Translate this page into:
Healing activity of a muco-adhesive formula of Teucrium polium against acetic acid-induced oral ulcer in rats
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Oral ulcers are highly common painful conditions that harmfully affect the quality of life and require long-term therapy. Muco-adhesive preparations provide efficient and convenient delivery systems that prolong the drug retention time necessary for the different stages of ulcer healing. Therefore, the study aimed to evaluate the healing activity of Teucrium polium in a muco-adhesive formula against acetic acid-induced oral ulcers in rats. Acetic acid was applied to the inner cheeks of the rats and the muco-adhesive formula was applied once daily for 14 days. Acetic acid was found to disrupt the oral tissue histological features, increase collagen deposition, induce oxidative stress through increasing malondialdehyde (MDA) levels and decreasing superoxide dismutase and catalase activities, increase the expression of the inflammatory biomarkers, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), and decreased hydroxyproline content and the expression of the factors that contribute in wound healing including transforming growth factor-beta1 (TGF-β1), vascular endothelial growth factor receptor 1 (VEGFR1), and platelet-derived growth factor receptor beta (PDGFRβ). However, the daily application of a mucoadhesive formula impregnated with T. polium, significantly restored the regular histological features of the oral tissue, decreased the concentration of MDA, TNF-α, IL-6, and COX-2, increased superoxide dismutase and catalase activities, and restored the expression of TGF-β1, VEGFR1, and PDGFRβ. Therefore, T. polium extract muco-adhesive formula can be considered a potential therapy for chronic oral ulcers.
Keywords
Teucrium polium
Oral ulcer
Acetic acid
Healing
TGF-β1/VEGFR1/PDGFRβ
1 Introduction
Oral ulcers are highly common painful conditions that may range from shallow sores affecting the epithelium to open lesions extending to the underlying connective tissues and can affect the lips, floor of the mouth, soft palate, tongue, pharynges, and uvula (Muñoz-Corcuera et al., 2009). Oral ulcers may develop due to different causes such as immune disease, trauma, and infection, and sometimes are drug-induced (Liu 2016).
Oral lesions can lower patients' quality of life as they interfere with chewing, swallowing, and speaking (Yan et al., 2020). Current management of oral ulcers includes the use of corticosteroids (Fantozzi et al., 2019), anti-infectives (Barrons, 2001), anti-inflammatory agents (Altenburg et al., 2014), and multi-ingredient mouthwash preparations (Zegarelli, 1991). However, the healing effect is not satisfactory and may pose lower risks of systemic adverse effects (Baccaglini et al., 2011, Philipone and Peters, 2023). In addition, the drug therapy within the oral cavity suffers rapid removal (Mizrahi et al., 2008). Therefore, using an effective and safe natural medication, preferably as a topical preparation, will have a great advantage. Topical preparations are preferred for the management of oral ulcers as target tissues are easily accessible and the incidence of adverse effects is lower, as compared to systemic administration (Xu et al., 2015). In particular, muco-adhesive preparations provide efficient and convenient delivery systems that prolong the drug retention time, which is necessary for the different phases of ulcer recovery (Shao et al., 2021).
Because of their efficacy and relative safety, natural products have gained wide acceptance (Bauer 2000) and are recommended for the management of oral ulcers (Aghamohamamdi and Hosseinimehr, 2016). Teucrium polium L. (syn Teucrium capitatum L.) belongs to the genus Teucrium, family Lamiaceae (El Oualidi et al., 1999). T. polium is endogenous to Southwestern Asia, North Africa, Mediterranean countries and Europe, and (Bahramikia and Yazdanparast 2012). Phytochemical analysis of T. polium indicated a variety of secondary metabolites as flavonoids, iridoids, terpenoids, fatty acid esters and phytosterols (Hachicha et al., 2009). Fatty acid, sterol and tocopherol, matter of three Tunisian Teucrium species (Hachicha et al., 2009). Traditionally, the plant has been used in many diseases such as diabetes, common cold, abdominal pain, indigestion, anorexia, hypertension, rheumatic diseases, and peptic ulcer (Asadi-Samani et al., 2017, Rahmouni et al., 2021, Sadeghi et al., 2022). Experimentally, it has been shown to possess antioxidant (Noumi et al., 2020), anti-inflammatory, anti-proliferative (Menichini et al., 2009), anti-nociceptive properties (Parsaee and Shafiee-Nick 2006). Further, the plant has proven hepatoprotective (Rahmouni et al., 2022), antidiabetic (Albadr et al., 2022), hypolipidemic (Shahraki et al., 2007), antibacterial (Essawi and Srour 2000), anti-ulcer (Mehrabani et al., 2009), and wound-healing (Fallah Huseini et al., 2020) activities. The current work aimed to evaluate the preventive effects of a muco-adhesive formula containing T. polium extract on acetic acid-induced oral ulcer in rats.
2 Materials and methods
2.1 Plant material and the methanolic extract
Areal parts of T. polium, obtained from Al-Taif region, KSA, were recognized by Dr. Faraj Al-Ghamdi (Biological Sciences Department, King Abdulaziz University). A sample was kept in the herbarium of Faculty of Science, King Abdulaziz University under the number (873563). High-resolution HPLC-ESI-QTOF-MS-MS analysis and identification of plant metabolites were previously published by our laboratory (Algandaby et al., 2023).
2.2 Chemicals
Acetic acid (Cat #, 1603051000) was purchased from Sigma Aldrich (St. Louis, MO, USA). All chemicals used in different experiments were of the finest grades.
2.3 Animals
Thirty male Wistar rats (7-week old, ̃ 200 g each) were taken from the Faculty of Pharmacy, King Abdulaziz University Animal Facility. Rats were adapted to the laboratory conditions for a week before the initiation of the study. During the study, animals were maintained under a standard condition at 24 ˚C room temperature, 50 % relative humidity, and 12 h dark/12 h light cycles. Animals freely accessed regular chow and water. The experimental procedure was approved by the Committee of Research Ethics, KAU (Reference # PH-1443-57).
2.4 Preparation of T. Polium in a muco-adhesive formula
For preparing an adhesive sponge formulation, CMC (2 % w/v) was added to double distilled water and mixed till uniform gel formation. The extract was dispersed in water to a final strength of 10 % (w/v) and stirred until a uniform dispersion was obtained. Then, the previously prepared CMC was added until a consistent gel was obtained (final concentration of the plant extract was 5 %). The mucoadhesive characteristic was evaluated by the assessing the mucoadhesive time of the formula (Ossama et al., 2021).
2.5 Design of the experiment
Rats were haphazardly distributed into five groups (six each). With the exclusion of the negative control, ketamine (80 mg/kg) and xylazine (8 mg/kg) were used to anesthetize the rats. Filter papers (5.0 mm diameter) were immersed in 20 mL of acetic acid (50 %) then pressed onto the inner cheeks for 60 s (Miao et al., 2019). The animals were divided as follows: group one, represented the negative control with no treatments applied; group two, animals were only challenged with acetic acid; group three, animals were challenged with acetic acid and treated with the plain muco-adhesive formula once daily on the ulcer area; group four, animals were challenged with acetic acid and treated with the 5 % T. polium in the muco-adhesive formula once daily; group five, represented the positive control and animals were challenged with acetic acid and treated with the Jogel® (Sedico, Giza, Egypt).
All treatments were repeated for 14 consecutive days. On day 14, all rats were euthanized by decapitation, and the oral tissues were dissected and preserved in 10 % neutral formaldehyde or instantly frozen using liquid nitrogen and then preserved at − 80 °C for the biochemical analyses.
2.6 Histopathological assessment
The formalin- fixed oral tissues were dehydrated in ethyl alcohol serial dilutions, immersed in xylene, and then molded into paraffin blocks. 5-µm-thick sections were taken on glass slides. This was followed by dewaxing, rehydration and stained with either hematoxylin and eosin (H and E) or Masson’s trichrome (MTC).
2.7 Immunohistochemical assessments
Sections of oral tissues were deparaffinized, rehydrated, and then boiled in 0.1 M citrate buffer (pH 6.0) for 12 min. Afterwards, sections were retained in bovine serum albumin (5 %) and Tris-buffered saline for two hours, then incubated with the primary antibodies (ABCAM, Cambridge, UK) tumor necrosis factor- α (anti-TNF-α, ab220210), interleukin-6 (anti-IL-6, ab9324), cyclooxygenase-2 (anti-COX-2, ab179800), transforming growth factor −beta1 (anti-TGF-β1, ab215715), vascular endothelial growth factor receptor 1 (anti-VEGFR1, ab32152), and platelet-derived growth factor receptor beta (anti-PDGFRβ, ab51046) at 4 °C for 12 h. Next to rinsing with TBS, according to the primary antibody reactivity, the tissue sections were incubated with either anti-mouse or anti-rabbit biotinylated secondary antibody (ab47827, and ab288151, respectively, ABCAM, Cambridge, UK). For target antigen staining, the mouse and rabbit specific HRP/DAB detection IHC kit was used (ab64264, ABCAM, Cambridge, UK). Image assessments were performed using Image J (1.52a, NIH, USA).
2.8 Assessment of lipid peroxidation, reduced glutathione, superoxide dismutase and hydroxyproline
Oral tissues were homogenized in a pH 7.4 phosphate-buffered saline to prepare a 10 % homogenate to be centrifuged for 15 min (8,000 × g and 4 °C), then the supernatants were collected to perform the different analyses. Commercial kits were utilized to assay the tissue levels of the malondialdehyde (MDA, Cat. No. MD 2529; Biodiagnostic, Giza, Egypt), and reduced glutathione (GSH; Cat. No. GR 2511; Biodiagnostic, Giza, Egypt). The activities of the enzymes superoxide dismutase (SOD) and catalase (CAT) were assessed using assay kit (Cat. No. SD 2521 & CA 2516 respectively, Biodiagnostic, Giza, Egypt). Concentrations of hydroxyproline were assessed using a commercial kit (Cat. No. Ab22294, ABCAM, Cambridge, UK). All procedures were performed in adherence to the manufacturer’s guides.
2.9 Statistical analyses
Data are shown as means ± SD after being analyzed by one-way ANOVA followed by the Tukey test (Ali and Bhaskar, 2016). The software GraphPad Prism, version 8.00 (GraphPad Software, La Jolla, CA, USA) was used for analyzing the data. Obtaining p-value of less than 0.05 was counted as statistically significant.
3 Results
3.1 The effect of T. polium on acetic acid-induced oral ulcer in rats
Staining with H and E revealed that the oral tissues obtained from the control rats exhibited normal histological features with no signs of ulceration. However, sections taken from the ulcerated tissues from acetic acid only and acetic acid and plain formula treated rats showed granulation tissue filling the ulcer gap with intense inflammatory cells infiltration and retarded re-epithelization. Nevertheless, application of T. polium was shown to induce improvement with less inflammatory cell infiltration and the surface of the ulcer showed re-epithelization in a manner comparable to that induced by the commercially available treatment, Jogel® that served as a positive control. To assess collagen deposition, tissue sections were stained with MTC. The control tissues showed minimal collagen deposition. Tissue sections from the acetic acid only and acetic acid and plain formula showed significantly increased collagen deposition, especially around the blood vessels. Interestingly, tissues from animals treated with the muco-adhesive formula impregnated with T. polium showed lower extents of collagen deposition like that induced by Jogel®. (Fig. 1).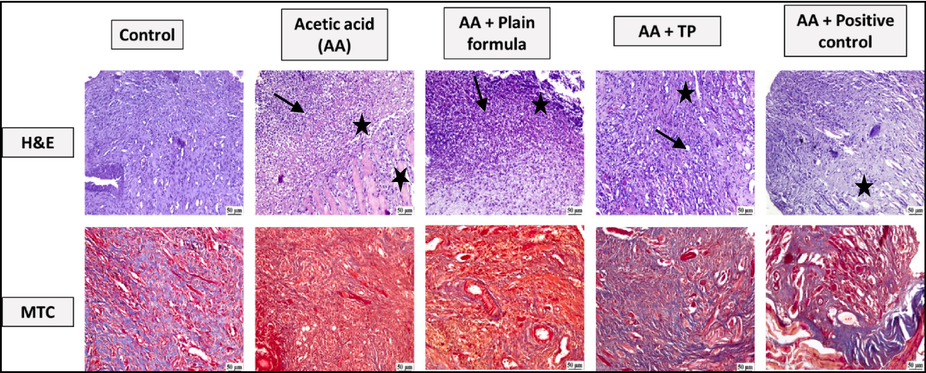
Photomicrographs of the oral tissues of rats from the control, acetic acid (AA) only, AA and plain muco-adhesive formula, AA and T. polium (TP), and AA and positive control groups. Sections were stained with either H and E, or MTC. AA-induced increased inflammatory cell infiltration (black arrow) and necrosis (star).
3.2 The effect of t. Polium muco-adhesive formula on oxidative status in oral tissues
Acetic acid application significantly increased MDA levels, indicating lipid peroxidation. In contrast, the application of the muco-adhesive formula impregnated with T. polium significantly reduced MDA concentration as compared to the tissues obtained from the acetic acid only or the acetic acid and plain formula-treated rats (Fig. 2A). Acetic acid application also compromised the antioxidant capacity in the oral tissues as presented by a reduction in the activities of the enzymes SOD and CAT (Fig. 2B and 2C). However, treating the animals with T. polium muco-adhesive formula significantly restored the activities of SOD and CAT compared to both acetic acid only and acetic acid plus plain formula-treated rats. The antioxidant effect induced by T. polium was similar to that induced by the positive control.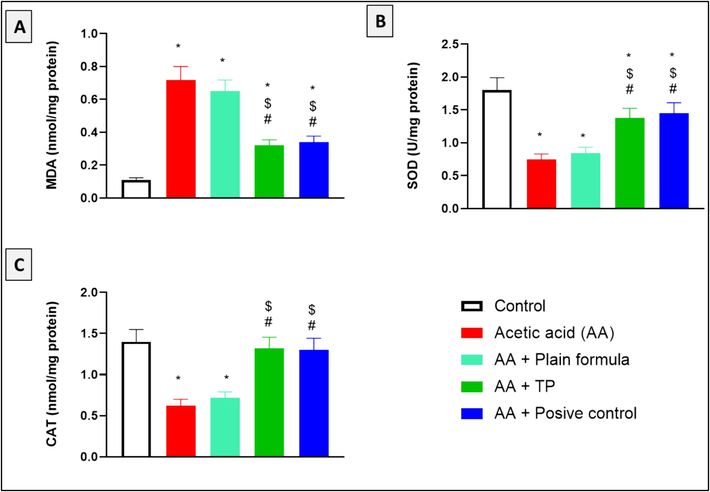
The effect of T. polium (TP), Jogel® (positive control), and acetic acid (AA) on MDA levels, and SOD and CAT activities in the oral tissues. n = 6. *, #, and $ are considered statistically significant from the control, AA only, and AA and plain formula-treated group, respectively, at p < 0.05.
3.3 The effects of t. Polium muco-adhesive formula on immunohistochemical reactivity of the inflammatory markers in oral tissue of the rats treated with acetic acid
As shown in Fig. 3, acetic acid application on the inner cheeks of the rats induced inflammation as reflected by increased immunohistochemical reactivity of the inflammatory markers, TNF-α, IL-6, and COX-2, in comparison to the control group. Application of the plain muco-adhesive formula did not induce any decline in the levels of the inflammatory biomarkers in the oral tissues. On the contrary, the application of the formula impregnated with T. polium significantly attenuated the elevated tissue expression of TNF-α, IL-6, and COX-2 compared to both acetic acid only and acetic acid plus plain formula-treated groups. Treatment with the commercially available formula, positive control, showed results similar to that induced by T. polium.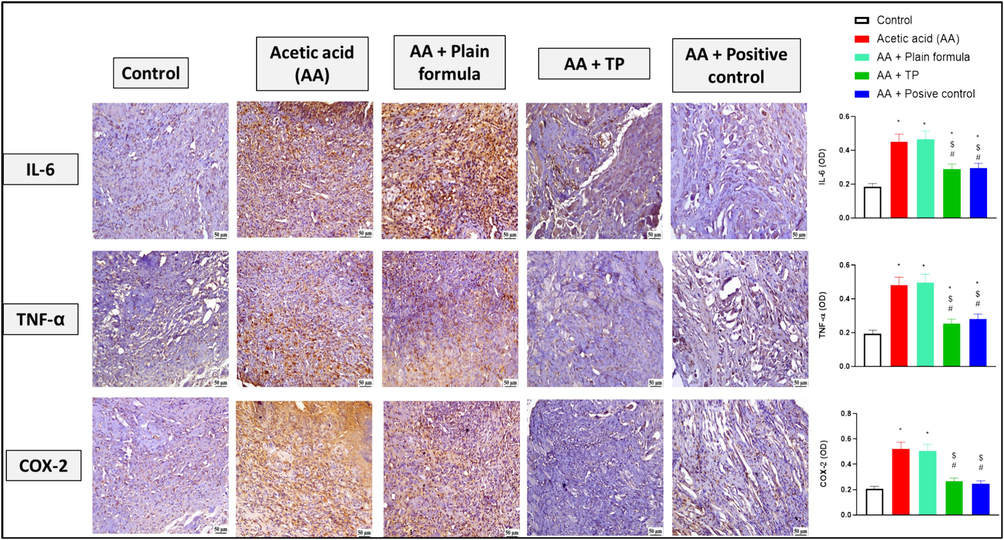
The effect of the muco-adhesive T. polium on the immunohistochemical reactivity of IL-6, TNF-α, and COX-2 in oral tissues of rats from the control, acetic acid (AA) only, AA and plain muco-adhesive formula, AA and T. polium (TP), and AA and Jogel® (positive control) groups. n = 6. *, #, and $ are considered statistically significant from the control, AA only, and AA and plain formula-treated group, respectively, at p < 0.05.
3.4 The effects of t. Polium muco-adhesive formula on the hydroxyproline content in oral tissue of the rats treated with acetic acid
As illustrated by Fig. 4, acetic acid or acetic acid and plain formula application significantly decreased the hydroxyproline content in the oral tissues of rats in comparison to the control. Contrastly, the application of the muco-adhesive formula impregnated with the T. polium significantly increased the hydroxyproline content compared to both acetic acid only and acetic acid plus plain formula-treated animals, in a manner comparable to that of the commercially available treatment.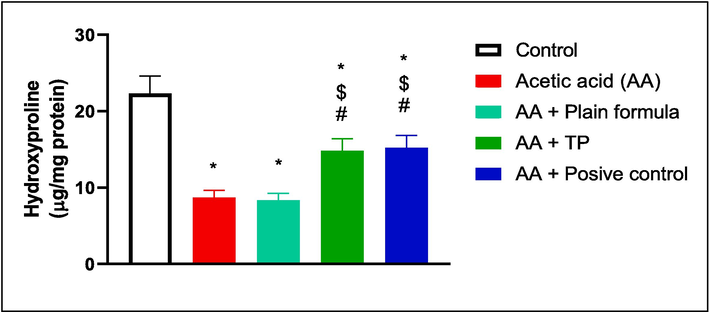
The effect of T. polium (TP), Jogel® (positive control), and acetic acid (AA) on hydroxyproline content in the oral tissues. n = 6. *, #, and $ are considered statistically significant from the control, AA only, and AA and plain formula-treated group, respectively, at p < 0.05.
3.5 The effect of t. Polium muco-adhesive formula on the immunohistochemical reactivity of the ulcer-healing markers in oral tissue
As illustrated in Fig. 5, use of acetic acid on the inner cheeks of the rats, either alone or together with the plain formula, significantly reduced the immunohistochemical reactivity of TGF-β1, VEGFR1, and PDGFR-β compared to the control. However, the application of the muco-adhesive formula impregnated with T. polium significantly increased the immunohistochemical reactivity of TGF-β1, VEGFR1, and PDGFR-β in comparison to both the acetic acid only and the acetic acid plus plain formula-treated groups. The effect of the T. polium formula was comparable to that induced by the positive control and the degree of immunohistochemical reactivity of TGF-β1, and PDGFR-β upon treatment with T. polium formula was comparable to that of the control untreated animals.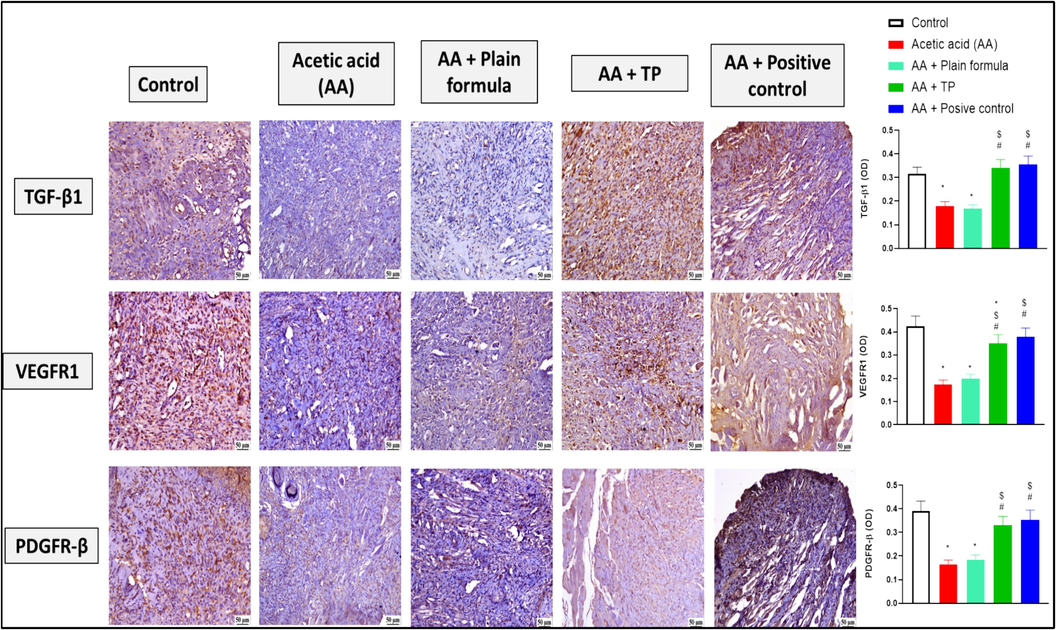
The effect of the muco-adhesive T. polium on the immunohistochemical reactivity of TGF-β1, VEGFR1, and PDGFR-β in oral tissues of rats from the control, acetic acid (AA) only, AA and plain muco-adhesive formula, AA and T. polium (TP), and AA and Jogel® (positive control) groups. n = 6. *, #, and $ are considered statistically significant from the control, AA only, and AA and plain formula-treated group, respectively, at P < 0.05.
4 Discussion
Many conditions result in oral ulceration that in most cases become chronic or recurrent and adversely affect quality of life of the patient. This requires long-term administration of medications that may, in turn, induce side effects contributing to the reduced patient quality of life (Riordain and Hodgson, 2014). Commonly affected areas in the oral cavity include the buccal mucosa followed by the tongue, and finally the lower lip (Muñoz-Corcuera et al., 2009). The current study focused on testing the effect of applying a muco-adhesive formula impregnated with the extract of T. polium on the inner cheeks of rats for 14 days together with the application of acetic acid that induces buccal ulceration. The results of treating rats with T. polium were compared to that induced by a positive control which is a commercially available formula that was approved for treating oral ulcers, Jogel®.
Acetic acid is used as it reproducibly induces the histopathological features associated with the oral ulcer (Okabe and Amagase, 2005; Ayoub et al., 2021). In this study, upon staining the oral tissues with H and E after 14 days from acetic acid application, tissues from rats treated with acetic acid alone or those treated with acetic acid and plain formula showed signs of necrosis and tissue inflammation. On the contrary, the application of the muco-adhesive formula impregnated with the T. polium extract prominently improved the ulcer condition, reduced necrosis, and inflammation, and induced re-epithelialization in a manner greatly comparable to the positive control. T. polium extract was formerly proven to improve the histopathological features of skin wounds improving their healing (Alizadeh et al., 2011), (Chabane, et al., 2021).
Oxidative stress and inflammation are recognized to remarkably participate in the pathogenesis of oral ulcers (Cimen et al., 2003). Oxidative stress together with a compromised cellular antioxidant capacity in the oral cavity result in disrupted cellular metabolism (Lushchak, 2014) and damage to the cellular components of protein, lipids, and nucleic acid underlying the pathogenesis of the majority of the oral diseases (Zalewska et al., 2014). Moreover, the overproduction of ROS is known to induce the production of proinflammatory mediators (Chapple, 1996) (Momen-Beitollahi et al., 2010). Mononuclear cell infiltration and release of inflammatory mediators have been given the most attention in oral diseases (Natah et al., 2000).
Acetic acid is well known for inducing mucosal ulceration through inducing oxidative stress and inflammation (da Silva et al., 2013) (Ansari et al., 2021) (Ayoub et al., 2021). In the current study, acetic acid application to the buccal mucosa was found to increase lipid peroxidation; compromise the antioxidant defense mechanisms through decreasing the enzymatic activities of CAT and SOD; induced the expression of the proinflammatory mediators, IL-6, TNF-α, and COX-2. On the other hand, the application of a muco-adhesive formula impregnated with T. polium extract was found to considerably lower lipid peroxidation and induce the catalytic activities of CAT and SOD enzymes restoring the cellular antioxidant defense mechanisms. Moreover, the application of T. polium formula also reduced the oral tissue inflammation as evidenced by decreased immunohistochemical reactivities of the proinflammatory markers, IL-6, TNF-α, and COX-2. Interestingly, T. polium extract has well-proven antioxidant and anti-inflammatory activities in many pathological conditions including diabetes (Asghari et al., 2020), hypercholesterolemia (Amraei et al., 2018), cardiovascular diseases (Mahmoudabady et al., 2018), and many other diseases.
Hydroxyproline is a nonessential, nonproteinogenic amino acid (Ananthanarayanan, 1983) that acts as a key constituent in the production and steadiness of the major structural protein, collagen (Némethy and Scheraga, 1986) (Srivastava et al., 2016). During wound healing, protein deficiency is known to negatively affect the speed of healing due to the subsequent diminished fibroblast proliferation, capillary development, and the synthesis and remodeling of collagen and proteoglycans (Chhabra et al., 2017). Collagen breakdown is known to release free hydroxyproline, therefore hydroxyproline can be used as a wound healing marker that reflects the tissue content of collagen that indicates collagen turnover after healing (Kumar et al., 2006). An increased level of hydroxyproline means better collagen maturation and proliferation throughout wound healing (Veis and Anesey, 1965), (Srivastava et al., 2016). Acetic acid was previously proven to reduce the hydroxyproline content in the tongue tissue, therefore, slowing down the ulcer healing (Ayoub et al., 2021). Similarly, in the current study, acetic acid was found to significantly lower the hydroxyproline content in the oral tissue. In contrast, the application of the T. polium extract restored the hydroxyproline levels and expedited wound healing. Our laboratory has shown that T. polium is rich with phenolic compounds, particularly flavonoids and kaempferol was found to be 7.85 ± 0.02 mg/g. In the same study, T. polium extract was proven to increase the hydroxyproline content speeding healing of wound in diabetic animals (Algandaby et al., 2023). The extract content of flavonoids as kaempferol lends additional support to the pro-collagen activities of the extract (Özay et al., 2019).
Early after induction of ulcer the inflammatory cells are recruited and stimulated to release proteolytic enzymes. Such proteolytic enzymes catalyze the breakdown of extracellular matrix proteins including collagen-producing protein fragments that recruit more inflammatory cells including macrophages that produce TNF-α, increasing the number of fibroblasts. Fibroblasts promote the secretion of growth factors, including TGF-β, VEGF, and PDGF that induce the proliferation of epithelial and vascular endothelial cell at the injured site so promoting wound healing (Schultz and Mast, 1999; Brett, 2008). In the current study, acetic acid application significantly reduced the immunohistochemical reactivity of TGF-β1, VEGFR1, and PDGFR-β. Interestingly application of the extract of T. polium significantly restored the control levels of the three markers, explaining its wound healing activities that were demonstrated upon the histological examination. The current study results come in line with previous studies that proved that T. polium extract promoted wound healing by inducing fibroblasts proliferation and increasing the expression of fibroblast-secreted growth factors including the VEGF and PDGF (Gharaboghaz et al., 2020) (Algandaby et al., 2023). The current study highlights the healing activities of T. polium in a subacute model of experimentally-induced oral ulcer. However, additional studies are suggested to explore the usefulness of the prepared muco-adhesive formula in sub-chronic and chromic models. Also, a comprehensive toxicity study of the formula is recommended.
5 Conclusion
Application of muco-adhesive formula impregnated with T. polium remarkedly promotes healing of oral ulcers provoked by acetic acid on the inner cheeks of the rats as evidenced by restoring the histopathological features of the oral tissues in a manner comparable to that was induced by the oral ulcer commercially available formula. This outcome can be ascribed to its antioxidant, anti-inflammatory, pro-collagen and angiogenic effects. These data provide an opening for exploring the potential benefits of the prepared T. polium muco-adhesive formula in patient with acute and subacute oral ulcers.
CRediT authorship contribution statement
Mardi M. Algandaby: Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration.
Acknowledgements
The author extends his appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFPRC-087–130-2020 and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Declaration of Competing Interest
The author declares that he has no recognized competing business interests or personal connections that could have looked to impact the work described in this article.
References
- Natural products for management of oral mucositis induced by radiotherapy and chemotherapy. Integr. Cancer Ther.. 2016;15:60-68.
- [CrossRef] [Google Scholar]
- LC-MS based metabolic profiling and wound healing activity of a chitosan nanoparticle-loaded formula of Teucrium polium in diabetic rats. Biomed. Pharmacother.. 2023;168:115626
- [CrossRef] [Google Scholar]
- Basic statistical tools in research and data analysis. Indian J Anaesth. 2016;60(9):662-669.
- [CrossRef] [Google Scholar]
- The effect of teucrium polium honey on the wound healing and tensile strength in rat. Iran. J. Basic Med. Sci.. 2011;14:499-505.
- [Google Scholar]
- The treatment of chronic recurrent oral aphthous ulcers. Dtsch. Arztebl. Int.. 2014;111(40):665.
- [Google Scholar]
- The effect of hydroalcoholic extract of Teucrium polium L. on the inflammatory markers and lipid profile in hypercholesterolemic rats. J. Inflamm. Res.. 2018;11:265-272.
- [CrossRef] [Google Scholar]
- Structural aspects of hydroxyproline-containing proteins. J. Biomol. Struct. Dyn.. 1983;1:843-855.
- [CrossRef] [Google Scholar]
- Role of Oxidative Stress and Inflammatory Cytokines (TNF-α and IL-6) in Acetic Acid-Induced Ulcerative Colitis in Rats: Ameliorated by Otostegia fruticosa. Life (basel). 2021;11:195.
- [CrossRef] [Google Scholar]
- Anti-diabetic properties and bioactive compounds of Teucrium polium L. Asian Pac. J. Trop. Biomed.. 2020;10:433-441.
- [Google Scholar]
- HPLC/MSn Profiling and Healing Activity of a Muco-Adhesive Formula of Salvadora persica against Acetic Acid-Induced Oral Ulcer in Rats. Nutrients. 2021;14:28.
- [CrossRef] [Google Scholar]
- Urban legends: recurrent aphthous stomatitis. Oral Dis.. 2011;17:755-770.
- [CrossRef] [Google Scholar]
- Treatment strategies for recurrent oral aphthous ulcers. Am. J. Health Syst. Pharm.. 2001;58(1):41-50.
- [Google Scholar]
- Teucrium polium-wound healing potential, toxicity and polyphenolic profile. S. Afr. J. Bot.. 2021;137:228-235.
- [CrossRef] [Google Scholar]
- Role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Clin. Mol. Pathol.. 1996;49:M247-M255.
- [CrossRef] [Google Scholar]
- Wound Healing Concepts in Clinical Practice of OMFS. J Maxillofac Oral Surg. 2017;16:403-423.
- [CrossRef] [Google Scholar]
- Oxidant/antioxidant status in patients with recurrent aphthous stomatitis. Clin. Exp. Dermatol.. 2003;28:647-650.
- [CrossRef] [Google Scholar]
- Ethanolic extract of roots from Arctium lappa L. accelerates the healing of acetic acid-induced gastric ulcer in rats: Involvement of the antioxidant system. Food Chem. Toxicol.. 2013;51:179-187.
- [CrossRef] [Google Scholar]
- Intralesional triamcinolone acetonide therapy for inflammatory oral ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128(5):485-490.
- [Google Scholar]
- Topical co-administration of Teucrium polium hydroethanolic extract and Aloe vera gel triggered wound healing by accelerating cell proliferation in diabetic mouse model. Biomed. Pharmacother.. 2020;127:110189
- [CrossRef] [Google Scholar]
- Beta-adrenoceptor agonist treatment reverses denervation atrophy with augmentation of collagen proliferation in denervated mice gastrocnemius muscle. Indian J. Exp. Biol.. 2006;44:371-376.
- [Google Scholar]
- Classification of oxidative stress based on its intensity. EXCLI J.. 2014;13:922-937.
- [Google Scholar]
- Extract from Teucrium polium L. Protects Rat Heart against Oxidative Stress Induced by Ischemic-reperfusion Injury. Adv. Biomed. Res.. 2018;7:15.
- [CrossRef] [Google Scholar]
- Effect of Shuangjinlian mixture on oral ulcer model in rat. Saudi J. Biol. Sci.. 2019;26:790-794.
- [CrossRef] [Google Scholar]
- Assessment of salivary and serum antioxidant status in patients with recurrent aphthous stomatitis. Med. Oral Patol. Oral Cir. Bucal. 2010;15:e557-e561.
- [CrossRef] [Google Scholar]
- Oral ulcers: clinical aspects. A tool for dermatologists. Part I. Acute Ulcers. Clin Exp Dermatol. 2009;34:289-294.
- [CrossRef] [Google Scholar]
- Immunolocalization of tumor necrosis factor-alpha expressing cells in recurrent aphthous ulcer lesions (RAU) J. Oral Pathol. Med.. 2000;29:19-25.
- [CrossRef] [Google Scholar]
- Stabilization of collagen fibrils by hydroxyproline. Biochemistry. 1986;25:3184-3188.
- [CrossRef] [Google Scholar]
- An overview of acetic acid ulcer models–the history and state of the art of peptic ulcer research. Biol. Pharm. Bull.. 2005;28:1321-1341.
- [CrossRef] [Google Scholar]
- Management of recurrent aphthous ulcers exploiting polymer-based Muco-adhesive sponges: in-vitro and in-vivo evaluation. Drug Deliv.. 2021;28:87-99.
- [CrossRef] [Google Scholar]
- Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res.. 2019;233:284-296.
- [CrossRef] [Google Scholar]
- Ulcerative and Inflammatory Lesions of the Oral Mucosa. Oral Maxillofac. Surg. Clin. North Am.. 2023;35:219-226.
- [Google Scholar]
- Content and quality of website information on the treatment of oral ulcers. Br. Dent. J.. 2014;217:E15.
- [CrossRef] [Google Scholar]
- Molecular analysis of the environments of healing and chronic wounds: cytokines, proteases and growth factors. Primary Intention. 1999;7:7-15.
- [Google Scholar]
- Hydroxyproline: A Potential Biochemical Marker and Its Role in the Pathogenesis of Different Diseases. Curr. Protein Pept. Sci.. 2016;17:596-602.
- [CrossRef] [Google Scholar]
- Modes of intermolecular cross-linking in mature insoluble collagen. J. Biol. Chem.. 1965;240:3899-3908.
- [Google Scholar]
- Antioxidant profile of salivary glands in high fat diet-induced insulin resistance rats. Oral Dis.. 2014;20:560-566.
- [CrossRef] [Google Scholar]







