Translate this page into:
Haematology and biochemistry panels in the Ethiopian hedgehog, Paraechinus aethiopicus (Ehrenberg, 1833) from central Saudi Arabia: Establishing reference intervals and assessing variability across sex and hibernation
⁎Corresponding author. obmkkwrc@yahoo.co.uk (Osama B. Mohammed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The Ethiopian hedgehog (Paraechinus aethiopicus) is an insectivore which is known to occur in northern an eastern Africa and in most of the Arabian Peninsula. Few recent studies on the reproduction, parasitology and ecology are available, but none has been published on the hematology and serum biochemistry reference intervals. Hence the present study was conducted in order to establish haematological and biochemical for free ranging apparently healthy hedgehogs using automated haematology and biochemistry analyzers. Variation and differences in blood values between males and females as well as before and after hibernation were evaluated. Blood samples from 50 males and 35 females were used for the establishment of the reference interval for the haematological and biochemical variables. Data from males and females were compared using student’s t-test in the computer program SPSS. Significant sex-related haematological differences were observed in the total WBCs, monocytes, RBCs, MCV and MCH values. Males showed significantly higher WBCs compared to females whereas females showed significantly higher values form monocytes, RBCs, MCV and MCH than males (p < 0.05–0001). Changes in the biochemical profiles were significant in AlP, BUN and Na. ALP and BUN were higher in males while Na was higher in females (p < 0.05–0.01). The effect of hibernation was evidenced by increase in the RBCs, haemoglobin, haematocrit and MCHC and decrease in the total protein and phosphorus in animals after hibernation. These findings are considered the first regarding the reference intervals for the desert hedgehog and it will constitute a basis for any further haematological and biochemical studies.
Keywords
Paraechinus aethiopicus
Haematology
Biochemistry
Reference intervals
Saudi Arabia
- RBC
-
Red blood corpuscle counts
- HB
-
Haemoglobin
- HCT
-
Haematocrit
- WBC
-
White Blood Cell (Leucocytes) counts
- LYMPH
-
Lymphocytes
- MONO
-
Monocytes
- GRANU
-
granulocytes
- PLT
-
Platelet counts
- MPV
-
Mean Platelet Volume
- PCT
-
Plateletcrit
- PDW
-
Platelet Distribution Width
- MCH
-
Mean Corpuscular Haemoglobin
- MCHC
-
Mean Corpuscular Haemoglobin Concentration
- MCV
-
Mean Corpuscular Volume
- ALB
-
Albumin
- ALP
-
Alkaline Phosphatase
- ALT
-
Alanine Transaminase
- AMY
-
Amylase
- TBIL
-
Total Bilirubin
- BUN
-
Blood Urea Nitrogen
- Ca
-
Calcium
- P
-
Phosphorus
- CREAT
-
Creatinine
- GLU
-
Glucose
- Na
-
Sodium
- K
-
Potassium
- TP
-
Total Protein
- GLOB
-
Globulin
- ALB/GLOB
-
Albumin/Globulin Ratio
- BUN/CREAT
-
Blood Urea Nitrogen/Creatinine ratio
Abbreviations
1 Introduction
The Ethiopian or desert hedgehog (Paraechinus aethiopicus) belongs to the mammalian order Insectivora (Lipothyphyla) which is one of the most complicated orders in mammalian taxonomy (Symonds, 2005). This group of animals was once regarded as “waste-basket” as it contains the mammalian species lacking some mammalian characteristics and being insectivores (Butler, 1972; Symonds, 2005). They were reclassified and placed into a separate order (Erinaceomorpha) with two subfamilies (Hutterer, 2005; Bannikova et al., 2014): Erinaceidae (spiny hedgehogs or true hedgehogs) and Galerucinae (hairy hedgehogs; gymnures and moonrats). The Erinaceidae contains five genera and Paraechinus is among them. The desert hedgehog is distributed in Africa from Morocco and Algeria to Egypt, Sudan, Ethiopia, Eretria and Somalia. In the Arabian Peninsula its distribution extends from Yemen, Saudi Arabia through to Jordan, Syria and Iraq (Harrison and Bates, 1991; Kock and Ebenau, 1996). P. aethiopicus is highly adapted to desert and arid environment and they prefer oases and areas with vegetation where food is available.
Only few and scattered reports on the reproduction, parasites and temperature regulation on the desert hedgehog have been published recently (Yamaguchi et al., 2013; Amin et al., 2016; Amin and Heckman, 2016; Abu Baker et al., 2017; Alagaili et al., 2017; Boyles et al., 2017).
Yamaguchi et al. (2013) and Alagaili et al. (2017) studied the reproductive biology of the desert hedgehog and the latter confirmed that it is s seasonal breeder as a result of the progesterone profile in females and the size of the testes in males while the first suggested that free-ranging Ethiopian hedgehogs breed more than twice a year in Qatar based on frequency of courtship behavioural observations. Abu Baker et al. (2017) conducted a research using the GPS to study the effect of land use and sex on the home range size and movement of the Ethiopian hedgehog in Qatar.
Routine health check or screening is generally practiced in many domestic, zoo animals and wildlife in several collections. It involves haematological, biochemical and faecal investigations.
Haematological values play and important role in the determination of the health condition as well as following up any treatment or surgical procedures (Mori et al., 2004; Satue et al., 2009). Haematological profiles from other hedgehog species have been limited to Rossi et al. (2014) and Okorie-Kanu et al. (2015). The haematological and biochemical reference intervals for the European hedgehog (Erinaceus europaeus) were established by Rossi et al. (2014). Whereas, Okorie-Kanu et al. (2015) studied the haematological and biochemical profiles of the African hedgehog (Atelerix albiventris) in Nigeria.
There was no study available on the hematological and biochemical reference intervals of the Ethiopian or desert hedgehog. Therefore, the objective of the present study was to establish reference intervals for desert hedgehog following the guidelines published by the American Society of Veterinary Clinical Pathology (ASVCP). Furthermore, the effect of sex and hibernation on the haematological and biochemical variables was also investigated in the desert hedgehog.
2 Materials and methods
2.1 Animal capture and sampling
Hedgehogs were collected from and around the town of Unizah (26.0840 N, 43.9940 E) (Al Qassim Province), central Saudi Arabia. Animal were captured by hand at night using beam lights to locate hedgehogs around farms. Animals were transported to the animal facility at the Department of Zoology for a different study. Collection of hedgehogs was authorised by the Saudi Wildlife Authority and the research protocol was approved by the animal ethics committee of the University of Pretoria (Ethics number EC017-16) which follows the South African National Standards for animal welfare and research.
Blood sampling was performed under general anesthesia, induced and maintained with 5% of isoflurane (Isoflurane Vet FL VT 250 ml; Merial, Duluth, GA, USA), mixed with oxygen and administered through a facemask. Blood samples were taken from the jugular vein using a 2.5 ml syringe with a 22 ga needle (Lewis et al., 2002). Approximately 1 ml of heparinsed blood was collected from each individual for haematological and biochemical investigations. Animals were weighed while they were under anaesthesia.
2.2 Inclusion and exclusion criteria
According to the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), all animals included in the present study are apparently clinically healthy and not showing any signs of disease at the time of sampling. Hedgehogs were excluded when they showed one of the following criteria: showing signs of any disease, lactating, pregnant, juvenile, or showing infestation of external parasites. A total number of 85 hedgehogs (50 males and 35 females) were included in this study. Blood samples collected from animals (n = 21) during October and November were considered for the set of samples before hibernation. While those collected (n = 19) during January and February represented those animals after hibernation.
2.3 Haematological and biochemical investigations
The heparinised blood samples were analysed immediately after collection using VetScan HM5 (Abaxis Veterinary Diagnostics, Union City, CA 94587, USA) for determination of erythrocyte (Red blood corpuscles) counts (RBC), haemoglobin (HB), haematocrit (HCT), leucocyte (white blood cell) counts (WBC), lymphocytes, monocytes, granulocytes, platelet counts (PLT), mean platelet volume (MPV), plateletcrit (PCT) and platelet distribution width (PDW). Erythrocyte indices were calculated from the values of RBC, HB and HCT which included mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and mean corpuscular volume (MCV).
Blood biochemistry from the heparinised blood was also performed using the biochemistry analyser VetScan VS2 (Abaxis Veterinary Diagnostics, Union City, CA 94587, USA) using the comprehensive profile rotor. The biochemical variables determined included: albumin (ALB), alkaline phosphatase (ALP), alanine transaminase (ALT), amylase (AMY), total bilirubin (TBIL), blood urea nitrogen (BUN), calcium (Ca), phosphorus (P), creatinine (CREAT), glucose (GLU), sodium (Na), potassium (K), total protein (TP) and globulin (GLOB). ALB/GLOB and BUN/CREAT ratios (B/C ratio) were calculated from the values obtained for these variables.
2.4 Statistical analyses
Reference Intervals (RI) were determined using an Excel (Excel; Microsoft Corp., Redmond, WA, USA) spreadsheet with the Reference Value Advisor (version 2.0) set of macroinstructions (Geffre et al., 2011) that performs computations following the IFCC-CLSI (International Federation of Clinical Chemistry, Clinical and Laboratory Standards Institute) recommendations as also suggested by ASVCP guidelines (Clinical and Laboratory Standards Institute, 2008). The computations include common descriptive statistic (e.g., sample size, mean, median, SD, and minimum and maximum values), test of normality according to Anderson–Darling with histograms and Q-Q- plots, and Box–Cox transformation.
To detect outliers, 2 different tests were used: Dixon–Reed and Tukey’s test. Following the ASVCP guidelines, outliers classified as “suspected” by the software were retained. Conversely, far outliers were removed from the analysis. Calculation of RIs was based on the assumption about data distribution and multiple RIs are reported, obtained by using standard and robust methods on both non transformed and transformed data. The 95% RI and the 90% CI for the upper and lower RIs were calculated using nonparametric methods. Comparison between males and females was performed using the student’s t-test in the Statistical Package of Social Science computer program SPSS (2009) ver 18.
3 Results
There was no significant difference in the body weight (gm) between males (345 ± 90.9) and females 347 ± 101.5) in the present study (p = 0.89). Animals which were weighed before hibernation (363.14 ± 115.2) were found to be significantly heavier than animals after hibernation (331.35 ± 66.2) (p = 0.01).
3.1 Hematology
The hematological reference intervals of male and female desert hedgehog are presented in Tables 1 and 2 respectively. Results showed no significant differences In haematological variables between the male and female hedgehogs except for changes reported in the WBCs, monocytes, RBCs, MCV and MCH. The WBCs were significantly higher in males (p < 0.05) while the monocytes (p < 0.05), RBCs (p < 0.001), HCT (p < 0.05) and MCH (p < 0.05) were significantly higher in females.
Analyte
SI Units
Mean
SD
Median
Min
Max
RI
Lower Ref Lim 90% CI
Upper Ref Lim 90% CI
WBC
109/l
7.439
2.978
7.390
2.25
13.82
2.5–13.6
2.3–3.4
12.7–13.8
LYMPH
109/l
3.3
1.5
3.3
1.1
6.7
1.1–6.6
1.1–1.5
6.2–6.7
MONO
109/l
0.29
0.23
0.29
0.06
0.93
0.06–0.93
0.06–0.08
0.83–0.93
GRANULO
109/l
3.42
1.9
3.09
0.85
8.93
0.86–8.56
0.9–1.1
6.7–8.9
LYMPH
%
36.04
14.87
37.10
3.90
66.19
16.7–79.8
16.6–25.6
75.7–80.1
MONO
%
2.52
1.51
2.59
0.61
5.75
0.65–12.1
0.6–1.1
10.2–12.4
GRANULO
%
46.2
13.9
46.1
17.6
75.3
18.4–74.5
17.6–26.6
68.1–75.3
RBC
1012/l
3.7
1.2
3.69
0.66
6.398
3.7–7.9
3.5–4.4
7.5–7.9
HGB
g/dl
11.29
1.54
11.24
8.0
14.8
8.2–14.6
8.0–8.9
13.914.8
HCT
%
35
5.1
35
24
46
24.3–45.1
23.6–26.3
43–45.6
MCV
fl
59
4.3
59
52
68
52.4–68
52–53.4
67–68
MCH
pg
19.1
1.5
18.9
16.5
23.1
16.8–22.6
16.5–16.8
21.523.1
MCHC
g/dl
32
1.4
31.7
29.8
35.6
29.9–35.6
29.8–30.1
34.7–35.6
RDWc
%
19.8
1.9
19.3
17.7
26.6
17.7–25.01
17.7–18.3
22.4–26.6
PLT
109/l
128.4
140.8
74.6
12
452
12.0–451
12.0–13.1
411–452
PCT
%
0.13
0.17
0.07
0.01
0.45
0.01–0.45
0.01–0.01
0.42–0.54
MPV
fl
9.6
2.6
9.8
0.2
13.5
1.1–13.5
0.2–6.9
13.0–13.5
PDWc
%
38.2
5.2
38.6
29
45.5
29.1–45.4
29–30.3
44.3–45.5
Analyte
SI Units
Mean
SD
Median
Min
Max
RI
Lower Ref Lim 90% CI
Upper Ref Lim 90% CI
WBC
109/l
6.0
2.6
6.0
0.9
10.5
1.8–15.2
0.5–3.2
13.2–17.1
LYMPH
109/l
3.5
1.3
1.996
0.31
4.2
0.4–7.4
0.04–1.1
6.3–8.4
MONO
109/l
0.67
0.35
0.61
0.22
1.43
0.18–1.58
0.13–0.25
1.24–1.96
GRANULO
109/l
4.3
1.9
4.3
0.53
8.1
0.45–9.1
0.24–1.37
7.56–10.0
LYMPH
%
41.9
10.9
41.5
24.6
68.7
23.9–69.0
21.42–28.0
60.12–78.1
MONO
%
6.88
2.9
6.60
1.9
12.3
0.73–12.9
0.44–2.15
11.25–14.4
GRANULO
%
51.36
10.9
52.00
23.1
70.1
24.9–70.9
14.6–33.5
66.5–75.2
RBC
1012/l
5.9
1.1
6.2
3.6
7.7
3.2–7.9
2.6–3.9
7.6–8.3
HGB
g/dl
11.57
1.81
11.70
8.1
14.6
7.6–15.1
6.7–8.6
14.4–15.7
HCT
%
36.3
6.7
34.9
24.3
55.7
24.2–51.9
22.2–26.7
47.9–57.2
MCV
fl
62.0
5.8
61.0
54
76
52.7–78.0
51.2–54
72.3–84
MCH
pg
19.9
2.3
19.70
17
30
17.1–26.5
16.7–17.5
24.3–30.3
MCHC
g/dl
31.5
2.2
31.6
21.1
34.9
25.0–34.4
22.1–28.3
33.7–35
RDWc
%
20.10
2.48
19.70
16.5
31.5
16.6–31.2
16.5–17.8
22.9–31.5
PLT
109/l
150
105
112
10
361
80.7–363
22.6–44.5
280.9–429
PCT
%
0.14
0.10
0.14
0.01
0.48
0.12–0.45
0.01–0.08
0.35–0.57
MPV
fl
10.02
2.69
9.80
0.3
14
4.14–14.7
1.91–6.1
13.56–15.7
PDWc
%
38.73
4.87
39.7
22
45
24.9–45.9
24.9–30.8
46.32–46.7
3.2 Serum biochemistry profiles
Serum biochemistry reference interval for male and female hedgehogs are presented in Tables 3 and 4 respectively. Significant differences in the values of ALP, BUN and Na were detected between males and females. ALP and BUN were significantly higher in females (p < 0.05) while Na was higher in males’ hedgehogs (p < 0.01).
Analyte
SI Units
Mean
SD
Median
Min
Max
RI
Lower Ref Limit 90% CI
Upper Ref Limit 90% CI
ALB
g/L
19.3
3.3
19.5
10
26
13.0–26.0
11.7–14.4
24.7–27
GLOB
g/L
49.9
7.9
50.1
28
66
34.2–66.0
31.3–36.9
62.5–68.7
TP
g/L
69.0
8.0
69.3
48
86
53.1–85.6
50.4–55.7
82.3–88.2
ALP
U/L
42.9
14.6
41.5
20
78
12.1–70.8
10.5–18.4
63.5–78
ALT
U/L
141.6
49.8
133.7
69
258
33.9–233.4
17.6–58
211.1–261
AMY
U/L
378.6
69.1
378.1
259
496
238.7–517.4
212–268
486–545
TBIL
µmol/L
0.25
0.05
0.30
0.2
0.3
0.15–0.36
0.14–0.17
0.34–0.36
BUN
mmol/L
13.2
3.4
13.4
4.3
19.6
6.7–20.2
5.6–7.8
18.9–21
CA
mmol/L
2.7
0.3
2.7
1.8
3.6
2.0–3.3
1.8–2.2
3.2–3.3
PHOS
mmol/L
2.4
0.6
2.3
1.3
5.3
1.1–3.6
0.7–1.6
3.1–4.1
CRE
µmol/L
38.2
14.5
36.2
17.7
79.6
7.2–65.2
5.6–14.6
57.9–73.4
GLU
mmol/L
6.7
0.2
6.7
4.6
9.0
4.3–9.1
4.0–4.8
8.6–9.5
NA
mmol/L
135.6
4.0
135.2
127
148
127.1–143.2
125.7–128.9
141.5–145.7
K
mmol/L
5.1
0.63
5.07
4.0
6.5
3.76–6.37
3.52–4.1
6.1–6.6
ALB/GLOB
ratio
0.39
0.1
0.38
0.19
0.71
0.17 – 0.59
0.13–0.23
0.35–065
BUN/CREAT
ratio
0.38
0.14
0.37
0.12
0.77
0.08–0.66
0.04–0.14
0.59–0.72
Analyte
SI Units
Mean
SD
Median
Min
Max
RI
Lower Ref Lim 90% CI
Upper Ref Lim 90% CI
ALB
g/L
18.1
4.9
17.7
10
28
7.7–27.8
5.6–10.4
25.1–30.5
GLOB
g/L
51.0
6.5
50.8
39
64
37.5–64.1
34–41
60.7–67.2
TP
g/L
69.8
8.1
69.7
55
85
53.2–86.2
49.9–57
82.1–89.9
ALP
U/L
49.9
14.7
50.4
17
74
20.3–80.5
13.2–27.3
73.2–87.2
ALT
U/L
143.3
54.3
135.4
50
267
25.2–245.7
6.1–58
216.9–278
AMY
U/L
401.4
81.5
408.8
227
560
242.8–574.9
201.2–275
531.9–605
TBIL
µmol/L
0.26
0.08
0.26
0.2
0.4
0.1–0.4
0.11–0.14
0.36–0.43
BUN
mmol/L
14.7
3.5
14.8
6.1
21.8
7.8–22.0
6.1–9.2
20.1–23.5
CA
mmol/L
2.8
0.34
2.7
2.1
3.6
2.0–3.4
1.8–2.2
3.2–3.7
PHOS
mmol/L
2.4
0.43
2.4
1.7
3.3
1.5–3.3
1.4–1.8
3.1–3.5
CRE
µmol/L
40
17.2
38.1
17.7
79.6
6.2–73.9
0.7–13.2
63.8–81.9
GLU
mmol/L
7.2
1.1
7.5
4.1
8.9
5.2–9.7
4.8–6.1
8.9–9.8
NA
mmol/L
132.7
4.5
133.0
123
139
123.9–142.1
121.9–125
140.1–143
K
mmol/L
4.86
0.73
4.74
3.5
6.5
3.26–6.23
2.99–3.72
5.87–6.7
ALB/GLOB
ratio
0.36
0.11
0.34
0.21
0.67
0.11–0.57
0.06–0.18
0.53–0.64
BUN/CREAT
ratio
0.44
0.22
0.42
0.13
0.93
0.04 – 0.87
0.01–0.09
0.73–0.99
3.3 Effect of hibernation on the haematological and biochemical variables
The weight of the animals after hibernation was lower (331 ± 25) than what they were before hibernation (363 ± 16), however, the differences in weight was not significant (p = 0.3140) (Fig. 1). There was a noticeable increase in most of the haematological values in animals which were sampled post hibernation (Table 5). However, the increase in the values of the RBCs, HB, HCT and MCHC collected from animals post hibernation was significant (p < 0.05). On the other hand, there was a decrease in most biochemical variable investigated in the animals post hibernation. There was a significant decrease (p < 0.05) in the TP and P in animals post hibernation unlike most other biochemical variables (Table 6).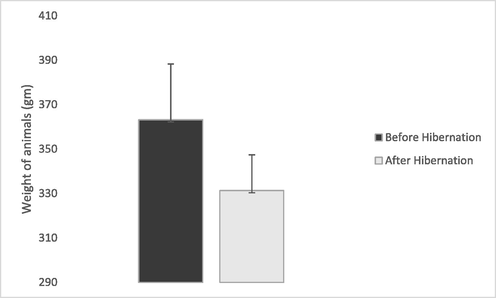
Body weight in grams of hedgehogs (Mean ± s.e.) before and after hibernation.
Parameter
Units
Before Hibernation (n = 21)
After Hibernation (n = 17)
p value
Mean ± SD
Range
Mean ± SD
Range
WBC
109/l
7.81 ± 2.7
3.8–12.6
7.83.3 ± 3.3
4.4–13.8
1.00
LYMPH
109/l
3.42 ± 1.9
1.1–6.7
3.43 ± 1.5
1.5–6.7
1.00
MONO
109/l
0.46 ± 0.25
0.17–0.93
0.39 ± 0.19
0.24–0.87
0.47
GRANULO
109/l
3.98 ± 2.0
2.2–8.9
3.56 ± 1.7
1.7–6.9
0.62
LYMPH
%
42.87 ± 16.6
16.6–69.6
48.7 ± 10.9
31.4–66.6
0.319
MONO
%
5.31 ± 2.3
2.0–9.2
4.8 ± 2.0
2.4–6.8
0.559
GRANULO
%
51.77 ± 15.4
26.6–75.3
46.52 ± 11.3
27.0–66.0
0.349
RBC
1012/l
5.1 ± 1.01
3.6–7.01
6.3 ± 1.06
3.5–7.9
0.002**
HGB
g/dl
10.3 ± 1.7
8.1–14.4
12.4 ± 1.4
10.7–14.8
0.0008***
HCT
%
30.6 ± 4.6
24.3–43.5
37.7 ± 5.6
23.6–45.6
0.0003***
MCV
fl
59.8 ± 4.6
54.0–67.0
61.1 ± 5.3
52.0–68.0
0.475
MCH
pg
20.4 ± 2.9
17.3–30.0
19.5 ± 31.7
16.8–21.5
0.065
MCHC
g/dl
33.0 ± 1.4
30.7–35.6
31.9 ± 1.2
30.1–34.6
0.034*
RDWc
%
20.5 ± 2.1
16.5–25.3
19.8 ± 1.3
17.5–27.2
0.238
PLT
109/l
63.3 ± 83.9
11.0–361.0
107.2 ± 105.8
38.0–452
0.247
PCT
%
0.05 ± 0.1
0.01–0.48
0.15 ± 0.07
0.04–0.28
0.357
MPV
fl
9.1 ± 3.5
0.3–13.5
10.8 ± 3.9
6.1–13.4
0.618
PDWc
%
37.9 ± 3.9
30.2–44.7
34.8 ± 10.7
9.2–44.2
0.952
Parameter
Units
Before Hibernation (n = 18)
After Hibernation (n = 19)
p value
Mean ± SD
Range
Mean ± SD
Range
ALB
g/L
19.1 ± 4.5
10–26
18.3 ± 3.4
10–25
0.537
GLOB
g/L
52.8 ± 8.1
39–66
47.7 ± 5.9
40–64
0.051
TP
g/L
71.9 ± 8.7
57–86
66.1 ± 6.4
56–80
0.04*
ALP
U/L
58.5 ± 12.9
34–74
50.2 ± 14.5
30–78
0.129
ALT
U/L
135.1 ± 48
70–267
116.7 ± 42.8
52–198
0.245
AMY
U/L
388.1 ± 75.9
248–549
447.6 ± 70.9
320–560
0.064
TBIL
µmol/L
0.24 ± 0.06
0.2–0.4
0.23 ± 0.05
0.2–0.3
0.594
BUN
mmol/L
13.7 ± 4.0
4.3–18.5
14.4 ± 3.4
7.0–19.9
0.522
CA
mmol/L
2.9 ± 0.39
1.8–3.5
2.7 ± 0.18
2.4–3.1
0.051
PHOS
mmol/L
2.5 ± 0.47
1.2–3.2
2.1 ± 0.23
1.7–2.4
0.0003***
CREAT
µmol/L
43.7 ± 14.1
26.5–79.6
44.2 ± 13.9
26.5–79.6
0.915
GLU
mmol/L
7.2 ± 0.95
5.3–8.9
7.15 ± 1.2
5.6–8.8
1.0
NA
mmol/L
134.8 ± 4.4
124–140
134.4 ± 2.8
128–138
0.744
K
mmol/L
5.1 ± 0.68
3.6–6.5
4.8 ± 0.56
3.9–8.0
0.162
ALB/GLOB
ratio
0.4 ± 0.2
0.21–0.67
0.39 ± 0.08
0.2–0.5
0.557
BUN/CREAT
ratio
0.33 ± 0.13
0.12–0.55
0.35 ± 0.10
0.16–0.52
0.697
4 Discussion
In the present study, the reference intervals for the haematology and biochemistry variables were established for the desert hedgehog (Paraechinus aethiopicus) in Saudi Arabia. This report constitutes the first report of haematological and biochemical data on the desert hedgehogs throughout its range of distribution in Africa and Asia. The haematological as well as the biochemical variables are important for disease diagnosis and prognosis of almost all animal species. They are also useful in the follow up of treatment of any condition from which a particular animal species is suffering from (Cebuli-Kaudune et al., 2002). Comparisons were made between male and female hedgehogs collected from the same environment. The effect of hibernation on the haematology and biochemistry variables was also evaluated in the present study.
Few reports are available on the normal haematological and biochemical values of the European (Erinaceus europaeus) as well as the African (Atelerix albiventris) hedgehogs (Lewis et al., 2002; Bexton and Robinson, 2003; Rossi et al., 2014; Okorie-Kanu et al., 2015). Rossi et al. (2014) documented the reference intervals of the European hedgehog and they found out there was relatively high counts of RBCs and platelets. In the present study as well as in Okorie-Kanu et al. (2015) lower values for RBCs have been recorded unlike what has been reported by Rossi et al. (2014). The reference interval for platelets was comparable to what has been reported by Rossi et al. (2014). The increase in the RBCs and the reticulocytes in other species may suggest erythroid regeneration due to erythropoietin release or in cases of nonregenerative anaemia (Cowgill et al., 2003; Barger, 2010; Tvedten, 2010). Rossi et al. (2014) attributed to the increase in RBCs and reticulocytes counts to the age of the animals they investigated as they were mostly juvenile and such group of animals have intense erythropoiesis. They also related their finding to the possibility of splenic contractions due to animal stress and suggested that RBCs in hedgehogs are probably released to blood earlier than in other animal species, a hypothesis which requires further investigation.
In our investigation, unexpectedly, the RBCs count in the females was significantly higher than in males. The sex specific difference in RBCs count was generally attributed to the effect of androgens which is believed to stimulate erythropoiesis (Shahidi, 1973). Other investigators have reported no relationship between the gender and differences in haematologic profile in the pronghorn antelope (Antelope americana). In women with adrenal hyperplasia and Cushing’s syndrome polycythemia was reported (Shahani et al., 2009) hence, the status of the female adrenal glands of hedgehogs requires further studies to find out if there is any evidence to explain the increased RBCs count in females. The platelets count in the present study which showed high 90% upper reference limit same as what has been found by Rossi et al. (2014) can be a characteristic to this group of animal species. Similar finding was recognized earlier by Alagaili et al. (2013) in the Libyan jird (Meriones libycus) and they explained their finding as a natural feature of that particular species. The same may applies to the desert hedgehog as it lives under the same desert conditions. The MCV was in agreement with what has been reported by Rossi et al. (2014) and females showed significantly higher value compared to males. The MCV values overlap in the male and female which suggest that the difference is statistically significant but it does not have biological relevance and it may not affect the interpretation of the haematologic variables. The white blood cell count in the present study was comparable to what has been reported by Rossi et al. (2014), however, they were much lower that what has been recorded by Okorie-Kanu et al. (2015) for the African hedgehog. Monocytes were significantly higher in males compared to females in the present study. Monocytes are generally increased in cases of chronic infections. The increased monocytes in the desert hedgehogs requires further investigations.
The reference intervals for the biochemical profiles were comparable with previous reports from other species of hedgehogs (Rossi et al., 2014; Okorie-Kanu et al., 2015). However, significant increase in the female BUN values compared to males. BUN is generally utilized to evaluate the renal function in different animal species. Although no apparent clinical signs of renal impairment were noted especially if we considered the creatinine values recorded in the present study. BUN level is affected by circadian rhythm, diet, liver function, hydration and intestinal absorption (Melillo, 2007). However, the observed increase in the BUN concentrations could be due to one of these reasons which requires further investigation. The ALP levels in the desert hedgehog are far much lower than those reported from the European hedgehogs and they are slightly higher than those reported from the African hedgehog. The increased levels of ALP are always associated with liver, gallbladder or bone affections or it is age related. In the present study the ALP level in females was found to be significantly higher than in males which may have been age-related or due to intestinal isoenzyme that contributes to the total ALP activity in some animal species such as rodents in case of fasting or damage (Wada et al., 2001). This point requires further investigations in future studies.
There was a change in the animals’ body weight post hibernation being lower than before hibernation despite the period of hibernation was one month. Weight loss in hibernating animals was noticed previously in some animal species such as arctic ground squirrel and the common dormouse (Buck and Barnes, 1999; Csorba, 2003). During hibernation animals such as the borwn bear decrease metabolism and reduce renal and liver function and a rise in HB, HCT and RBCs counts were noticed (Græsli et al., 2015). In the present study hedgehogs after hibernation showed high values for haematological variables and the change was significant in the RBCs, HB, HCT and MCHC. Conversely, animals after hibernation showed lower values for most of the variables studied and the reduction in the values of the TP and P were significant. The variation in the haematological variables in the erythrocytes and their indices has been noticed in other hibernating animal species such as, echidnas and bears (Seal et al., 1967; Anderson et al., 2000). A notable observation was the increase in the MCHC in hibernating echidnas which is in agreement with Anderson et al. (2000). The high values for MCHC means higher HB concentration in an individual RBC hence, a higher oxygen-delivery capacity by circulating RBCs and this could be an adaptation for hibernating animals to maintain oxygen delivery when the blood flow is reduced during hibernation. Furthermore, RBCs in hibernating individuals are not replaced quickly and they are ageing and in a senescent state hence show increased HB content in contrast to newly formed RBCs which has low MCHC (Nikinmaa, 1990). An acceptable explanation of the increase in RBCs indices may be related the effect of the reduction in blood volume during hibernation as has been shown in the northern birch mouse (Elżbieta, 1985). Investigation of the blood volume in hibernating hedgehogs may prove this assumption. Body proteins in the European hedgehog (Erinaceus europaeus) represent a significant proportion of body mass loss, and their utilization covers 9% of the energy expenditure (Cherel et al., 1995). The reduction in the total protein in the desert hedgehogs after hibernation was likely linked to energy utilization. Loss or reduction in total protein is associated with body water because each gram of protein requires 2–4 g of intracellular water (Cahill et al., 1970).
5 Conclusion
Results from the present study provide RI for a number of haematological and biochemical variables in the hedgehogs in central Saudi Arabia, and demonstrate the importance of the effect of gender as well as the effect of hibernation on these variables. These RI constitutes a baseline data to which will give accurate indicators for healthy hedgehog. It will undoubtedly help in evaluating the health conditions of hedgehogs in central Saudi Arabia. Results indicated that hedgehogs lose weight during hibernation and also hibernation was associated with increase in the HB, HCT and RBCs count. Additional controlled studies may explain the physiology of hibernation in the desert hedgehog.
Acknowledgements
This project was financially supported by the Vice Deanship of Research Chairs, Deanship of Scientific Research of the King Saud University, Kingdom of Saudi Arabia.
Authors’ contributions
OBM conceived the study, ANA, NMSA, OBM, SAO conducted the laboratory work and data analysis. OBM drafted the manuscript and all authors read it and approved it.
Conflict of interest
The authors OBM, NMSA, SAO, and ANA declare that they have no conflict of interest.
References
- Abu Baker, M.A., Reeve, N., Conkey, A.A.T., Macdonald, D.W., Yamaguchi, N., 2017. Hedgehogs on the move: Testing the effects of land use change on home range size and movement patterns of free-ranging Ethiopian hedgehogs. PLoS ONE 12, e0180826. DOI: 10.1371/journal.pone.0180826.
- Body temperature patterns of a small endotherm in an extreme desert environment. J. Arid Environ.. 2017;137:16-20.
- [CrossRef] [Google Scholar]
- Reference data of haematology and serum biochemistry in adult wild-caught Libyan jird (Meriones libycus) from central Saudi Arabia. J. King Saud Univ.- Sci.. 2013;25(4):307-311.
- [CrossRef] [Google Scholar]
- Anderson, N.A., Mesch, U., lovell, D.J., Nicol, S.C., 2000. The effect of sex, season, and hibernation on the haematology and blood viscosity of free-ranging echidnas (Tachyglossus aculeatus). Canad. J. Zool. 78, 174–181. DOI: 10.1139/z99-199
- Nematodes and cestodes from the desert hedgehog, Paraechinus aethiopicus (Ehrenberg) in Central Saudi Arabia, revealed by SEM and microscopy,with notes on histopathology. Sci. Parasitol.. 2016;17:26-35.
- [Google Scholar]
- Morphological and moleculardescriptions of Moniliformis saudi sp. n. (Acanthocephala: Moniliformidae) from the deserthedgehog, Paraechinus aethiopicus (Ehrenberg) in Saudi Arabia, with a key to species and noteson histopathology. Folia Parasitol.. 2016;63:014
- [CrossRef] [Google Scholar]
- Contrasting evolutionary history of hedgehogs and gymnures (Mammalia: Erinaceomorpha) as inferred from a multigene study: Evolutionary History of Erinaceidae. Biol. J. Linn. Soc. Lond.. 2014;112(3):499-519.
- [CrossRef] [Google Scholar]
- Erythrocyte morphology. In: Weiss D.J., Wardrop K.J., eds. Schalm’s Veterinary Hematology (sixth ed.). Ames, IA: Wiley-Blackwel; 2010. p. :142-151.
- [Google Scholar]
- Hedgehogs. In: Mullineaux E., Best R., Cooper J.E., eds. BSAVA Manual of Wildlife Casualties. London, UK: British Small Animal Veterinary Association Publication; 2003. p. :49-65.
- [Google Scholar]
- Torpor Patterns in Desert Hedgehogs (Paraechinus aethiopicus) Represent Another New Point along a Thermoregulatory Continuum. Physiol. Biochem. Zool.. 2017;90(4):445-452.
- [CrossRef] [Google Scholar]
- Annual Cycle of Body Composition and Hibernation in Free-Living Arctic Ground Squirrels. J. Mammal.. 1999;80(2):430-442.
- [CrossRef] [Google Scholar]
- The problem of insectivore classification. In: Joysey K.A., Kemp T.A., eds. Studies in Vertebrate Evolution. Edinburgh: Oliver and Boyd; 1972. p. :253-265.
- [Google Scholar]
- Fat and nitrogen metabolism in fasting man. In: Jeanrenaud B., Hepp D., eds. Adipose tissue: regulation and metabolic functions. New York: Academic Press; 1970. p. :181-185.
- [Google Scholar]
- The influence of age and gender on haematological variables in Lipizzan horses. J. Vet. Med.. 2002;49:217-221.
- [CrossRef] [Google Scholar]
- Protein and lipid utilization during fasting with shallow and deep hypothermia in the European hedgehog (Erinaceus europaeus) J. Comp. Physiol. B.. 1995;164:653-658.
- [CrossRef] [Google Scholar]
- Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guidelines (third ed.). Wayne, PA: CLSI; 2008.
- Clinical application of reticulocyte counts in dogs and cats. Vet. Clin. North Am. Small Anim. Pract.. 2003;33:1223-1244.
- [CrossRef] [Google Scholar]
- Influence of body weight on hibernation of the common dormouse (Muscardinus avellanarius) Acta Zool. Hung.. 2003;49(Suppl. 1):39-44.
- [Google Scholar]
- Hematology of a hibernating rodent—the northern birch mouse. Acta Theriol.. 1985;30:337-348.
- [Google Scholar]
- Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet. Clin. Pathol.. 2011;40:107-112.
- [CrossRef] [Google Scholar]
- Seasonal variation in haematological and biochemical variables in free-ranging subadult brown bears (Ursus arctos) in Sweden. BMC Vet. Res.. 2015;11:301.
- [Google Scholar]
- The Mammals of Arabia (second ed.). Sevenoaks, Kent, UK: Harrison Zoological Museum Publications; 1991. p. :354.
- Hutterer, R., 2005. Order Erinaceomorpha, In: Wilson, D.E., Reeder, D.M. (Eds.) Mammal Species of the World, third ed., JHU Press, Baltimore, USA, pp. 212–219.
- The desert hedgehog, Paraechinus aethiopicus (Ehrenberg, 1833), new to the fauna of Syria. Z. Säugetierkd.. 1996;61:189-191.
- [Google Scholar]
- Normal haematological values of European hedgehogs (Erinaceus europaeus) from an English rehabilitation centre. Vet. Rec.. 2002;9:567-569.
- [Google Scholar]
- Reference values on haematologic variables of the Brazilian donkeys (Equus asinus) breed. J Equine Vet. Sci.. 2004;24:271-276.
- [CrossRef] [Google Scholar]
- Vertebrate red blood cells. Berlin: Springer-Verlag; 1990.
- Normal haematological and serum biochemistry values of African hedgehog (Atelerix albiventris) Comp. Clin. Pathol.. 2015;24:127-132.
- [CrossRef] [Google Scholar]
- Hematologic and biochemical variables of hedgehogs (Erinaceus europaeus) after overwintering in rehabilitation centers. Vet. Clin. Pathol.. 2014;43:6-14.
- [CrossRef] [Google Scholar]
- Age-related differences in the haematological profile of Andalusian broodmares of Carthusian strain. Vet. Med-Czech.. 2009;54:175-182.
- [Google Scholar]
- Androgens and erythropoiesis: past and present. J. Endocrinol. Invest.. 2009;32:704-716.
- [Google Scholar]
- SPSS., 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.
- Phylogeny and life histories of the “Insectivora”: controversies andconsequences. Biol. Rev. Camb. Philos. Soc.. 2005;80:92-128.
- [CrossRef] [Google Scholar]
- Laboratory and clinical diagnosis of anemia. In: Weiss D.J., Wardrop K.J., eds. Schalm’s Veterinary Hematology (6th ed.). Ames, IA: Wiley-Blackwell; 2010. p. :152-161.
- [Google Scholar]
- Distribution and properties of rat intestinal alkaline phosphatase isoenzymes. Exp. Anim.. 2001;2:153-158.
- [Google Scholar]
- Show more Timing of breeding in free-ranging Ethiopian hedgehogs, Paraechinus aethiopicus, from Qatar. J. Arid Environ.. 2013;9:1-4.
- [CrossRef] [Google Scholar]







