic species, resulting in side effects (Cruz et al., 2017). Initially, twelve POPs were known as the “dirty dozen,” which induced adverse human and ecosystem effects. Environment Canada, the United States Environmental Protection Agency, the European Union, and the United Nations Environment Program banned the well-known toxic POPs. However, some developing nations utilized POPs, and they are present in the environment (Annex, 2008).
Polychlorinated biphenyls (PCBs) are commercial chemical compounds that are synthetically manufactured and have different applications. Around 209 individual PCBs congeners are depending upon the number of chlorine atoms in the molecule. Up to eight chlorine atoms can possess biphenyl congeners with a double aromatic ringed structure (Balejcikova et al., 2020). Trademarked industrial PCBs mixtures use the term “Aroclor,” along with a numeric symbol. These industrial mixtures reflect PCBs variations with various concentrations of chlorine. It is not only because of its immediate toxicity but also because it can accumulate in the tissues of aquatic species and then biomagnetize with each trophic stage that the PCBs are found in the aquatic environment. The PCBs differ based on the species, the conger chlorination degree, and the place where the organism lives and feeds (Jafarabadi et al., 2019). Organochlorine pesticides (OCPs) are also persistent toxic substances (PTS) rapidly worried worldwide owing to their salient characteristics of toxicity, persistence, and bioaccumulation (Necibi and Mzoughi, 2020). Nine organochlorine pesticides were suggested in the 12th Stockholm Convention to be regulated as POPs. Several organic chemicals with permanent adverse effects on the atmosphere and the ecology, including hexachlorobenzene, mirex, endrin, dieldrin, chlordane, DDT, toxaphene, and aldrin (Li et al., 2018). Residues of the OCPs have been detected and documented worldwide, including Antarctica and the Arctic Region, owing to their intensive agricultural and industrial usage (Bengtson Nash et al., 2017).
2 Source of organochlorine pesticides and polychlorinated biphenyl
PCBs have many applications as oils, lubricants, and in electrical machinery because of their chemical durability, low manufacturing cost, and non-inflammability. Considering the environmental fate of many synthetic chemicals, such as PCBs, it is of considerable concern due to wide-range environmental transportation and prevalence (Keithmaleesatti et al., 2007). Owing to the thermal stability, dielectric, oxidative resistance properties, PCBs have been widely used in lubricants, regulators, electric capacitors, circuit breakers, transformers, industrial manufacturing and metallic coating, adhesives, plastics, and paints compounds (Erickson and Kaley, 2011).
OCPs were broadly employed worldwide as pesticides in agriculture and the health sector over several years(Oliveira et al., 2016). The development and use of these pesticides were stopped or limited in developed countries during the 1970 s and 1980 s; nevertheless, most OCPs, particularly HCHs and DDTs (Bigus et al., 2014). However, high pesticide use has often contributed to accumulating these chemicals in the atmosphere across many routes. It can impact ecology and its services(Carriquiriborde et al., 2014).
3 Toxic effect of PCBs and OCPs
An assessment of various pesticides indicates that they can induce hypertension and other cardiac-related issues in humans. Organochlorines serve as endocrine disruptors by interacting with molecular circuitry and endocrine system functions (Mrema et al., 2013). Experimental animals' chronic exposure to harmful concentrations of chlorinated hydrocarbon pesticides such as DDT, aldrin, dieldrin, and others, induced various degrees of harm to the liver and other organs. It often caused a toxic effect on the brain and nervous system (Baltazar et al., 2014). That may be a correlation between liver diseases and the retention of these pesticides in human tissues. Many studies have investigated the toxicity of specific OCPs on various fish models such as zebrafish (Danio rerio), Japanese medaka (Oryzias latipes), and fathead minnow (Pimephales promelas). The acute toxicity of endosulfan in juvenile rainbow trout was examined under static conditions in glass aquaria. The women exposed to PCBs during their pregnancy may result in severe motor control and neurological issues, and in children, they cause short-term memory loss and lowered IQ (Reddy et al., 2019). Exposed employees have noticed changes in urine and blood that could be symptoms of liver injury (Deng et al., 2019). Several studies on zebrafish have revealed that PCBs exposure can cause neurotoxicity, developmental toxicity, and genotoxicity during all stages of zebrafish life. Some studies have shown that PCBs responsible for physiological dysfunction such as deformities of cardiac, reproduction abnormality, and metabolically fragile β-cells. Some recent studies have also revealed that PCBs can disrupt fish endocrine and metabolism(Hoydal et al., 2017). During the freshwater migration, fish undergo a preparatory phase, named smoltification using a system known to improve the tolerance of marine water and metabolic adjustments, enhancing survival, food use, and accumulation of energy during residence in the sea (Espe et al., 2020). Interesting experiments were performed to test the impact of PCBs on smoltification and seawater choice. Because seawater entry may cause these fish extreme osmotic stress, effective smoltification is critical for survival, increase energy allocation during seawater residences, and eventual reproduction until it returns to freshwater.
4 Bioavailability of PCBs and OCPs in seafood
Different organism affected by PCBs (Fig. 1). PCBs are transferred into the food web through living organisms that are present in the environment. Still, their transport is mainly attributed to sediment transport and deposition processes, which vary by location. PCBs that enter the marine system gradually dissolved by sediments and accumulate in the environment. Bottom feeders are more prone to accumulate PCBs such as Gobionotothen gibberifrons (Desmet et al., 2012). Cappelletti et al. (2015) also demonstrated that polluted sediments via grazing practices could increase PCBs bioaccumulation in bottom-feeders (Cappelletti et al., 2015). Trophic position showed variation in contaminant levels among biota because of PCBs’ biomagnification (Lopes et al., 2011). PCBs penetration in freshwater bodies, including rivers, lakes, and streams, causes these elements to bioaccumulate in freshwater fish(Goutte et al., 2020). Many invertebrates are essential food sources and the practical path of nutrient absorption PCBs for fish and other species. Species at higher trophic levels in a food chain are more prone to biomagnify higher PCBs levels in the food chain. A species higher PCBs levels increase PCBs level in the food chain that can threaten humans or other organisms.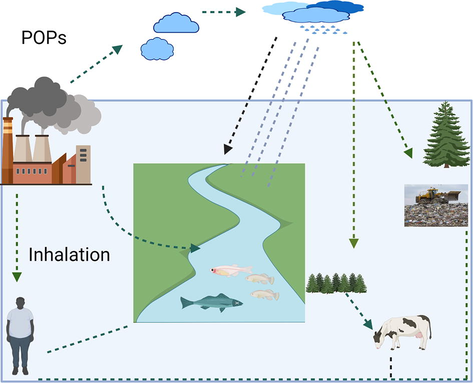
Different organisms effected by POPs.
OCPs are a category of extremely persistent POPs. The impact of POPs on the ecosystem, both on humans and animals, is of considerable concern due to their toxic, bioaccumulative, and semi-volatile character(Luo et al., 2019). These pesticides are present in the marine ecosystem and may be ingested by suspended pollutants from the water and then accumulated into the sediment's bottom. However, sediment is a conduit for introducing pollutants and other toxins to the surrounding ecosystem and the organisms around. Biota may absorb these pesticides and transport them to food chains because of their bioaccumulation (Ganaie, 2020). Organisms at a higher trophic level in the food chains are at greater risk of biomagnification of these pesticides in food chains. Fishes are present at the top of the food chain in the aquatic ecosystem; lead concentrating in their tissues is at high risk the gills and livers of fishes store OCPs at high concentration (Arisekar et al., 2019). People typically eat fish as part of their regular diet. Since lead is ingested into the bloodstream and taken into tissues during absorption, people are at risk of OCPs accumulation in their body tissues by eating fish.
5 Bioaccumulation of PCBs and OCPs in seafood
The bioaccumulation of PCBs in freshwater fishes presents important biological, social, and environmental consequences. When PCBs are found in broad ambient quantities, they typically influence the organism's aggregation of toxins. It also induces the biomagnification of POPs in the trophic web, which has a detrimental impact on the marine environment since it depends upon them in several forms, whether explicitly or indirectly. Sediments can consume PCBs and act as basins(Apell and Gschwend, 2016). It indicates that PCBs levels in sediments may be diverse and non-significant for total PCBs exposure for migratory fishes. Fish absorb PCBs from their surrounding habitats, mainly through the gills or the organism's epithelial or dermal tissues and through the prey (Visha et al., 2015) (Fig. 2). Research intended to evaluate PCBs concentrations in five commercially valuable fish species in Iran, namely the Scomberomorus commerson, Thunnus tonggol, Euthynnus affinis, Scomberomorus guttatus, and Otolithes ruber (Jafarabadi et al., 2019). Overall findings suggest that PCBs' greatest contribution in fish was from PCBs-contaminated surface sediments. Slightly higher amounts of PCBs were observed for both sex kidney and liver tissue than other body tissues. Rather predictably, PCBs concentrations in fish were strongest in the tissues with the highest volume of lipids; the liver, muscle, and gills. The degree of PCBs deposition in different fish tissues typically differs depending on tissues' structure and function. Solé et al. (2001) reported higher concentrations of PCBs in tissues with greater lipid content (muscles, liver, and gills) in fish from the Mediterranean (Solé et al., 2001).Higher OCPs levels are recorded in fish gills tissues of 22 specimens of M. cephalus and 30 specimens of P. commersonnii (Olisah et al., 2019). Samples of the muscle and liver tissues (from three separate fish groups Cathorops spixii, Trichiurus lepturus, and Paralonchurus brasiliensis) from two different sites located in Santos, Brazil, were analyzed for the concentrations of PCBs and polybrominated diphenyl ethers (PBDEs) and also the organochlorine pesticides (OCPs), n-hexachlorobenzene (n-HBCB), mirex (MIR), methylhexane (CHM), and dichlorodiphenyldichloroethylene (DDD). The concentrations of n-HBCB, MIR, DDD, and CHM were significantly higher in the muscle of the fishes from Santos Bay than those of the fishes from Moela Island, whereas concentrations of DDD, PCBs, and PBDEs were higher in the muscle of the fishes from the Santos Bay than the fishes from Moena Island (Magalhães et al., 2017). The concentrations of total DDE, PCDDs, PCDFs, and PCBs in the muscle of the fishes from the Santos Bay were significantly lower than those of the fishes from Moena Island, whereas, from these fishes, significantly higher PCDDs and PCDFs concentrations were determined than those in the muscle of the fishes from both sites (Magalhães et al., 2017). Like several other seafoods, bivalves provide a big part of the human diet, and they play an essential role throughout the biogeochemical cycle and they are aquatic environment filter feeders (van der Schatte Olivier et al., 2020). They like to accumulate contaminants by eating in seawater and being consumed by other aquatic species at heights above their own position in the food chain (Yuan et al., 2020). These bivalve mollusks and other small organisms can pick up particles suspended in the marine water column, suspended particulate materials, sediments, and even food sources. The bioaccumulation rate of metals in bivalves depends on biotic factors (e.g., animals, age, sex, weight, gametogenesis, and physiological status) and abiotic factors (e.g., chemical species, pH, salinity, temperature, filtration rate, supply of atmosphere contaminants) (Fernández-Tajes et al., 2011). Bivalve mollusks (e.g., clam species) are species that accumulate bioavailable toxins in the aquatic setting. Therefore, they have been commonly used as bioindicators for tracking aquatic environmental emissions(Milun et al., 2016). Presence of various chemical contaminants has been reported in several studies investigating wild and cultured bivalves (Suárez et al., 2013). However, studies have examined the presence of OCPs in cultured bivalves in the Adriatic Sea Croatian part (Herceg-Romanić et al., 2014).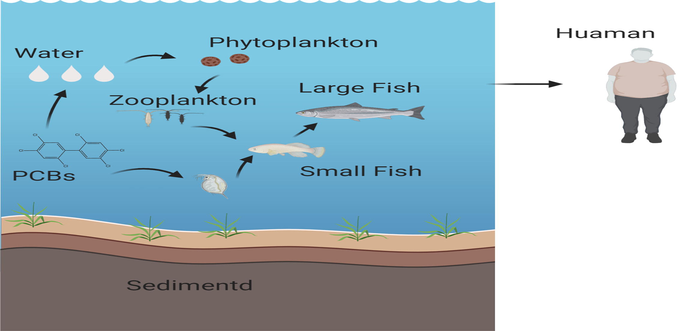
Bio-accumulation of PCBs in aquatic environment.
6 Recommendations
6.1 Biological indicators as a warning system of PCBs and OCPs
Bioindicators are organisms whose appearance, absence, and physiological conditions are markers of environmental quality(Edegbene et al., 2020). The relationships between parasites and their host fish have drawn growing ecological interests (Oyoo-Okoth et al., 2010). Studies have provided data suitable for developing sentinels for contaminant biomonitoring on the relationship between parasites, their hosts, and contaminants (Oyoo-Okoth et al., 2010). Simultaneously, fish parasites have been used to examine the aggregation of inorganic contaminants such as PCBs (Akinsanya et al., 2020). To evaluate the levels of PCBs in sediments and commercial fish species and decide whether the parasites of fish host species would bioaccumulate higher levels of PCBs than their piscine hosts' fish parasites used as a bioindicator in Lake Victoria. He proved that fish parasites bioaccumulate higher amounts of PCBs than their hosts and thus have a promising biomonitor for PCBs.
Some studies have revealed that PCBs consumption predominantly derives from aquatic foods because of high pollution levels in the surroundings. PCBs in marine fishes were analyzed using Pagrosomus major, Bullet mackerel, and Navodon septentrionalis as bioindicators (Shang et al., 2016). Two fish species Merlangius merlangus euxinus and Oncorhynchus mykiss eggs were used as an indicator to evaluate the concentration of PCBs (Atmaca et al., 2019).
Bivalve mollusks accumulate bioavailable toxins in the aquatic world as filter-feeding species. They are the first alternative as bioindicators, for example. Mytilus edulis has been commonly used as bioindicators for marine environmental contamination monitoring because of its low procurement costs, global spread, and sedentary activity(Kljaković-Gašpić et al., 2010). The use of bivalves as bioindicators has a long history. Young et al. (1974) used M. californianus transplanted mussels as a bioindicator for (PCBs) in a Southern California Bight (Young et al., 1976). Lee et al. (1996) using the M. edulis as an indicator collected from the south-west Baltic Sea for organochlorine determination PCBs (Lee et al., 1996).
Twenty years later, Carro et al. (2016) examined PCBs from the coast of Galicia in two oysters and blue mussel (Carro et al., 2016). The wild Corbicula largillierti were used as a bioindicator of PCBs pollution(de Castilhos Ghisi et al., 2020). PCBs were determined in the bivalve molluscs such as Callista chione, Callista chione,Callista chione,Callista chione, Arca noae, Callista chione, Arca noae, Venus verrucosa, Ostrea edulis, and Mytilus galloprovincialis tissue (Milun et al., 2016). Seabirds are at the top of the food chain; that's why they are useful. Seabirds also are valuable bioindicators for the monitoring of organochlorine pollutants. Non-migrating birds may reflect their habitat's background contamination. If local emission sources are not present, birds represent global pollution induced by transboundary transport of contaminants (Tsygankov et al., 2017). Most fish type’s bioaccumulated OCPs. Many fish species fulfill bioindicator requirements needed to determine aquatic environments. Therefore, fish are deemed one of the finest bioaccumulation properties and environmental impact research species(Manfra et al., 2016). Organochlorine pesticides (OCPs), such as DDTs and HCHs, are now being used as pesticides. Clarias gariepinus has been selected as an indicator species because it is abundant in South Africa, resilient, apex aquatic predator in the study region, and most importantly a valued food source (Rouhani and Britz, 2011). Local fishers attack them in rural areas and fishers in the study region. Due to their place on the food chain and choice for deep life in aquatic environments, sensitivity to contaminants, bio-amplification, and bioaccumulation of organic chemical pollutants are best studied, facilitating research into the potential transmission pollutants to humans. (Gerber et al., 2017) investigate tiger species (Hydrocynus vittatus) as the bioindicator species because of their ecological and economic significance. H. vittatus is the main piscivore predator in several African rivers (Verhaert et al., 2017). Because it is present at the top of the food chain, it is necessary to elucidate these populations' health considering what has occurred in the Olifants River regarding C. niloticus. Tigerfish is an excellent aquatic bioindicator organism because it fulfills all good bioindicators' requirements(Burger and Gochfeld, 2001).
6.2 Remediation of PCBs and OCPs
For using them in bioremediation, fungal organisms often have many fascinating properties but not in their natural habitat (Germain et al., 2020). Fungi are widespread microorganisms present in marine sediments, terrestrial ecosystems, and water surfaces, and their hyphal growth facilitates access to toxins (Grossart et al., 2019). Fungal species, such as Trametes versicolor, Pleurotus ostreatus, Lentinus tigrinus, and Phanerochaete chrysosporium certain filamentous ascomycetous fungi can degrade many PCBs congeners(Federici et al., 2012). Over the years, fungi have been used as a biological resource to degrade and eliminate pesticides' influence. The microbial degradation of PCBs is known as one of the most economical and least costly ways of PCBs removal from the ecosystem (Passatore et al., 2014). Many gram-positive strains such as Micrococcus, Arthrobacter, Paenibacillus, Bacillus, Rhodococcus, Corynebacterium, and gram-negative strains including Enterobacter, Ralstonia, Sphingomonas, Comamonas, Acinetobacter, Burkholderia, Janibacter, Achromobacter, Alcaligenes, and Pseudomonas have been already reported for the removal of PCBs(Erickson and Mondello, 1993). However, the PCBs degrading bacteria are more efficient at eliminating PCBs congeners with five or fewer chlorines. Bacterium o-17 is a member of the phylum Chloroflexi, and it has 90% similar to that of Dehalococcoides ethenogenes (Bedard, 2008). This is the first microorganism to be isolated and identified in the community of Dehalococcoides. When the species Dehalococcoides ethenogenes195 and Chloroflexi, grown with perchloroethylene, they dechlorinated the PCBs (Fennell et al., 2004). Armoracia rusticana and Salix caprea were established as rhizoremediation candidates because of their strong resin content with active PCBs degraders (Rezek et al., 2008). Matsumoto et al. (2009) analyzed in-depth studies of endrin and dieldrin degradation by aerobic microorganisms (Matsumoto et al., 2009). Microorganisms such as Trichoderma koningi, Mucor alternans, Aerobacter aerogenes, Trichoderma viride, Bacillus sp., and Pseudomonas sp. were performed degradation of dieldrin (Anderson et al., 1970). In comparison, aerobic microorganisms such as Micrococcus sp; Pseudomonas sp have been used to suppress endrin in few experiments (Matsumoto et al., 2009). In another research, microorganisms such as T. viride, Micrococcus sp., Arthrobacter sp and Bacillus sp. and Pseudomonas sp., have been identified to endrin degrading strains. It has been documented that the microorganisms degrade endrin and dieldrin to organic solvent-soluble and water-soluble substances.
In research by Olette and her colleagues (2008), aquatic plants, such as Cabomba aquatic, Elodea canadensis, and Leman minor, were found to “take up” and “absorb” three pesticides, such as dimethomorph, flazasulfuron, and copper sulfate (Olette et al., 2008). The authors noticed that the Lemna minor species had the most effective absorption ability, led by the Elodea Canadensis species, which was more efficient than the Cabomba aquatic species.
Further, Olette et al. (2010) demonstrated that the microalgae Scenedesmus quadricauda is more successful in the elimination of one herbicide (isoproturon) and two fungicides' (dimethomorph and pyrimethanil) from their medium (Dosnon-Olette et al., 2010). The green alga (Chlamydomonas reinhardtii) is an alga that may degrade the herbicide prometryne (Jin et al., 2012). This quick uptake and catabolism of prometryne contributed to the rapid elimination of the compound from the media. The result of this degradation may be viewed as an internal tolerance process, indicating that green algae are useful in prometryne-contaminated marine ecosystem bioremediations (Jin et al., 2012).
6.3 Genetic engineering for seafood to avoid speciation of PCBs and OCPs
Various approaches have been introduced to speed up environmental dominance in engineering microbial strains cloning and transmission of specific microbial genes that can effectively degrade the toxic compounds. A bacterial strain was engineered for degrading the pesticide mixture organochlorine (OCs) and organophosphate (OPs) by over-expressing the OC (linA) and the OP degradation gene (mpd) of E. coli (Yang et al., 2012). In addition to the similar genes over-expression, protoplast fusion is often an optional tool for the engineering of microbial cultures to control contamination (Dillon et al., 2008) necessary enzymes abundance of extraordinary catalytic potential achieve robust and more environmentally safe degradation. Degradation of pollutants by the fusion of protoplast is a successful strategy. The genetically modified microbial cultures can significantly expand PCBs exclusion and mitigation. Catalytically healthy and genetically engineered microbial cultures will have a long-term effect on the aquatic environment and the entire living planet. Multiple bacterial cultures such as Ralstonia, Sphingomonas, Alcaligenes, Comamonas, Rhodococcus, Burkholderia, Pseudomonas, Dehalococcoides, Dehalococcoides, and Achromobacter, may change contaminants into non or less harmful compounds and thus reduce or remove aquatic pollutants (Lloyd et al., 2003). One research implies that microbiologically induced bioremediation allows the catalytic enzymes to release, which encourages the deprivation of harmful toxins (Dangi et al., 2019). Another study suggests that using the Aroclor 1242 sequential anaerobic, aerobic treatment in sediments of the river. The sediments sludge were then incubated under anaerobic conditions to produce the lower chlorinated congeners. The sediment was subsequently treated aerobically after inoculation with genetically modified adapted bacteria, strain RHA1 (ffc) and Burkholderia xenovorans strain LB400, capable of developing 2-CBp and 4-chlorobenzoate, respectively (Mackova et al., 2010). The bacterial strains reduced the lower residual chlorine significantly, or chlorines, left during the anaerobic process by 57%. It means the anaerobic step eliminated much of the chlorines, although the lower amounts of chlorines persisted. The degradation of Aroclor 1242 was also examined in a granular reactor with minimal aeration supplying both aerobic and anaerobic conditions concurrently (Tartakovsky et al., 2001). It was observed that PCBs elimination with the present process from the effluent and biomass was strong at over 19% of PCBs. The specific pace of PCBs removal (i.e., PCBs/VSS) was 1.43 mg PCBs/(g VSS × day). Like several other molecules, PCBs (a class of organic chemicals) may be difficult to degrade. But to counteract this, species in the environment that are capable of degrading PCBs have been collected. These organisms have been inserted with pieces of genetic material that cause them to degrade PCBs. Not only is that, but the rate of PCBs degradation slow. But this ability of PCBs to resist degradation is not the only thing that PCBs are resistant too. Because of PCBs' highly environmentally isolating ability, the naturally occurring organisms cannot degrade the PCBs. That is why some scientists genetically engineered these organisms so that they could degrade PCBs (Pieper, 2005). It is well established that the advent of extremely useful degradation strains through genetic modification has vastly increased PCBs' degradation performance. The enzymes are known as Biphenyl 2,3-dioxygenase (BDO), which were encoded by gene bphA used in PCBs biodegradation (Solyanikova et al., 2015). A hybrid pseudomonas pseudoalcaligenes KF707-D34 containing a mutated gene bph constructed by homologous recombination of biphenyl dioxygenase encoding genes Burkholderia cepacia LB400 and P. pseudoalcaligenes KF707-D34 is contributing to an improved capability of PCBs degradation (Furukawa, 2000). These bph genes inject into the genome of Pseudomonas sp. B13FR1 was exposed to a rise in multi-delete mutations, B13FR2 was under genetic transformation by the bacterium, and so on. A recombinant strain B13FR1 was formed after adding the bph gene in the genome of Pseudomonas sp. having a higher biological bio-degradation potential than that of the parent strain (MENN et al., 1999).
The intermediate product used in the aerobic metabolism of PCBs molecule a natural toxic organic pollutant named chlorobenzene acid (Adebusoye, 2017). An essential enzyme Dehalogenase (4-chlorobenzoate-4-hydroxylase) encoded by fcbA gene is the significant enzyme for chlorobenzoate biodegradation. An overexpression of 4-chlorobenzoate-4-hydroxylase from Arthrobacter globiformis KZT1 in E. coli. The recombinant has increased efficiency for degradation of 4-chlorobenzene acid in KZT1 (Tsoi et al., 1991). Dehalogenase overexpression created an engineered strain Pseudomonas putida PaW340/pDH5 with improved metabolic function, which may have increased in 4-chlorobenzene acid degradation (Massa et al., 2009).
7 Conclusion
In brief, the planet is polluted at varying degrees by numerous POPs like conventional POPs and emerging POPs. The amounts of POPs (PCBs and OCPs) between various aquatic habitats vary greatly. Contamination levels of POPs (PCBs and OCPs) are typically higher in industrialized regions worldwide than in less developed regions. Certain POPs (PCBs and OCPs) levels across the globe, certain POPs may be smaller in some areas, whereas some may be equivalent or higher. Aquatic ecosystems are directly connected to human life, but our knowledge of POPs contamination degradation is still not adequate. Several experiments have been conducted on PCBs and OCPs from the various aquatic ecosystems in multiple matrices. These previous studies have typically concentrated on certain contaminants or certain matrices in specific conventional POPs. To better understand aquatic ecology, rigorous analysis involving various contaminants and matrices and risk analyses is also needed.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2020YFD0901002) and Shenzhen science and technology application demonstration project (KJYY20180201180253571). KAK appreciates the support of the Research Center for Advanced Materials Science (RCAMS) at King Khalid UniversityAbha, Saudi Arabia through a grant RCAMS/ KKU/002-21.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biological degradation of 4-chlorobenzoic acid by a PCB-metabolizing bacterium through a pathway not involving (chloro) catechol. Biodegradation. 2017;28(1):37-51.
- [Google Scholar]
- Accumulation of PCBs and infections of parasitic helminthes in Synodontis filamentosus (Boulenger, 1901) and Tilapia zillii (Gervais, 1848) of Epe Lagoon, Lagos, Nigeria. Egypt. J. Aquat. Biol. Fisher.. 2020;24(1):49-63.
- [Google Scholar]
- Effect of Mucor alternans on the persistence of DDT and dieldrin in culture and in soil. J. Econ. Entomol.. 1970;63:1595-1599.
- [Google Scholar]
- Annex, C. (2008). of the Stockholm Convention on Persistent Organic Pollutants. United Nations Environmental Programme, Geneva 29.
- In situ passive sampling of sediments in the Lower Duwamish Waterway Superfund site: Replicability, comparison with ex situ measurements, and use of data. Environ. Pollut.. 2016;218:95-101.
- [Google Scholar]
- Accumulation of organochlorine and pyrethroid pesticide residues in fish, water, and sediments in the Thamirabarani river system of southern peninsular India. Environ. Nanotechnol. Monit. Manage.. 2019;11:100194.
- [CrossRef] [Google Scholar]
- An evaluation of the levels of organochlorine compounds (OCPs and PCBs) in cultured freshwater and wild sea fish eggs as an exposure biomarker for environmental contamination. Environ. Sci. Pollut. Res.. 2019;26(7):7005-7012.
- [Google Scholar]
- Dechlorination of 2, 4, 4′-trichlorobiphenyl by magnetoferritin with different loading factors. Chemosphere. 2020;260:127629.
- [CrossRef] [Google Scholar]
- Pesticides exposure as etiological factors of Parkinson's disease and other neurodegenerative diseases—a mechanistic approach. Toxicol. Lett.. 2014;230(2):85-103.
- [Google Scholar]
- A case study for microbial biodegradation: anaerobic bacterial reductive dechlorination of polychlorinated biphenyls—from sediment to defined medium. Annu. Rev. Microbiol.. 2008;62(1):253-270.
- [Google Scholar]
- Persistent organic pollutants in the East Antarctic atmosphere: inter-annual observations from 2010 to 2015 using high-flow-through passive sampling. Environ. Sci. Technol.. 2017;51(23):13929-13937.
- [Google Scholar]
- New insights into the degradation of synthetic pollutants in contaminated environments. Chemosphere. 2020;268:128827.
- [CrossRef] [Google Scholar]
- Historical records of organic pollutants in sediment cores. Mar. Pollut. Bull.. 2014;78(1-2):26-42.
- [Google Scholar]
- On developing bioindicators for human and ecological health. Environ. Monit. Assess.. 2001;66:23-46.
- [Google Scholar]
- Bioaccumulation of dioxin-like PCBs and PBDEs by detritus-feeding fish in the Rio de la Plata estuary, Argentina. Environ. Sci. Pollut. Res.. 2015;22(9):7093-7100.
- [Google Scholar]
- Aquatic risk assessment of pesticides in Latin America. Integr. Environ. Assess. Manage.. 2014;10(4):539-542.
- [Google Scholar]
- Levels of PCBs in Oysters Coming from Galicia Coast: Comparison to Mussels from the Same Region. Bull Environ. Contaminat. Toxicol.. 2016;96(5):608-615.
- [Google Scholar]
- Polybrominated diphenyl ethers and metabolites–an analytical review on seafood occurrence. TrAC, Trends Anal. Chem.. 2017;87:129-144.
- [Google Scholar]
- Bioremediation through microbes: systems biology and metabolic engineering approach. Crit. Rev. Biotechnol.. 2019;39(1):79-98.
- [Google Scholar]
- de Castilhos Ghisi, N., Larentis, C., de Oliveira, E. C., Neves, M. P., Zavaski, A. G., de Almeida Roque, A., Wachtel, C. C., da Silva, A. P., de Lima, E. B. S., and Costa, G. d. O. N. (2020). Environmental assessment of Neotropical streams using fish as bioindicators: a multibiomarker and integrated approach. Hydrobiologia, 1-18.
- Hepatic metabolomics reveals that liver injury increases PCB 126-induced oxidative stress and metabolic dysfunction. Chemosphere. 2019;217:140-149.
- [Google Scholar]
- Spatial and temporal trends in PCBs in sediment along the lower Rhône River, France. Sci. Total Environ.. 2012;433:189-197.
- [Google Scholar]
- Generation of recombinants strains to cellulases production by protoplast fusion between Penicillium echinulatum and Trichoderma harzianum. Enzyme Microb. Technol.. 2008;43(6):403-409.
- [Google Scholar]
- Fungicides and herbicide removal in Scenedesmus cell suspensions. Chemosphere. 2010;79(2):117-123.
- [Google Scholar]
- Exploring the distribution patterns of macroinvertebrate signature traits and ecological preferences and their responses to urban and agricultural pollution in selected rivers in the Niger Delta ecoregion, Nigeria. Aquatic Ecol.. 2020;54(2):553-573.
- [Google Scholar]
- Enhanced biodegradation of polychlorinated biphenyls after site-directed mutagenesis of a biphenyl dioxygenase gene. Appl. Environ. Microbiol.. 1993;59(11):3858-3862.
- [Google Scholar]
- Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res.. 2011;18(2):135-151.
- [Google Scholar]
- Atlantic salmon fed a nutrient package of surplus methionine, vitamin B12, folic acid and vitamin B6 improved growth and reduced the relative liver size, but when in excess growth reduced. Aquac. Nutr.. 2020;26(2):477-489.
- [Google Scholar]
- Bioaugmentation of a historically contaminated soil by polychlorinated biphenyls with Lentinus tigrinus. Microb. Cell Fact.. 2012;11(1):35.
- [CrossRef] [Google Scholar]
- Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ. Sci. Technol.. 2004;38(7):2075-2081.
- [Google Scholar]
- Use of three bivalve species for biomonitoring a polluted estuarine environment. Environ. Monit. Assess.. 2011;177(1-4):289-300.
- [Google Scholar]
- Biochemical and genetic bases of microbial degradation of polychlorinated biphenyls (PCBs) J. Gener. Appl. Microbiol.. 2000;46(6):283-296.
- [Google Scholar]
- Ganaie, H. A. (2020). Threats and Risks of Contamination Load on Different Biota. In “Bioremediation and Biotechnology, Vol 4”, pp. 107-124. Springer.
- Insights into the drivers of histopathological changes and potential as bio-indicator of riverine health of an aquatic apex predator from a premier conservation area: a multiple lines of evidence and multivariate statistics approach. Ecol. Ind.. 2017;72:530-544.
- [Google Scholar]
- Screening and metabolic potential of fungal strains isolated from contaminated soil and sediment in the polychlorinated biphenyl degradation. Ecotoxicol. Environ. Saf.. 2020;208:111703
- [Google Scholar]
- Trophic transfer of micropollutants and their metabolites in an urban riverine food web. Environ. Sci. Technol.. 2020;54(13):8043-8050.
- [Google Scholar]
- Distribution of persistent organic pollutants (POPs) in cultured mussels from the Croatian coast of the Adriatic Sea. Chemosphere. 2014;114:69-75.
- [Google Scholar]
- Steroid hormones and persistent organic pollutants in plasma from North-eastern Atlantic pilot whales. Environ. Res.. 2017;159:613-621.
- [Google Scholar]
- First polychlorinated biphenyls (PCBs) monitoring in seawater, surface sediments and marine fish communities of the Persian Gulf: Distribution, levels, congener profile and health risk assessment. Environ. Pollut.. 2019;253:78-88.
- [Google Scholar]
- Bioaccumulation and catabolism of prometryne in green algae. Chemosphere. 2012;87(3):278-284.
- [Google Scholar]
- Concentration of organochlorine in egg yolk and reproductive success of Egretta garzetta (Linnaeus, 1758) at Wat Tan-en non-hunting area, Phra Nakhorn Si Ayuthaya Province, Thailand. Ecotoxicol. Environ. Saf.. 2007;68(1):79-83.
- [Google Scholar]
- Biomonitoring of organochlorine compounds and trace metals along the Eastern Adriatic coast (Croatia) using Mytilus galloprovincialis. Mar. Pollut. Bull.. 2010;60(10):1879-1889.
- [Google Scholar]
- The pattern of organochlorines in mussels Mytilus edulis L. from the south west Baltic Sea. Arch. Environ. Contam. Toxicol.. 1996;31(1):68-76.
- [Google Scholar]
- Health risk characterization of maximum legal exposures for persistent organic pollutant (POP) pesticides in residential soil: An analysis. J. Environ. Manage.. 2018;205:163-173.
- [Google Scholar]
- Biotechnological application of metal-reducing microorganisms. Adv. Appl. Microbiol.. 2003;53:85-128.
- [Google Scholar]
- Is PCBs concentration variability between and within freshwater fish species explained by their contamination pathways? Chemosphere. 2011;85(3):502-508.
- [Google Scholar]
- Accumulation and fate processes of organochlorine pesticides (OCPs) in soil profiles in Mt. Shergyla, Tibetan Plateau: A comparison on different forest types. Chemosphere. 2019;231:571-578.
- [Google Scholar]
- Mackova, M., Uhlik, O., Lovecka, P., Viktorova, J., Novakova, M., Demnerova, K., Sylvestre, M., and Macek, T. (2010). Bacterial degradation of polychlorinated biphenyls. In “Geomicrobiology: molecular and environmental perspective”, pp. 347-366. Springer.
- Organochlorine pesticides, PCBs, and PBDEs in liver and muscle tissues of Paralonchurus brasiliensis, Trichiurus lepturus and Cathorops spixii in Santos Bay and surrounding area, São Paulo, Brazil. Regl. Stud. Mar. Sci.. 2017;16:42-48.
- [Google Scholar]
- Lethal and sublethal endpoints observed for Artemia exposed to two reference toxicants and an ecotoxicological concern organic compound. Ecotoxicol. Environ. Saf.. 2016;123:60-64.
- [Google Scholar]
- Efficiency of natural and engineered bacterial strains in the degradation of 4-chlorobenzoic acid in soil slurry. Int. Biodeterior. Biodegrad.. 2009;63(1):112-115.
- [Google Scholar]
- Bioremediation of the organochlorine pesticides, dieldrin and endrin, and their occurrence in the environment. Appl. Microbiol. Biotechnol.. 2009;84(2):205-216.
- [Google Scholar]
- MENN, F., Easter, J. P., Sayler, G. S. (1999). 21 Genetically Engineered Microorganisms and Bioremediation. Knoxville, TN, 37996-1605.
- Polychlorinated biphenyls, organochlorine pesticides and trace metals in cultured and harvested bivalves from the eastern Adriatic coast (Croatia) Chemosphere. 2016;153:18-27.
- [Google Scholar]
- Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74-88.
- [Google Scholar]
- Determination of organochlorine pesticides in the surface water from Medjerda river, Tunisia. Int. J. Environ. Anal. Chem. 2020:1-12.
- [Google Scholar]
- Toxicity and removal of pesticides by selected aquatic plants. Chemosphere. 2008;70(8):1414-1421.
- [Google Scholar]
- Distribution of organochlorine pesticides in fresh fish carcasses from selected estuaries in Eastern Cape Province, South Africa, and the associated health risk assessment. Mar. Pollut. Bull.. 2019;149:110605.
- [CrossRef] [Google Scholar]
- The legacy of organochlorine pesticide usage in a tropical semi-arid region (Jaguaribe River, Ceará, Brazil): Implications of the influence of sediment parameters on occurrence, distribution and fate. Sci. Total Environ.. 2016;542:254-263.
- [Google Scholar]
- Monitoring exposure to heavy metals among children in Lake Victoria, Kenya: environmental and fish matrix. Ecotoxicol. Environ. Saf.. 2010;73(7):1797-1803.
- [Google Scholar]
- Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): state of knowledge and research perspectives. J. Hazard. Mater.. 2014;278:189-202.
- [Google Scholar]
- Aerobic degradation of polychlorinated biphenyls. Appl. Microbiol. Biotechnol.. 2005;67(2):170-191.
- [Google Scholar]
- Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem. Eng. J.. 2019;358:1186-1207.
- [Google Scholar]
- Hydroxy-PCBs, methoxy-PCBs and hydroxy-methoxy-PCBs: metabolites of polychlorinated biphenyls formed in vitro by tobacco cells. Environ. Sci. Technol.. 2008;42(15):5746-5751.
- [Google Scholar]
- Rouhani, Q., Britz, P. J. (2011). “Participatory Development of Provincial Aquaculture Programmes for Improved Rural Food Security and Livelihood Alternatives: Report to the Water Research Commission and Department of Agriculture, Forestry and Fisheries,” Water Research Commission.
- Polybrominated diphenyl ethers (PBDEs) and indicator polychlorinated biphenyls (PCBs) in various marine fish from Zhoushan fishery, China. Food Control. 2016;67:240-246.
- [Google Scholar]
- Hydrocarbons, PCBs and DDT in the NW Mediterranean deep-sea fish Mora moro. Deep Sea Res. Part I. 2001;48(2):495-513.
- [Google Scholar]
- Peculiarities of the degradation of benzoate and its chloro-and hydroxy-substituted analogs by actinobacteria. Int. Biodeterior. Biodegrad.. 2015;100:155-164.
- [Google Scholar]
- Organochlorine compounds in mussels cultured in the Ría of Vigo: accumulation and origin. Chemosphere. 2013;90(1):7-19.
- [Google Scholar]
- Degradation of Aroclor 1242 in a single-stage coupled anaerobic/aerobic bioreactor. Water Res.. 2001;35(18):4323-4330.
- [Google Scholar]
- Cloning and expression of the Arthrobacter globiformis KZT1 fcbA gene encoding dehalogenase (4-chlorobenzoate-4-hydroxylase) in Escherichia coli. FEMS Microbiol. Lett.. 1991;81(2):165-169.
- [Google Scholar]
- Bioindicators of organochlorine pesticides in the Sea of Okhotsk and the Western Bering Sea. Arch. Environ. Contam. Toxicol.. 2017;73(2):176-184.
- [Google Scholar]
- A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquacult.. 2020;12(1):3-25.
- [Google Scholar]
- Persistent organic pollutants in the Olifants River Basin, South Africa: Bioaccumulation and trophic transfer through a subtropical aquatic food web. Sci. Total Environ.. 2017;586:792-806.
- [Google Scholar]
- A Bayesian assessment of the mercury and PCB temporal trends in lake trout (Salvelinus namaycush) and walleye (Sander vitreus) from lake Ontario, Ontario, Canada. Ecotoxicol. Environ. Saf.. 2015;117:174-186.
- [Google Scholar]
- Analysis of polychlorinated biphenyls, polycyclic aromatic hydrocarbons and organochlorine pesticides in the tissues of green sea turtles, Chelonia mydas,(Linnaeus, 1758) from the eastern coast of the United Arab Emirates. Mar. Pollut. Bull.. 2020;160:111574.
- [CrossRef] [Google Scholar]
- Construction of a genetically engineered microorganism that simultaneously degrades organochlorine and organophosphate pesticides. Appl. Biochem. Biotechnol.. 2012;166(3):590-598.
- [Google Scholar]
- An offshore biomonitoring system for chlorinated hydrocarbons. Mar. Pollut. Bull.. 1976;7(8):156-159.
- [Google Scholar]
- Bioaccumulation and health risk assessment of heavy metals to bivalve species in Daya Bay (South China Sea): Consumption advisory. Mar. Pollut. Bull.. 2020;150:110717.
- [CrossRef] [Google Scholar]







