Health and environmental effects of heavy metals
⁎Corresponding authors. sfli@szu.edu.cn (Shuangfei Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Seafood safety is a critical requirement for sustained global quantitative and qualitative development. In recent years, unintended poisons have damaged human health and food quality. Heavy metals (HMs) distribution, speciation, bioaccumulation, and toxicity evaluation in aquatic settings are at their peak. Safe ecosystems have a significant influence in the possible composition of safe aquaculture products, which serve as the foundation of every food web. HMs eventually impose a number of stresses on the living organisms, contributing to increased mortality. Therefore, this study reflects and explains the exposure of heavy metals to aquatic food as well as the resulting health risks to humans. A more in-depth review on the translocation processes of metal toxins into seafood is provided. Finally, for achieving stability in aquatic environments, management techniques, genetic engineering, and remediation are recommended.
Keywords
Aquatic
Bioaccumulation
Bioindicators
Ecosystem food chain remediation
1 Introduction
Water is a basic need for all living forms on the planet. Clean water is essential for living a healthy life since polluted water can pose citizen’s health at risk through direct or indirect contact with dangerous chemicals (Sajid et al., 2018). Environmental contamination has been exacerbated by industrial revolution and anthropological activities. Significant pollutant discharges into the ocean have resulted in huge hazards to the coastal environments. Because of their chronic toxicity, non-biodegradability, and environmental bioaccumulation, heavy metals (HMs) are incredibly harmful environmental pollutants (Valdés et al., 2014). Heavy metals can be transferred and biomagnified via food chains and seriously threaten human health (Liu et al., 2018; Mansour and Sidky, 2002). Effective monitoring and surveillance of heavy metal concentrations in the marine environment is also highly sought (Ahmed et al., 2015). At the local, regional, and national levels, problems are now being raised because of the HMs concentration and their effects, distribution, and environmental origin (Kumar et al., 2019). The bioaccumulation patterns of HMs, such as mercury (Hg), arsenic (As), Nickel (Ni), cobalt (Co), copper (CU), cadmium (Cd), and chromium (Cr), have a significant influence on the lives of most organism (Rahman and Singh, 2019). Heavy metals from different distribution sources have a negative influence on marine biota (Kahlon et al., 2018).
These HMs have an impact on beneficial organisms such as fishes and other invertebrates (Morkunas et al., 2018). Heavy metals from the surrounding water and foodstuffs accumulate in marine species (Hao et al., 2019). In certain cases, excessive levels of heavy metals in marine ecosystems are directly related to environmental contamination. According to several research studies, the concentration of heavy metal bioaccumulation differed substantially amongst marine species. Variations in heavy metal accumulation of aquatic organisms are possibly related to their different living environments, feeding patterns, and trophic levels (Liu et al., 2018; Rajeshkumar et al., 2018).
The purpose of this review is to give insight into the overall geographical pattern of heavy metal outlets in the aquatic ecosystem as well as human sources. It also discusses heavy metal pollution in marine food components. Furthermore, the effects of such components on the environment and human life are thoroughly discussed in order to explain the physiological/molecular processes involved in the use of metallic toxins in aquatic foods. Finally, the review examines remedil techniques (e.g., ecosystem remediation and the application of genetic engineering). These management strategies are intimately linked to human population safety by eliminating or mitigating the transfer of HMs pollutants from the aquatic environment to the food chain.
2 Source of heavy metals
Heavy metals (HMs) are elements with larger density and higher atomic mass that can affect individuals and the environment, such as cadmium (Cd), zinc (Zn), mercury (Hg), arsenic (As), silver (Ag), chromium (Cr), copper (Cu), iron (Fe), and platinum (Pt). Heavy metal contamination of water is one of the most serious environmental concerns affecting plants, animals, and humans (Gu et al., 2018; Wang et al., 2020). Heavy metals are hazardous even in low concentrations because they are not biodegradable (Brodin et al., 2017; Ferrey et al., 2018).
Metals and metalloid ions re classified into three groups. The first group includes metals such as mercury, cadmium, and lead, which are toxic at minimum concentrations. The second group of metals is less dangerous (bismuth, indium, arsenic, thallium, and antimoney), and the third category includes essential metals such as zinc, cobalt, copper, iron, and selenium, which are part of several chemical or biochemical processes in the body and are only toxic above a certain concentration (Odobašić et al., 2019). HMs accumulate in the soil, human and animal tissues as a result of absorption and, in certain cases, inhalation, and as well as accidents or mishandling. Metals have been present on the planet since the origin through regular biogeochemical cycles (Dalziel, 1999; Masindi and Muedi, 2018). The underlying weathering mechanism resulted in the occurrence of HMs in the soil. Because of mineralized veins and metal deposits in high concentrations in the bedrock, the soil in the Mendip region (Great Britain) is rich in cadmium, lead, and zinc. Metal enrichment during soil formation can occur as a result of bedrock weathering with a slightly high concentration of HMs.
The major reasons of increased environmental toxicity owing to heavy metals are human and anthropogenic factors. Natural sources of HMs include wind-blown soil debris, forest fires, volcanic eruptions, biogenic processes, and marine salt (Blaser et al., 2000; Muhammad et al., 2011). Anthropogenic causes of HMs contamination include mining operations, pesticides, fertilizers, and herbicides use, crop field irrigation with industrial and sewage water (Sarkar et al., 2018; Srivastava et al., 2018) (Fig. 1). HMs trace levels in fertilizers are important sources of heavy metal contaminants in our food. Inappropriate industrial waste management, traffic pollution, use of lead (Pb) as fuel antiknock, aerosol cans, metallurgy and smelting, discharge of sewage and construction materials are the anthropogenic practices responsible for HMs contamination (Srivastava et al., 2016; Srivastava et al., 2017).
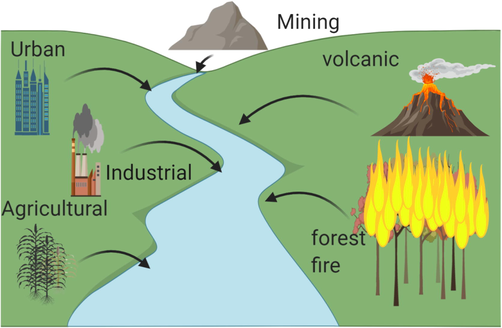
- Sources of Heavy metals.
Several industries, including drugs manufacturing, paper, and pulp preservatives, the farming sector, chlorine and caustic soda industry, release mercury (Hg) into the atmosphere (Ibrahim et al., 2019). Soils and rocks, including coal and mineral fertilizer, contain a certain amount of cadmium. Cadmium (Cd) is widely used in electroplating for a variety of applications, including batteries, pigments, textiles, and metal coatings (Saini and Dhania, 2020). All these practices are responsible factors for increased HMs contamination of the environment.
3 Heavy metals toxic effect
Heavy metal contamination is becoming a global issue. Heavy metals can enter fish through three routes: the gills, the body surface, and the digestive tract (Dane and Şi̇şman, 2020). Fish juveniles and larvae rise pretty fast and their growth in both body length and mass is closely related to suitable temperature and sufficient food supply, i.e. under optimal growth conditions (Krieger et al., 2020). On the other hand, fish development is hampered by toxic food loaded with heavy metals. One of the most obvious signs of metal toxicity in fish is growth inhibition. As a result, HMs concentrations in tissues cause a variety of metabolic, physiological, and histological changes in fish and other freshwater species by altering various enzymes and metabolites (Mehmood et al., 2019).
The feeding mechanism differs amongst fish species based on a variety of factors such as developmental agents, psychological agents, and fish lifespan. HMs accumulate in the tissues of Fish living in polluted environment (Kumar et al., 2020; Topal and Onac, 2020). Metal intensity, expression duration, metal absorption, environmental variables (temperature, pH, hardness, and salinity), and intrinsic agents, such as fish age and feeding activities are all factors in the selection of body organs for HMs deposition. Most metals accumulate mainly in the kidneys, gills, and liver (Kucukosmanoglu and Filazi, 2020; Squadrone et al., 2019). Zinc accumulates in fish gills disrupting the oxygen supply to tissues and causing hypoxia, which leads to death. However, if water pH falls, HMs may be mobilized and discharged into the water column, endangering marine organisms such as crustaceans and insects (Bonsignore et al., 2018). These toxic sediments kill the benthic organisms and reducing food availability for the gigantic organism. In modest levels, HMs found in the environment and food are necessary for optimal health, but in large amounts, they can be harmful or unhealthy. Their toxicity can deplete energy and affect the brain, lungs, kidneys, liver, blood, and other vital organs. Long-term exposure eventually results to degenerative physical, tissue, and neurological processes imitating diseases such as Alzheimer's, Parkinson's, muscle dystrophy, and multiple sclerosis. Acute lead (Pb) exposure can induce appetite loss, headaches, hypertension, stomach discomfort, renal dysfunction, fatigue, insomnia, arthritis, hallucinations, and vertigo. Mercury toxicity results in acrodynia or pink disease. Increased mercury exposure may affect the brain's structure and cause shyness, tremors, cognitive loss, irritability, and visual or hearing (Guzzi et al., 2020). Exposure to metallic mercury vapors at higher levels for a shorter length of time might result in lung damage, vomiting, diarrhea, nausea, skin rashes, and increased blood pressure. Organic mercury toxicity signs and symptoms include depression, memory problems, tremors, fatigue, headache, and hair loss. Because these signs and symptoms are frequently associated with other diseases, circumstances may be difficult to recognize (Atti et al., 2020).
4 Bioavailability of HMs in food webs
Heavy metals contamination of rivers, lakes, and streams causes bioaccumulation of toxic elements in fishes. HMs might enter fish through different routes, including dietary intakes and the incorporation of sediment particles (Liu et al., 2020). Many invertebrates are important food sources for fish and other aquatic species, and the provide a practical route for lead, copper, zinc, and cadmium absorption (Corrias et al., 2020; Jardine et al., 2020). Immediate water absorption another path of exposure to these toxic compounds (Maurya et al., 2019). Sediment, which is the primary trace element repository in marine settings, provides a third possible route (Luoma and Rainbow, 2008). The rate of element concentration between the fish and the abiotic (water and sediment) environments, also known as the Bioaccumulation Factor (BAF), was typically used to assess the pollution status of water bodies (Mortuza and Al-Misned, 2015; Ziyaadini et al., 2017). Significant elements and trace elements are transmitted from abiotic to live species in this environment and accumulated in biota, polluting the food chain (Ali and Khan, 2018) (Fig. 2).
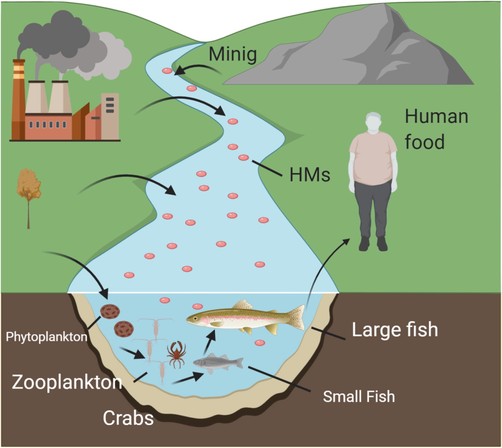
- Bioavailability of HMs in food webs.
Organisms at higher trophic levels in food chains are more vulnerable to biomagnification. Because of bioamplification, higher concentrations of trace elements in species with higher trophic levels can endanger these organisms or humans. The activity of water-living microorganisms convert atmospheric mercury into methyl, dimethyl, and ethyl mercury, which is subsequently ingested by smaller and larger animals (Bernhoft, 2012). More than 20 organic and inorganic chemical compounds containing arsenic (As) have been detected in aquatic bodies. The form of chemical compounds present in water is influenced by factors such as bacteria, phytoplankton, salinity, temperature, and redox conditions (Zhang et al., 2013). Marine microorganisms can transform one source of arsenic into a new one. Arsenic is incorporated into the marine food chain by depositing invertebrates (Casado-Martinez et al., 2010). These invertebrates are an important source of aquatic food for higher species, and fish consume arsenic particles when feeding on these invertebrates. According to the research findings, fish constitute the most significant source of arsenic exposure in humans (Juma et al., 2002). Lead is harmful to marine species (Nair et al., 2006), and fish is present at the top of the aquatic food chain, accumulating lead at a high rate in their gills and livers (Nair et al., 2006). People typically eat fish as part of their regular diet. Due to the fact that lead is transported into the circulation and incorporated into tissues after absorption (Castro-González and Méndez-Armenta, 2008), HMs accumulation in the body (Nair et al., 2006).
5 Bioaccumulation of HMs in seafood
The bioaccumulation of heavy metals in freshwater fish has important ecological, environmental, and social implications (Ali et al., 2017). When metals are present in high concentrations in the environment, species accumulate their higher amounts. Causing biomagnification of metals in the trophic web, which has a negative impact on the aquatic ecology since it relies on them in various ways, either directly or indirectly (Luoma and Rainbow, 2008). Increased pollution has resulted in a reduction in freshwater fishes and other aquatic organisms in the Indus river, Pakistan (Al-Ghanim et al., 2016).
Fish characteristics such as size, sex, reproductive cycle, feeding habits, and swimming patterns, as well as environmental factors such as HMs bioavailability and concentration in water columns, physicochemical properties of water, and other climatic factors all play an essential role in the bioaccumulation of HMs (Ali et al., 2019; Moiseenko and Gashkina, 2020). The degree of HMs deposition in various fish organs typically varies according to tissue shape and function. Metabolically active tissues, including kidneys, liver, or gills, generally accumulate more HMs than other tissues, such as the skin and muscles. Fish gills have been identified as the target tissue for the aggregation and disposal of HMs like nickel (Ni) (El-Moselhy et al., 2014; Mansouri et al., 2012). HMs bioaccumulate in muscles of fish in species-specific manner (Chakraborty et al., 2016). Toxic elements were determined in Indian anchovy (Stolephorus indus) collected from the Arabian Gulf, United Arab Emirates. Zinc was discovered in high quantities mostly in the muscles, and high levels of Cd, Cu, and Cr were found in muscle, and liver (Alizada et al., 2020)
Three fish species, Labeo rohita, Pangasius hypophthalmus, and Katsuwonus pelamis were taken from Visakhapatnam, and their eyes, gills, stomach, gonads, liver, and muscles were studied. The metal concentrations in small and large L. rohita and P. hypophthalmus were in the order Fe > Zn > Cu, whereas Co, Hg, and Pb were below the detectable limit (BDL). HMs concentrations in K. pelamis were as follows: Fe > Zn > Cu > Cd, whereas Pb, Hg, and Co were BDL (Pragnya et al., 2020).
As filter feeders of the aquatic environment, bivalves constitute an essential component of the human diet and play an important part in the biogeochemical cycle. They can accumulate HMs by feeding in seawater and then act as prey for other marine bodies at higher trophic levels (Kodama et al., 2012; Yuan et al., 2020). Pollutants in the water column, suspended particulate matter, sediments, and even food sources can be picked up by bivalve molluscs. The bioaccumulation rate of metals in bivalves depends on biotic factors (e.g., organisms, age, sex, weight, gametogenesis, and physiological status) and abiotic factors (e.g., chemical species, pH, salinity, temperature, filtration rate, availability of environmental contaminants). Bivalves possessed a high capacity for bioaccumulation of HMs (Yuan et al., 2020). Consumption of edible bivalves is detrimental to human health. Crabs of the Ocypodid family are depositary feeders known to be important prey and inseparable food for many mammals and water birds. They contribute significantly to particle size reduction, organic mineralization, and sediment purification (Gouws and Stewart, 2001; Hewitt, 2004). Studies have shown that the bioaccumulation of heavy metals, such as Zn, Cu, Cd, and Pb, occurred in the aquatic organisms in coastal regions of Tuticorin. According to (Yogeshwaran et al., 2020), crabs in contaminated habitats are significant bio accumulators of heavy metals. The research was performed to explore the accumulation and biomonitoring capacity of HMs for Fe, Cu, Zn, Cr, Ni, Co, Pb, and Cd in Macrophthalmus depressus from Karachi, Pakistan (Saher and Siddiqui, 2019).
6 Recommendations
6.1 Biological indicators as a warning system of HMs
Bioindicators are organisms whose physiological features, absence, or appearance indicate the quality of the environment in which they live (Arimoro and Keke, 2017; Sures, 2003). They can be either impact indicators or accumulation indicators. Effect Bioindicators reflect changes in metabolism, substances, roles, or the number of species. Their presence, absence, and appearance indicate environmental quality (Arimoro and Keke, 2017; Sures, 2003). In contrast, accumulation bioindicators (sentinels) may successfully collect elements in the environment at concentrations considerably higher than those present in the environment without harmful consequences (Sures, 2003; Tellez and Merchant, 2015). Historically, free-living biotas such as fish, macroinvertebrates, and plankton have been used as bioindicators in water quality studies (Keke et al., 2020). The use of fish parasites (acanthocephalans, cestodes, and nematodes) as crucial biomonitoring instruments functioning as bioindicators of trace elements environmental pollution has been effectively proven in research. Host ingested food pollutants directly affect the intestinal parasites; they may respond to the contamination by accumulating such contaminants (Sures et al., 1995). Fish parasites have been proven to collect considerably more contaminants than their host species. Acanthocephalans are highly bioaccumulative, in particular, due to their lack of a digestive system, which enables them to absorb nutrients from the predigested system via diffusion from intestinal fish content. In addition, both the location and growth of the parasite in fish may play a significant role in the process of bioaccumulation (Nachev and Sures, 2016). Acanthocephalans are incredibly important in verifying and quantifying toxic substances in aquatic ecosystems due to their rapid response to chemical exposure and accumulation of high levels, particularly with trace elements like cadmium and lead, which have a significant toxic effect in these environments. Intestinal helminthic parasites may be an ideal remedy to heavy metal impact and accumulation bioindication (Keke et al., 2020). Parasites, primarily intestinal trematodes, collectively accumulated higher Se, Cu, As, and Zn levels and served as sensitive bioindicators for heavy metals contamination (Gilbert and Avenant-Oldewage, 2017). Bamidele and Kuton utilized Parachanna obscura and Clarias gariepinus fish as markers of heavy metal contamination, such as Cu, Cr, Ni, Pb, and Fe in fish tissues and parasites as indications of heavy metal bioaccumulation in Lekki lagoon, Nigeria, in 2016. (Bamidele and Kuton, 2016).
In recent years several biosensor fishes have been used to monitor aquatic toxins. Numerous genetic modifications have produced these transgenic fishes. In living fish, numerous promoters, including cyp1a, cyp19a1b, and mt, activate the fluorescent protein reporter gene in response to hazardous chemical exposure (Ng and Gong, 2013). Several transgenic reporter lines were established in zebrafish (Danio rerio) and Japanese medaka (Oryzias latipes) for the identification of contaminants (Pawar et al., 2016; Zhou et al., 2020). Because of the ease with which genes may be manipulated, the short maturation time, transparency, and controlled ovulation, zebrafish and medaka have become attractive model fish for detecting toxins. The use of model fish or embryonic transgenic lines carrying an easily detectable reporter gene whose expression is controlled by a pollutant-deficient element such as heavy metals (Seok et al., 2007). Many fishes have characterized the metallothionein promoter to identify heavy-metal pollution (copper, cadmium, mercury, and zinc). Medaka is used for monitoring reproductive events through GFP-linked estrogenic vitellogenin (vtg) gene promoter. Zhou et al. (2020) created lines for the first time using an upgraded cyp1a-12 DRE promoter that particularly supports the usage of Enhanced Green Fluorescent Protein (EGFP) in marine transgenic plants (Zhou et al., 2020). Several researchers worldwide have established transgenic zebrafish lines expressing fluorescent proteins under the control of promoter elements such as estrogen, aryl hydrocarbon, glycoprotein hormone, heat-shock protein (HSP), DNA damage and tissue-specific promoters and response elements for monitoring the aquatic pollution. Most transgenic zebrafish biosensors developed to date for detecting heavy metals have been based on hsp promoter elements activated by many other stressors (Blechinger et al., 2002).
Recently, Liu et al. (2016) have produced transgenic zebrafish mt:egfps as a biosensor using a zebrafish MT promoter responsive to zinc and cadmium (Liu et al., 2016). A transgenic zebrafish has been reported to respond to heavy metals by employing a metal-response promoter with a fluorescent reporter (DsRed2) gene (Pawar et al., 2016). Perna viridis, an Asian green mold, provided the MT-Ia1 metallothionein promoter containing metallic-responsive components. Tg(cyp1a-12DRE:EGFP) is a transgenic strain with a cyp1a zebrafish promoter recombined with multiple DREs dioxin-responsive elements to induce EGFP expression (Pawar et al., 2016).
Because of their intimate interaction with sediments, benthic crustaceans are sensitive to contaminants. They lack a sophisticated metabolic system and accumulate HMs in their bodies. As a result, utilizing these benthic crustaceans, the bioavailability of poisons in sediments may be measured and assessed (Baki et al., 2018; Cheng et al., 2017). Barytelphusa cunicularis and Spiralothelphusa hydrodroma are essential freshwater crabs in various parts of India, including Tamil Nadu (Cumberlidge, 2014; Pati et al., 2014). Because of their widespread distribution and high nutritional content, these crab species have a high market value and are popular among locals. Furthermore, B. cunicularis and S. hydrodroma were utilized as powerful biological markers for a variety of environmental contaminants, including heavy metals (Gayathri et al., 2020). This research was carried out to investigate HMs build-up in different organs of sentinel crab Macrophthalmus depressus and its ability for sediment bio-monitoring of heavy metals (Hg, Cd, Ni).Investigate Macrophthalmus depressus’s potential as a heavy metal pollution indicator in the various coastal areas of Karachi. In addition, possible associations between HMs concentrations in crab and environmental endpoints such as organic matter, grain size, sediment, pH, salinity, temperature, and metal sediment concentrations have also been evaluated to determine control factors for crab metal accumulation in the marine ecosystem (Saher and Siddiqui, 2019).
6.2 Remediations for HMs
Microbial biotechnology has emerged as an environmentally friendly and essential alternative for HM bioremediation in recent years. Heavy metal-tolerant bacterial species can be used for heavy metal bioremediation (Nanda et al., 2019; Ojuederie and Babalola, 2017). Various scientists have identified numerous putative heavy metal tolerance mechanisms, including redox reactions, pumped, compound building with other components, and extracellular and intracellular sequestration. Isolated Pseudomonas sp. Streptococcus sp., and Staphylococcus sp. strains from pulp and paper industry effluent for heavy metal bioremediation. They tested their ability to extract heavy metals and found that Pseudomonas sp. efficiently extracts cadmium, manganese, and mercury. In comparison, Streptococcus sp. and Staphylococcus sp. might extract Cu more easily (Hakeem and Smita, 2010). Gram-positive bacteria accumulate heavy metals in their cell walls more actively than Gram-negative bacteria (Rani and Goel, 2009). Bacteria can absorb and accumulate various metal ions, resulting in transferring metals into a polluted biomass matrix (Smith et al., 1994). Due to the negative sites on bacterial cell walls, wastewater cadmium cations biosorption occurred when actinomycetes dead biomass suspension from industrial fermentation was mixed (Butter et al., 1995). Kang et al. (2016) proposed using a bacterial consortium more effectively instead of single bacterial organisms for water bioremediation of HMs (Kang et al., 2016). They also eliminated various metal toxins utilizing the bacterial consortium and registered a reduction of 98.3% lead, 85.40% Cadmium, and 5.6% Copper. Streptomyces sp, Bacillus firmus, Oscillatoria anguistissima, Chlorella fusca, Sargassum natans, Ascophyllum nodosum, Rhizopus nigricans, Penicillium chrysogenum, and Aspergillus niger biomass have the highest potential for metal adsorption from 5 to 641 mgg−1 for Ni, Cu, Cr, Cd, Zn, and Pb metals. Previously, fungi were examined as bioremediation agents for water pollutants. The strong metabolic ability of fungi make them better microorganisms for growth and production in acidic conditions and radionuclide exposure (Deshmukh et al., 2016). The fungal cell surface has chitin and chitosan, known to be outstanding heavy metal ion biosorbents. The fungi Fusarium sp, Saccharomyces sp., Mucor spp., Rhizopus spp., Aspergillus spp., and Penicillium spp are excellent metal ion biosorbents (Cárdenas González et al., 2019). Saccharomyces sp., Rhizopus sp. and Penicillium sp. biomass can biosorb As, Cr, Pb, Zn, and Ni (Bano et al., 2018). The promising treatment of metal-contaminated sites could be suggested for Penicillium piscarium. Coelho et al. (2020) examined the dead biomass of P. piscarium in metal biosorption (Coelho et al., 2020). The findings were remarkable and showed that the dead biomass of P. piscarium might be an essential answer to traditional water treatment systems polluted with heavy metals. This eco-friendly, cost-effective, and reliable wastewater management technology can be promoted from industrial activities. The performance of Aspergillus sp. was also stated by Srivastava and Thakur (2006) for chromium reduction of tannery wastewater (Srivastava and Thakur, 2006). Algae are autotrophic and thus need low nutrients and generate large biomass compared with other microbial biosorbents. These biosorbents were often used for the removal of heavy metal with strong sorption potential (Cardoso et al., 2017). Algae biomass is used for the bioremediation of contaminated heavy metal effluent through adsorption or cell incorporation. Phycoremediation uses reduction or oxidation of the toxicant for different algae and cyanobacteria species to remove heavy metals. Algae provide several chemical moieties surfaces such as hydroxy, carboxylic, phosphate, and amide as metal-binding sites. Many researchers concluded that Sargassum brown algae are adsorbent and capable of efficiently extracting heavy metals like Pr, Sm, Cr, Cd, Cu, Pd, and Ni due to cell wall structures containing active bioabsorption sites (Cardoso et al., 2017). Bioadsorbents are widely available as by-products or waste, and no growth media or growth conditions are required. Consequently, low-cost products with a strong capacity for usage for several cycles (Nazal, 2019). The literature suggests that heavy metals can be extracted by living or dead marine algae. Goher et al. (2016) used Chlorella vulgaris dead cells at different times of contact, pH, biosorbent used to extract led ions (Pb2+), copper (Cu2+), and cadmium (Cd2+), from aqueous solution. The findings showed C. vulgaris biomass is 99.4 %, 97.7 %, and 95.5 %, effective for removal of led ions (Pb2+), copper (Cu2+), and cadmium (Cd2+) respectively (Goher et al., 2016).
6.3 Genetic engineering
Advances in genetic modification and optimization techniques demonstrate that such advancements have a bright future. Genetically engineered microorganisms might be more able to bioremediate different pollutants (Kapahi and Sachdeva, 2019). In addition, the genetic modification of photosynthesizing species has been studied to improve resistance, sequestration, transport, absorption, and chelation of metals. Microbes are modified in genetic engineering, and they are capable of tolerating metals stress. Closterium ehrenbergii exhibits a high sensitivity to various hazardous chemicals, making it a model species for ecotoxicology research (Abassi et al., 2019). CeHOP, CeHSP70, and CeHSP90 all responded to different stresses, but over-expression of the HOP gene than HSP70 and HSP90 means that this gene could be much more significant than the HSP70/HSP90 co-chaperone activity. HSP genes have already been suggested as microalgae biomarkers (Chankova et al., 2013; Guo et al., 2013); therefore, HOP may be used as biomarkers for the prediction of the action of species and data collected from various gene transcription for the creation of an answer profile to external stressors that can assist in the protection and monitoring of surroundings. Microalgae have molecular machinery that allows differentiation between essential and non-essential heavy metals (Perales-Vela et al., 2006). Chlamydomonas reinhardtii was identified as a species for heavy metal tolerance (Hanikenne et al., 2005). Zinc can detoxify heavy metals and decrease oxidative stress in Dunaliella tertiolecta (Tsuji et al., 2002). Thioredoxin (TRXs) is believed to detoxify heavy metals in Chlamydomonas, exemplified by two TRX genes, being stimulated mercury and Cd. To remediate heavy metal, genetically engineered E. coli targets As(III) (Ibuot et al., 2017). Corynebacterium glutamicum and Saccharomyces cerevisiae were genetically modified to target Zn2+ and Cd2+ using ars operons overexpression to detoxify As-contaminated sites (Mateos et al., 2017).
7 Conclusion
Environmental pollution, food quality, safety, and public well-being are all intertwined. Heavy metal concentrations in the ecosystem have grown substantially in recent decades. The origins of heavy metals in food crops varies across the developing and industrialized worlds. Heavy metals (HMs) buildup in organisms is one of the primary causes of seafood contamination in poor nations. However, the disposal of inadequately treated effluent or sludge is the major source of seafood pollution in developed countries. The heavy metal transfer is complex and uses multifaceted processes. Metal toxicity in seafood requires a thorough evaluation of the exact toxicity of a metal. Internationally, risks to public health have been extensively researched, but few of these initiatives have employed effective epidemiological techniques. Current remediation techniques lower heavy metal concentrations in aquatic environments and the food chain, therefore reducing health risks. To prevent metal contaminants from entering the food chain and create efficient remediation techniques, marine food contamination must be mapped quickly and precisely. Biological treatment can be an environmentally friendly and cost-effective solution for moderately contaminated water.
Acknowledgements
This work was supported by the special funds of Chinese National Key R & D Project(2020YFD0901002), Shenzhen Special Project for Sustainable Development (KCXFZ20201221173404012),Shenzhen Science and Technology application demonstration project (KJYY20180201180253571). The authors appreciate the support of the Research Center for Advanced Materials Science (RCAMS) at King Khalid University Abha, Saudi Arabia through a grant KKU/RCAMS/G002-21.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Small heat shock protein genes of the green algae Closterium ehrenbergii: cloning and differential expression under heat and heavy metal stresses. Environ. Toxicol.. 2019;34(9):1013-1024.
- [Google Scholar]
- Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere. 2015;128:284-292.
- [Google Scholar]
- Monitoring of trace metals in tissues of Wallago attu (lanchi) from the Indus River as an indicator of environmental pollution. Saudi J. Biol. Sci.. 2016;23(1):72-78.
- [Google Scholar]
- Bioaccumulation of Cu and Zn in Schizothorax plagiostomus and Mastacembelus armatus from river swat, river panjkora and river barandu in malakand division, Pakistan. Pak. J. Zool.. 2017;49(5):1555-1561.
- [Google Scholar]
- Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ. Chem. Lett.. 2018;16(3):903-917.
- [Google Scholar]
- Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019
- [Google Scholar]
- Bioaccumulation of heavy metals in tissues of Indian anchovy (Stolephorus indicus) from the UAE coast, Arabian Gulf. Mar. Pollut. Bull.. 2020;154:111033.
- [CrossRef] [Google Scholar]
- The intensity of human-induced impacts on the distribution and diversity of macroinvertebrates and water quality of Gbako River, North Central, Nigeria. Energy, Ecol. Environ.. 2017;2(2):143-154.
- [Google Scholar]
- All that glitters is not gold: Mercury poisoning in a family mimicking an infectious illness. Curr. Probl. Pediatr. Adolesc. Health Care 2020:100758.
- [Google Scholar]
- Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf.. 2018;159:153-163.
- [Google Scholar]
- Parasitic diseases and heavy metal analysis in Parachanna obscura (Gunther 1861) and Clarias gariepinus (Burchell 1901) from Epe Lagoon, Lagos, Nigeria. Asian Pacific J. Trop. Dis.. 2016;6(9):685-690.
- [Google Scholar]
- A novel, thermotolerant, extracellular PHB depolymerase producer Paenibacillus alvei PHB28 for bioremediation of biodegradable plastics. Turk. J. Biochem.. 2018;44:344-353.
- [Google Scholar]
- Mercury toxicity and treatment: a review of the literature. J. Environ. Publ. Health 2012
- [Google Scholar]
- Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci. Total Environ.. 2000;249(1-3):257-280.
- [Google Scholar]
- Developmental toxicology of cadmium in living embryos of a stable transgenic zebrafish line. Environ. Health Perspect.. 2002;110(10):1041-1046.
- [Google Scholar]
- Bioaccumulation of heavy metals in fish, crustaceans, molluscs and echinoderms from the Tuscany coast. Ecotoxicol. Environ. Saf.. 2018;162:554-562.
- [Google Scholar]
- Lignocellulosics as sustainable resources for production of bioplastics–A review. J. Cleaner Prod.. 2017;162:646-664.
- [Google Scholar]
- Butter, T., Evison, L., Hancock, I., and Holland, F., 1995. Removal and recovery of cadmium from dilute aqueous streams by biosorption, elution and electrolysis. In: Mededelingen-Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen Universiteit Gent (Belgium).
- Bioremoval of Cobalt (II) from aqueous solution by three different and resistant fungal biomasses. Bioinorg. Chem. Appl. 2019
- [Google Scholar]
- Biosorption of toxic metals using the alginate extraction residue from the brown algae Sargassum filipendula as a natural ion-exchanger. J. Cleaner Prod.. 2017;165:491-499.
- [Google Scholar]
- Bioaccumulation of arsenic from water and sediment by a deposit-feeding polychaete (Arenicola marina): a biodynamic modelling approach. Aquat. Toxicol.. 2010;98(1):34-43.
- [Google Scholar]
- Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol.. 2008;26(3):263-271.
- [Google Scholar]
- Polychlorinated biphenyls in settled dust from informal electronic waste recycling workshops and nearby highways in urban centers and suburban industrial roadsides of Chennai city, India: levels, congener profiles and exposure assessment. Sci. Total Environ.. 2016;573:1413-1421.
- [Google Scholar]
- Heat shock protein HSP70B as a marker for genotype resistance to environmental stress in Chlorella species from contrasting habitats. Gene. 2013;516(1):184-189.
- [Google Scholar]
- Bioaccumulation of sulfadiazine and subsequent enzymatic activities in Chinese mitten crab (Eriocheir sinensis) Mar. Pollut. Bull.. 2017;121(1-2):176-182.
- [Google Scholar]
- Bioremediation of water contaminated with uranium using Penicillium piscarium. Biotechnol. Prog.. 2020;36(5)
- [CrossRef] [Google Scholar]
- Integrated environmental evaluation of heavy metals and metalloids bioaccumulation in invertebrates and seaweeds from different marine coastal areas of Sardinia, Mediterranean Sea. Environ. Pollut.. 2020;266:115048.
- [CrossRef] [Google Scholar]
- Cumberlidge, N., 2014. Spiralothelphusa hydrodroma. IUCN Red List of Threatened Species. Version.
- Vestiges of a beginning and the prospect of an end. Geol. Soc., London, Spec. Publ.. 1999;150(1):119-155.
- [Google Scholar]
- A morpho-histopathological study in the digestive tract of three fish species influenced with heavy metal pollution. Chemosphere. 2020;242:125212.
- [CrossRef] [Google Scholar]
- Diverse metabolic capacities of fungi for bioremediation. Indian J. Microbiol.. 2016;56(3):247-264.
- [Google Scholar]
- Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt. J. Basic Appl. Sci.. 2014;1(2):97-105.
- [Google Scholar]
- Pharmaceuticals and other anthropogenic chemicals in atmospheric particulates and precipitation. Sci. Total Environ.. 2018;612:1488-1497.
- [Google Scholar]
- Assessment of Heavy metals pollution in Noyyal and Chinnar Rivers, Western Ghats of Tamil Nadu, India with reference to crabs (Gecarcinucidae)–a baseline study. Bull. Environ. Contamin. Toxicol.. 2020;105(4):538-545.
- [Google Scholar]
- Parasites and pollution: the effectiveness of tiny organisms in assessing the quality of aquatic ecosystems, with a focus on Africa. Environ. Sci. Pollut. Res.. 2017;24(23):18742-18769.
- [Google Scholar]
- Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris. J. Elementol.. 2016;21
- [Google Scholar]
- Potamonautid river crabs (Decapoda, Brachyura, Potamonautidae) of KwaZulu-Natal, South Africa. Water SA. 2001;27:85-98.
- [Google Scholar]
- Bioaccessibility and human health implications of heavy metals in different trophic level marine organisms: a case study of the South China Sea. Ecotoxicol. Environ. Saf.. 2018;163:551-557.
- [Google Scholar]
- Different transcriptional responses of heat shock protein 70/90 in the marine diatom Ditylum brightwellii exposed to metal compounds and endocrine-disrupting chemicals. Chemosphere. 2013;92(5):535-543.
- [Google Scholar]
- Heavy metal reduction of pulp and paper mill effluent by indigenous microbes. Asian J. Exp. Biol. Sci.. 2010;1:201-203.
- [Google Scholar]
- A comparative inventory of metal transporters in the green alga Chlamydomonas reinhardtii and the red alga Cyanidioschizon merolae. Plant Physiol.. 2005;137:428-446.
- [Google Scholar]
- Heavy metal distribution and bioaccumulation ability in marine organisms from coastal regions of Hainan and Zhoushan, China. Chemosphere. 2019;226:340-350.
- [Google Scholar]
- Crustacea (excluding Cirripedia) of the Dampier Archipelago, Western Australia. Rec. West. Aust. Mus. Suppl.. 2004;66(1):169.
- [CrossRef] [Google Scholar]
- Selenium protection against mercury toxicity on the male reproductive system of Clarias gariepinus. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2019;225:108583
- [Google Scholar]
- Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res.. 2017;24:89-96.
- [Google Scholar]
- Unlocking the power of fatty acids as dietary tracers and metabolic signals in fishes and aquatic invertebrates. Philos. Trans. R. Soc. B. 2020;375(1804):20190639.
- [CrossRef] [Google Scholar]
- Arsenic and cadmium levels in imported fresh and frozen fish in Jordan. Bull. Environ. Contamin. Toxicol.. 2002;68(1):132-137.
- [Google Scholar]
- Impact of heavy metals and nanoparticles on aquatic biota. Environ. Chem. Lett.. 2018;16(3):919-946.
- [Google Scholar]
- Bioremediation of heavy metals by using bacterial mixtures. Ecol. Eng.. 2016;89:64-69.
- [Google Scholar]
- Bioremediation options for heavy metal pollution. J. Health Pollut.. 2019;9(24):191203.
- [CrossRef] [Google Scholar]
- Biomonitoring of effects and accumulations of heavy metals insults using some helminth parasites of fish as bio-indicators in an Afrotropical stream. Front. Environ. Sci.. 2020;8:155.
- [Google Scholar]
- Disturbance of benthic macrofauna in relation to hypoxia and organic enrichment in a eutrophic coastal bay. Mar. Environ. Res.. 2012;76:80-89.
- [Google Scholar]
- Growth of young-of-year sablefish (Anoplopoma fimbria) in response to temperature and prey quality: insights from a life stage specific bioenergetics model. J. Exp. Mar. Biol. Ecol.. 2020;526:151340.
- [CrossRef] [Google Scholar]
- Investigation of the Metal Pollution Sources in Lake Mogan, Ankara, Turkey. Biol. Trace Elem. Res.. 2020;198(1):269-282.
- [Google Scholar]
- Biomonitoring of heavy metals in river ganga water, sediments, plant, and fishes of different trophic levels. Biol. Trace Elem. Res.. 2020;193(2):536-547.
- [Google Scholar]
- Assessment of heavy metals uptake by cauliflower (Brassica oleracea var. botrytis) grown in integrated industrial effluent irrigated soils: a prediction modeling study. Sci. Hortic.. 2019;257:108682
- [Google Scholar]
- Generation of mt: egfp transgenic zebrafish biosensor for the detection of aquatic zinc and cadmium. Environ. Toxicol. Chem.. 2016;35(8):2066-2073.
- [Google Scholar]
- Contamination features, geo-accumulation, enrichments and human health risks of toxic heavy metal (loids) from fish consumption collected along Swat river, Pakistan. Environ. Technol. Innov.. 2020;17:100554.
- [CrossRef] [Google Scholar]
- Heavy metals (As, Hg and V) and stable isotope ratios (δ13C and δ15N) in fish from Yellow River Estuary, China. Sci. Total Environ.. 2018;613-614:462-471.
- [Google Scholar]
- Metal Contamination in Aquatic Environments: Science and Lateral Management. Cambridge University Press; 2008.
- Ecotoxicological studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chem.. 2002;78(1):15-22.
- [Google Scholar]
- Bioaccumulation and elimination of nickel in the organs of black fish (Capoeta fusca) Toxicol. Ind. Health. 2012;28(4):361-368.
- [Google Scholar]
- The arsenic detoxification system in corynebacteria: basis and application for bioremediation and redox control. In: Advances in applied microbiology. Elsevier; 2017. p. :103-137.
- [Google Scholar]
- Bioaccumulation and potential sources of heavy metal contamination in fish species in River Ganga basin: possible human health risks evaluation. Toxicol. Rep.. 2019;6:472-481.
- [Google Scholar]
- Heavy metal contamination in two commercial fish species of a trans-Himalayan freshwater ecosystem. Environ. Monit. Assess.. 2019;191(2)
- [CrossRef] [Google Scholar]
- Distribution and bioaccumulation of heavy metals (Hg, Cd, and Pb) in fish: influence of the aquatic environment and climate. Environ. Res. Lett.. 2020;15(11):115013.
- [CrossRef] [Google Scholar]
- The role of heavy metals in plant response to biotic stress. Molecules. 2018;23(9):2320.
- [CrossRef] [Google Scholar]
- Heavy Metal concentration in two freshwater fishes from Wadi Hanifah (Riyadh, Saudi Arabia) and evaluation of possible health hazard to consumers. Pak. J. Zool.. 2015;47
- [Google Scholar]
- Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem. J.. 2011;98(2):334-343.
- [Google Scholar]
- Environmental parasitology: Parasites as accumulation bioindicators in the marine environment. J. Sea Res.. 2016;113:45-50.
- [Google Scholar]
- Bioaccumulation of toxic metals by fish in a semi-enclosed tropical ecosystem. Environ. Forensics. 2006;7(3):197-206.
- [Google Scholar]
- Multimetal tolerance mechanisms in bacteria: The resistance strategies acquired by bacteria that can be exploited to ‘clean-up’heavy metal contaminants from water. Aquat. Toxicol.. 2019;212:1-10.
- [Google Scholar]
- Marine algae bioadsorbents for adsorptive removal of heavy metals. In: Advanced Sorption Process Applications. IntechOpen; 2019.
- [Google Scholar]
- GFP transgenic medaka (Oryzias latipes) under the inducible cyp1a promoter provide a sensitive and convenient biological indicator for the presence of TCDD and other persistent organic chemicals. PLoS ONE. 2013;8(5) e64334
- [Google Scholar]
- Biosensors for determination of heavy metals in waters. In: Biosensors for Environmental Monitoring. IntechOpen; 2019.
- [Google Scholar]
- Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int. J. Environ. Res. Publ. Health. 2017;14(12):1504.
- [CrossRef] [Google Scholar]
- Freshwater crabs (Crustacea: Decapoda: Brachyura: Gecarcinucidae) in the collection of Western Ghat Regional Centre, Zoological Survey of India, Kozhikode. Rec. Zool. Survey India. 2014;114:651-668.
- [Google Scholar]
- Development of a fluorescent transgenic zebrafish biosensor for sensing aquatic heavy metal pollution. Transgenic Res.. 2016;25(5):617-627.
- [Google Scholar]
- Bioaccumulation of heavy metals in different organs of Labeo rohita, Pangasius hypophthalmus, and Katsuwonus pelamis from Visakhapatnam, India. Mar. Pollut. Bull.. 2020;157:111326.
- [CrossRef] [Google Scholar]
- The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ. Monit. Assess.. 2019;191:419.
- [Google Scholar]
- Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere. 2018;191:626-638.
- [Google Scholar]
- Strategies for crop improvement in contaminated soils using metal-tolerant bioinoculants. In: Khan M.S., Zaidi A., Musarrat J., eds. Microbial Strategies for Crop Improvement. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. p. :85-104.
- [CrossRef] [Google Scholar]
- Occurrence of heavy metals in sediment and their bioaccumulation in sentinel crab (Macrophthalmus depressus) from highly impacted coastal zone. Chemosphere. 2019;221:89-98.
- [Google Scholar]
- Cadmium as an environmental pollutant: ecotoxicological effects, health hazards, and bioremediation approaches for its detoxification from contaminated sites. In: Bioremediation of Industrial Waste for Environmental Safety. Springer; 2020. p. :357-387.
- [Google Scholar]
- Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: a critical review. Sep. Purif. Technol.. 2018;191:400-423.
- [Google Scholar]
- Effect of wastewater irrigation on crop health in the Indian agricultural scenario. In: Emerging Trends of Plant Physiology for Sustainable Crop Production. Apple Academic Press; 2018. p. :357-371.
- [Google Scholar]
- Quantitative GFP fluorescence as an indicator of arsenite developmental toxicity in mosaic heat shock protein 70 transgenic zebrafish. Toxicol. Appl. Pharmacol.. 2007;225(2):154-161.
- [Google Scholar]
- Differential bioaccumulation of trace elements and rare earth elements in the muscle, kidneys, and liver of the invasive Indo-Pacific Lionfish (Pterois spp.) from Cuba. Biol. Trace Elem. Res. 2019:1-10.
- [Google Scholar]
- Biosorption potency of Aspergillus niger for removal of chromium (VI) Curr. Microbiol.. 2006;53(3):232-237.
- [Google Scholar]
- Biological response of using municipal solid waste compost in agriculture as fertilizer supplement. Rev. Environ. Sci. Bio/Technol.. 2016;15(4):677-696.
- [Google Scholar]
- Biochemical, physiological, and yield responses of lady’s finger (Abelmoschus esculentus L.) grown on varying ratios of municipal solid waste vermicompost. Int. J. Recycl. Org. Waste in Agricult.. 2018;7(3):241-250.
- [Google Scholar]
- Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front. Environ. Sci.. 2017;5:64.
- [Google Scholar]
- Accumulation of heavy metals by intestinal helminths in fish: an overview and perspective. Parasitology. 2003;126(7):S53-S60.
- [Google Scholar]
- Determination of trace metals (Cd, Pb) in fish by electrothermal atomic absorption spectrometry after microwave digestion. Anal. Chim. Acta. 1995;311(2):135-139.
- [Google Scholar]
- Biomonitoring heavy metal pollution using an aquatic apex predator, the American alligator, and its parasites. PLoS ONE. 2015;10(11) e0142522
- [Google Scholar]
- Determination of heavy metals and pesticides in different types of fish samples collected from four different locations of Aegean and Marmara Sea. J. Food Qual. 2020
- [Google Scholar]
- Enhancement of tolerance to heavy metals and oxidative stress in Dunaliella tertiolecta by Zn-induced phytochelatin synthesis. Biochem. Biophys. Res. Commun.. 2002;293(1):653-659.
- [Google Scholar]
- Cu, Pb, and Zn content in sediments and benthic organisms from San Jorge Bay (northern Chile): accumulation and biotransference in subtidal coastal systems. Cienc. Marinas. 2014;40(1):45-58.
- [Google Scholar]
- One-step synthesis of cake-like biosorbents from plant biomass for the effective removal and recovery heavy metals: effect of plant species and roles of xanthation. Chemosphere. 2020;129129
- [Google Scholar]
- Bioaccumulation of heavy metals, antioxidants, and metabolic enzymes in the crab Scylla serrata from different regions of Tuticorin, Southeast Coast of India. Mar. Pollut. Bull.. 2020;158:111443.
- [CrossRef] [Google Scholar]
- Bioaccumulation and health risk assessment of heavy metals to bivalve species in Daya Bay (South China Sea): consumption advisory. Mar. Pollut. Bull.. 2020;150:110717.
- [CrossRef] [Google Scholar]
- Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem. Toxicol.. 2013;51:87-92.
- [Google Scholar]
- Generation and application of a Tg (cyp1a: egfp) transgenic marine medaka (Oryzias melastigma) line as an in vivo assay to sensitively detect dioxin-like compounds in the environment. J. Hazard. Mater.. 2020;391:122192
- [Google Scholar]
- Biota-sediment accumulation factor and concentration of heavy metals (Hg, Cd, As, Ni, Pb and Cu) in sediments and tissues of Chiton lamyi (Mollusca: Polyplacophora: Chitonidae) in Chabahar Bay, Iran. Iran. J. Fish. Sci.. 2017;16:1123-1134.
- [Google Scholar]







