Translate this page into:
Growth inhibitory potential of quinone-rich fraction of Plumbago zeylanica L. Against MG-63 osteosarcoma cells via Bax/Bcl-2 modulation
⁎Corresponding author at: University Center for Research & Development, Chandigarh University, Gharuan, Mohali 140413, Punjab, India. wrajamp2009@gmail.com (Vaseem Raja),
⁎⁎Corresponding author at: Department of Botanical & Environmental Sciences, Guru Nanak Dev University, Amritsar 143005, India. satwinderjeet.botenv@gndu.ac.in (Satwinderjeet Kaur)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
The objectives of this study were to explore the cytotoxic potential of different fractions (Hexane, chloroform, ethyl acetate and methanol) of Plumbago zeylanica L. root powder.
Methodology
In this study, PzMH, PzMC, PzME, and PzMM fractions from the root powder of P. zeylanica were obtained through the cold maceration technique. The cytotoxic properties of these fractions on MG-63 cells were assessed using the MTT assay. The most potent fraction was further analyzed to understand its cytotoxic mechanism. To explore its apoptosis-inducing potential, microscopic and flow cytometric studies were conducted. Additionally, western blot analysis was used to examine the expression levels of proteins associated with apoptotic pathways.
Results
PzMH showed the highest cytotoxicity against MG-63 osteosarcoma cells, with a GI50 value of 53.07 µg/ml. Flow cytometric studies revealed an upsurge in reactive oxygen species and disruption of mitochondrial membrane potential. The highest concentration of PzMH fraction (183.48 µg/ml) led to 62 % cell population arrest at G0/G1 stage. The anti-apoptotic protein Bcl2 was downregulated, with p53, cleaved-caspase 9, cleaved-caspase3, Bad and Bax expression upregulated. HPLC analysis revealed the presence of lawsone, p-coumaric acid, plumbagin, and 4-hydroxy benzaldehyde, indicating that the quinone-rich fraction of Plumbago zeylanica L. attenuates the proliferation of MG-63 osteosarcoma cells through the Bax/Bcl-2 pathway.
Conclusion
To the best of our current knowledge, this marks the first documented case where the hexane fraction enriched with quinones extracted from the roots of P. zeylanica demonstrates its ability to induce apoptosis by influencing the Bax/Bcl2 pathway.
Keywords
Cell cycle
Mitochondrial membrane potential
Osteosarcoma
Plumbago zeylanica L.
P53
- Rh-123
-
Rhodamine-123
- PVDF
-
Polyvinylidene fluoride
- PI
-
Propidium Iodide
- PAGE
-
Polyacrylamide gel electrophoresis
- MG-63
-
Human Osteosarcoma Cell line
- LA
-
Late apoptosis
- FBS
-
Fetal Bovine Serum
- EB
-
Ethidium bromide
- EDTA
-
Ethylenediaminetetraacetic acid
- EA
-
Early apoptosis
- DMSO
-
Dimethyl Sulfoxide
Abbreviations
1 Introduction
Last few decades have witnessed a tremendous advancement in health care system, still cancer continues to be a major global public health issue and remains one of the leading causes of death worldwide. The rising incidence of cancer is particularly evident in Africa, Asia, and Central and South America, which together account for more than 70 % of all cancer-related deaths worldwide. (Nguyen et al.,2020). Based on the data from the Global Cancer Observatory, lung cancer emerged as the predominant etiology of cancer related mortality globally in the year 2020 (Chhikara & Parang, 2022). A substantial proportion of lung cancer cases are typically diagnosed at an advanced stage, characterized by metastatic spread. Notably, bone metastasis gradually manifest in the majority of individuals affected by lung cancer. Compared to the 12 to 15 months of survival typically seen in patients with metastatic lung adenocarcinoma, those with bone metastass from lung cancer face a grim outlook, with median overall survival ranging between 6 and 8 months. (Chambard et al.,2018). One of the most common malignant primary bone cancers is osteosarcoma (OS), which has a peak frequency either in young people or people older than 50 years. (Rabelo et al., 2022). The 5-year overall survival rate of nonmetastatic OS patients is nearly 77 %, whereas the 5-year overall survival rate of metastatic OS patients is less than 20 %. The possible causes of osteosarcoma can be prior therapeutic radiotherapy, tall height, heavy weight at the time of birth and many pre-existing cancer susceptibility syndromes, such as Li-Fraumeni syndrome, Paget’s disease and inherited retinoblastoma (Cole et al.,2022). Due to some biochemical intricacies involved in tumor formation, the specific etiology of bone cancer is still unknown. Cell cycle deregulation and lack of apoptosis are considered essential hallmarks of cancer cells (Koh et al., 2020). Genetic alterations, coupled with dysregulated expression of cell cycle regulators and the loss of tumor suppressor alleles, have been associated with the inactivation of tumor suppression mechanism. This inactivation allows cells to bypass crucial checkpoints and undergo uncontrolled proliferation (Abreu Velez & Howard, 2015).
Malignant diseases frequently present formidable challenges when it comes to their treatment using conventional therapeutic approaches, primarily due to the dual burdens of economic constraints and the debilitating side effects associated with these treatments. Research shows phytochemicals have potential for chemoprevention and chemotherapy (Zhang et al., 2022). Natural plant compounds including capsaicin, Asiatic acid, honokiol, and betulinic acid cause apoptosis by downregulating pro-survival/anti-apoptotic markers like Bcl-2 and Bcl-xl and upregulating pro-apoptotic markers like cytochrome c, bax, and activating the caspase cascade. Similarly, ginsenoside, atractylodin, β-eudesmol, fucosterol, thymol and silymarine arrest cell cycle at G0/G1 and G2/M phases (Koh et al., 2020).
Plumbago zeylanica L. is a well-known herbal plant commonly known as Ceylon leadwort (Tokarz et al.,2020). P. zeylanica, a perennial plant, in Ayurveda, is known as chitrak and its roots as chitramula (Bhinde et al.,2020). It is widely spread in India and Sri Lanka and has antioxidant, antimicrobial, anti-inflammatory, antidiabetic, antihyperlipidemic, antiulcer and hepatoprotective activities (Shukla et al.,2021). Quinones, divided into naphthoquinones, benzoquinones, and anthraquinones, possess various pharmacological properties, including anticancer, apoptotic, antibiotic, antiallergenic, antiviral, antiplatelet inhibitory, anti-ringworm, antifungal, blood thinning, and radical scavenging properties (Rahman et al.,2022). Therefore, this study focused on exploring the anticancer potential of P. zeylanica L. which is rich in quinones against MG-63 osteosarcoma cell line.
2 Materials and methods
2.1 Extract preparation and cell culture
Roots of Plumbago zeylanica L. were obtained from Amritsar (Majith Mandi) and authenticated at CSIR-CIMAP in Lucknow, India. The voucher specimen (Accession no. P035) was submitted to the CSIR-CIMAP crude drug repository. The roots were dried and powdered and subsequently macerated in various solvents viz. hexane, chloroform, ethyl acetate and methanol for 72 h, resulting in PzMH (0.17 %), PzMC (0.40 %), PzME (0.58 %) and PzMM (6.25 %) fractions respectively. The extracts were filtered and concentrated using a rotavapor (Buchi R-210, Flawil, Switzerland).
The MG-63 (osteosarcoma) cells were obtained from the National Centre for Cell Science (NCCS) Pune and grown in culture flasks using DMEM medium, to encourage ideal cellular development, media was enhanced with 10 % FBS and treated with sodium bicarbonate. A precise temperature of 37 °C and 5 % carbon dioxide was maintained in the culture environment.
2.2 Assessment of cytotoxic activity and cell migration assay
The cytotoxic activity of P. zeylanica fractions was assessed using an MTT assay with few adjustments (Mickisch et al.,1990). MG-63 cells (8 × 103 cells/well) were seeded in a 96 wells microplate and placed in CO2 incubator for 24 h. Subsequently different concentrations of P. zeylanica root fractions (15.625 to 500 µg/ml) were used to the treat the cells for 24 h. After 48 h, the cells in each well were treated with 20 µl MTT and incubated for 3 h to assess the capacity of living cells to convert MTT into insoluble formazan crystals (purple colored). The optical density measurements at 570 were carried out with the help of a multi-well plate reader and percent growth inhibition was calculated with the following formula where, Absc: absorbance of untreated control cells, Abst: absorbance of cells treated with P. zeylanica root fractions the method of
Cell migration capability was tested using a cell scratch experiment as per the method of (Liang et al.,2007).
2.3 Morphological changes and nuclear morphology analysis
In a 6-well plate, MG-63 cells (8 × 105/well) were grown and incubated with various concentrations of PzMH fraction (15.35, 53.07 and 183.48 µg/ml) for 24 h following confluency. The cells were observed under an inverted microscope and images were acquired (Nikon Eclipse TS2, Tokyo, Japan) to study the nuclear morphology. The PzMH fraction, at concentrations of 15.35, 53.07, and 183.48 µg/ml, was introduced into the cellular milieu for a duration of 24 h. post-incubation, the cells underwent fixation for 20 min in cold paraformaldehyde (4 %) within a light-restricted environment, followed by a thorough rinse with saline phosphate-buffered (PBS). Subsequent to this step, the cells were subjected to incubation with DAPI dye (10 µg/ml) for a period of 30 min. Thereafter, alterations in nuclear morphology were meticulously assessed utilizing a fluorescence microscope, with subsequent capture of images for further analysis.
2.4 AO/EtBr parallel staining for apoptosis detection and mitochondrial transmembrane potential using a fluorescence microscope
MG-63 cells, (4 × 105 cells/well), were cultured, in a six-well plate, followed by 24 h treatment with PzMH fraction (15.35, 53.07 and 183.48 µg/ml). The cells were collected and subjected to a 5-min incubation in a light-restricted environment with a solution containing AO/EtBr (100 µg/ml) with two dyes combined in equal proportions. To detect, the mitochondrial membrane potential (MMP) changes in osteosarcoma cells treated with PzMH fraction, rhodamine 123 (Rh-123) dye was used. MG-63 osteosarcoma cells were exposed to various fractions of PzMH for 24 h. After treatment, the cells were fixed in 70 % ethanol after a thorough washing with PBS. They were then placed in a carbon dioxide (CO2) incubator for a duration of 30 min, during which they were exposed to Rh-123 dye (2 µg/ml). After that, remnants of dye were eliminated by washing the cells with PBS thrice before scanning them with a fluorescence microscope.
2.5 Apoptosis detection and cell cycle analysis using Annexin V-FITC/PI double staining
Apoptosis detection was investigated using Annexin V- FITC kit (Sigma). In a 6-well plate, MG-63 cells were cultured for 24 h and subsequently treated with varying concentrations of PzMH fraction for 24 h. Attached and floating cells were gathered after treatment and centrifuged at 1200g for 5 min and washed twice with PBS. Cells were again centrifuged, at 1200g, resuspended in 0.1 ml binding buffer for 15 min and subsequently treated with 5 µl Annexin V-FITC conjugate and 5 µl propidium iodide (PI) for 20 min. The labeled cells were examined using a flow cytometer. The method of Rasul et. al., (2011) was used for cell cycle analysis.
2.6 Cell cycle phase distribution analysis and reactive oxygen species generation analysis
The BD cycle DNA kit was employed for the analysis of cell cycle stages. MG-63 cells were subjected to treatment using different amounts of the PzMH component. Following a 24 h incubation period, cellular samples were harvested, subjected to centrifugation, and subsequently preserved in a solution of 70 % chilled ethanol. The samples were subjected to fixation using trypsin buffer, followed by incubation at 37 °C for a duration of 5 min. Subsequently, the samples were treated with RNase buffer and trypsin inhibitor. A 200 µl solution of cold PI stain was added, to the mixture and allowed to cool on ice for a duration of 1 h. The flow cytometer was employed for the analysis of cells that had been labeled.
In a 6-well plates the MG-63 cells were cultured for 24 h, subsequently subjected to a 24 h exposure to the PzMH fraction. After treatment, the cells underwent a rinsing process utilizing phosphate-buffered saline (PBS) and then were subjected to a 30-min treatment with DCFH-DA (5 µg/ml PBS). Following the process of centrifugation, the cells were collected, subjected to another round of centrifugation, and subsequently reconstituted in 500 µl of PBS. The cells were promptly analyzed utilizing the FL-1 channel of the flow cytometer, with an excitation wavelength of 488 nm and an emission wavelength of 535 nm.
2.7 Mitochondrial membrane potential analysis using flow cytometer, western blot analysis and HPLC of the PzMH fraction
In 6-well plates MG-63 osteosarcoma cells were grown for 24 h, and treated with PzMH fraction, and then decanted. Rh-123 was added for 30 min. Cells were collected, centrifuged, and resuspended in 500 µl PBS. The FL-1 channel was used to examine the cells. The cells were then examined using a flow cytometer (O’Connor et al.,1988).
For western blotting the MG-63 cells were treated with GI30, GI50 and GI70 concentrations of PzMH fraction for 24 h, and RIPA lysis buffer was used to extract proteins from the cells and separated on a 10 % SDS-PAGE with subsequent transfer to a PVDF membrane. 5 % skimmed milk was used to block the membrane, and then incubated with specific primary antibodies. The membrane was then rinsed twice with saline phosphate buffer and incubated with secondary antibodies. ECL (Enhanced chemiluminescence) plus reagent was used to detect protein bands, and ImageJ software was used for intensity analysis. The ultimate quantification of each protein's expression was determined by dividing its expression level by the expression level of β-actin in the respective sample. High-performance liquid chromatography (HPLC), of PzMH fraction was performed as per of Engida et al.(2013).
2.8 Statistical analysis
The analysis of the data was carried with the help of IBM SPSS software and represented as mean ± SE (n = 3). Significant differences between the different groups were calculated with ANOVA with p ≤ 0.05 significant level. In addition, the Tukey test was performed to calculate the HSD.
3 Results
PzMH, PzMC, PzME, PzMM obtained from the root powder of Plumbago zeylanica L. were investigated for their cytotoxic potential using MTT assay on MG-63 cell line. PzMH fraction exhibited the highest cytotoxic potential amongst all fractions with 53.07 µg/ml GI50 value in MG-63 cells (Table 1). As PzMH fraction possessed the highest cytotoxic activity in MG-63 osteosarcoma, further experimentation was performed on this cell line using PzMH fraction. (*p ≤ 0.05). The values are given as mean ± SE. Data labels with different letters in a column indicate a significant difference between the.
MG-63
Concentration (μg/ml)
PzMH
PzMC
PzME
PzMM
15.625
27.01 ± 0.76d
20.74 ± 1.08d
15.60 ± 0.97e
12.66 ± 2.11e
31.25
44.06 ± 2.48c
27.71 ± 2.28d
22.84 ± 2.4de
21.69 ± 1.56d
62.5
54.79 ± 2.38b
41.69 ± 2.05c
24.26 ± 1.38d
28.05 ± 0.91cd
125
62.98 ± 1.96b
51.09 ± 1.59b
33.33 ± 0.89c
34.08 ± 2.78bc
250
76.18 ± 1.20a
58.41 ± 1.11b
51.06 ± 1.83b
42.98 ± 1.32b
500
84.33 ± 2.23a
73.47 ± 2.05a
65.62 ± 2.00a
56.68 ± 2.07a
GI50
53.07 μg/ml
119.00 μg/ml
246.72 μg/ml
376.12 μg/ml
R2
0.9875
0.9899
0.9196
0.9791
F Ratio
117.29*
124.62*
131.43*
68.51*
Regression
Equation
y = 16.124ln(x) − 14.04
y = 15.05ln(x) − 21.927
y = 14.172ln(x) − 28.063
y = 11.954ln(x) − 20.886
HSD
0.09
0.082
0.078
0.09
3.1 PzMH fraction possessed anti-migratory potential
A scratch migration experiment was employed to investigate the inhibitory activity of the PzMH fraction on the migratory behavior of osteosarcoma (MG-63 cells). The measurement of cells migrated was analyzed by using ImageJ software. The pre- and post-treatment phase-contrast images revealed that following a 24 h treatment with PzMH fraction, there was a concentration-dependent decrease in the migration of MG-63 cells towards the scratched area. The MG-63 cell population under controlled conditions, showed that almost all of the areas that were subjected to scratching were successfully covered within a time frame of 24 h (Fig. 1).
(A) PzMH fraction inhibited the migration of MG-63 cells. (B) Representative bar graph showing quantitative analysis of cell migration.
3.2 Morphological changes and nuclear morphology
PzMH fraction treatment caused morphological alterations in MG-63 cells, including detachment, membrane rounding, and blebbing. Control cells exhibited thick multilayers, while PzMH treated cells showed rounding off, contact loss, and increased intercellular space (Fig. 2 A). Apoptosis is programmed cell death, and DAPI staining shows healthy nuclei in untreated MG-63 cells, while increased PzMH fraction causes fragmentation and shrinkage (Fig. 2 B).
Photomicrographs of untreated control and PzMH fraction treated MG-63 cells for 24 h (A) Phase contrast micrography. (B) Staining with DAPI. Arrows indicate changes in nuclear morphology. (C) AO/EtBr double staining. (D) Micrographs of untreated and PzMH treated MG-63 cells stained with Rhodamine-123.
3.3 AO/EtBr parallel staining for apoptosis
PzMH fraction treatment led to increased apoptosis in cells compared to control cells. Cells treated with varying concentrations of PzMH fraction displayed apoptosis-like characteristics. Early apoptosis was observed in cells treated with GI30 (15.35 µg/ml), while late apoptosis was observed in cells treated with GI50 (53.07 µg/ml). Most cells experienced apoptosis at GI70 (183.48 µg/ml), indicating increased apoptotic or necrotic cells (Fig. 2 C).
3.4 Mitochondrial membrane potential assessment
Depolarization of mitochondrial membrane was studied using Rh-123 dye. PzMH administration at a 15.35 µg/ml concentration significantly promoted depolarization. PzMH fractions treated with 183.48 µg/ml showed higher depolarization compared to those treated with 53.07 µg/ml (Fig. 2 D).
3.5 PzMH fraction induces apoptosis and cell cycle arrest at sub-G1 stage
The PzMH fraction increased late and early apoptotic cells in treated cells compared to untreated cells. An increase of about 21.2 % in early apoptotic (EA) cells was observed at 15.35 µg/ml concentration, while a small increase of about 7.6 % was also observed in control cells. At 53.07 µg/ml, early apoptotic cells increased by (56.5 %), while late apoptotic cells increased by (7.7 %). At 183.48 µg/ml, 73.8 % of cells were in the EA stage. PzMH fraction induces apoptosis in a concentration-dependent manner and inhibits osteosarcoma cell proliferation (Fig. 3A&B). PzMH fraction treatment at different concentrations led to sub-diploid cell accumulation in the sub-G1 phase, stopping cell cycle progression. MG-63 cells exposed to PzMH fraction for 24 h showed arrest rates of 59.2 %, 70.6 % and 83.5 % at the sub-G1 stage at 15.35, 53.07, and 183.48 µg/ml concentrations, respectively (Fig. 3 C&D).
(A) PzMH induced apoptosis in MG-63 cells (B) Histogram representing cell percentage at different stages in control and PzMH treated MG-63 cells. (C) represents arrest of cell cycle in at sub-G1 in MG-63 cells by PzMH fraction (D) Bar graph represents % cell cycle arrest by PzMH fraction at different concentrations.
3.6 Analysis of cell cycle phase distribution and effect on reactive oxygen species (ROS)
PzMH fraction treatment resulted in the arrest of 62 % of cells in the G0/G1 phase at the maximum tested dose of 183.48 µg/ml whereas, untreated control cells showed 20.9 % cells in this stage. At the G0/G1 stage, the GI50 concentration (53.07 µg/ml) produced 46.1 % cell population arrest. The lowest concentration (15.35 µg/ml) caused 39.6 % G0/G1 phase cell cycle arrest (Fig. 4 A&B). The generation of ROS in the PzMH treated cells increased by 38 %, 53.5 %, and 76.2 % respectively, while the population of intact cells decreased by 62 %, 46.5 %, and 23.8 % respectively, compared to untreated cells which had a ROS generation of 26.4 % and intact cell population of 73.6 % (Fig. 4 C&D).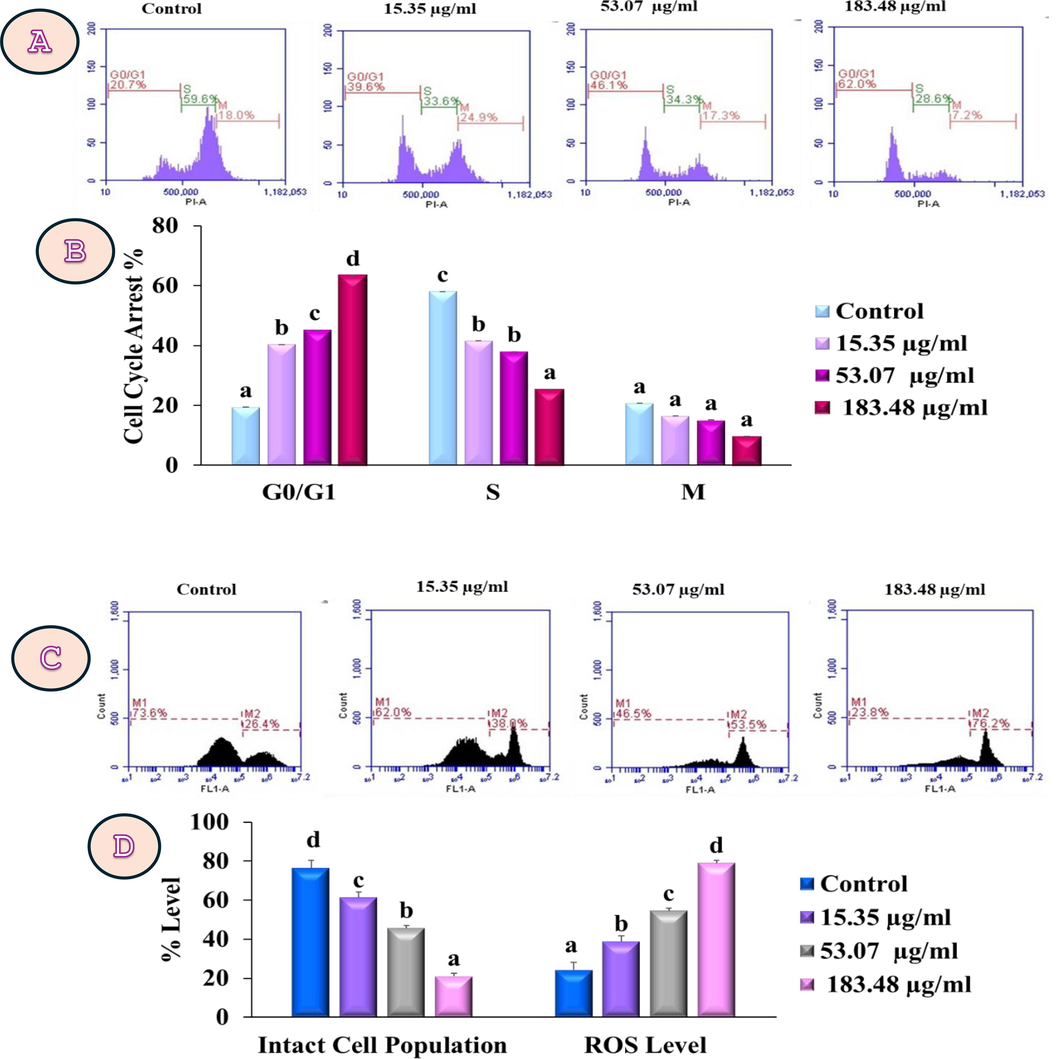
(A) Represents arrest of cell cycle at G 0 /G 1 stage by PzMH fraction (B) Bar graph represents cell cycle arrest at different stages in PzMH treated MG-63 cells. (C) PzMH fraction generated concentration dependent ROS in MG-63 cell lines. (D) Percentages of ROS that accumulated in MG-63 cell lines treated with PzMH fraction and untreated control cells are shown in the bar graph.
3.7 PzMH fraction influence on mitochondrial membrane potential (MMP; Δψm) and western blotting
PzMH (15.35, 53.07 and 183.48 µg/ml) fraction treated MG-63 cells showed a 46.1 %, 55.7 %, and 80.4 % reduction in mitochondrial membrane integrity respectively as compared to control cells. The presence of intact mitochondria was revealed by increased dye accumulation. However, increased PzMH fraction lost mitochondrial integrity, and dye fluorescence. The membrane potential of MG-63 cells was attenuated in a concentration-dependent manner after 24 h (Fig. 5A&B). PzMH treated MG-63 cells showed increased p53, Bax, Bad, cleaved-caspase 3, and cleaved-caspase 9 protein expression, while Bcl-2 expression was reduced. Upregulated cleaved-caspase 3 expression confirmed intrinsic apoptotic pathway stimulation. The expression level was quantified using the Image J software, and relative expression is presented in the bar graph (Fig. 5 C&D). As a housekeeping protein, β-Actin was used.
Rhodamine-123 labeling of MG-63 cells revealed the breakdown of mitochondrial membrane potential (ΔΨm). Bar graph represents % mitochondrial membrane disruption with increase in PzMH concentration. (C) Western blot and (D) Represents the transcriptional expression of proteins like Bcl-2, p-53, Bad, Bax, cleaved-caspase3, and cleaved-caspase 9.
3.8 High performance liquid chromatographic (HPLC) analysis of PzMH fraction and mechanism of P. zeylanica (PzMH)-induced cell death in MG-63 cells
HPLC analysis demonstrated the presence of polyphenolic compounds such as, p-coumaric acid retention time (RT), 24.276 min., 4-hydroxybenzaldehyde (RT, 25.676 min), lawsone (RT, 36.132 min) and plumbagin (RT, 47.542 min) (Fig. 6.). Lawsone was found to be in highest quantity (67.154 mg/L) followed by p-coumaric acid (22.746 mg/L), plumbagin (16.623 mg/L) and 4-hydroxybenzaldehyde (6.257 mg/L).
(A) HPLC chromatogram of PzMH fraction, (B) mechanism of P. zeylanica (PzMH)-induced cell death in MG-63 cells.
4 Discussion
Medicinal plants contain bioactive substances that can treat and prevent various ailments, including cancer (Roy et al.,2017). Conventional treatments like chemotherapy and radiation therapy have side effects like hair loss, appetite loss, depression, anxiety, and fever (Jain et al.,2016). Research into cytotoxic properties of phyto-therapeutics in cancer treatment has gained attention, with the quinone rich hexane fraction (PzMH) exhibiting the highest cytotoxicity against MG-63 cells.
HPLC analysis revealed presence of three, 1,4-naphthoquinones, lawsone and plumbagin in PzMH fraction. Naphthoquinones with two carbonyl groups possess the ability to form radical anion or di-anion species by accepting one or two electrons, is what contributes to the various biological activities. Moreover, the electrophilicity of the naphthoquinone ring is reduced by the presence of hydroxy groups at positions 5 and 8 (Kishore et al.,2014). Lawsone is used as the primary precursor in the synthesis of a diverse array of clinically significant anticancer pharmaceuticals, including Atovaquone, Lapachol, and Dichloroallyl lawsone (Pradhan et al., 2012). Lawsone, when used either independently or in conjunction with cisplatin, effectively curtailed the growth of SKOV-3 cells in a trend contingent on the administered dosage. This treatment hindered SKOV-3 cell growth by halting progression through the G1/G0 phase within the cellular life cycle. This arrest was accompanied by an elevation in the expression of p53 and Cip1/p21, which in turn, led to a reduction in the levels of two pivotal proteins, cyclin E and cyclin D1. Furthermore, lawsone was observed to trigger apoptosis by downregulating Bcl-2, enhancing the Bax:Bcl-2 ratio, and activating caspase 3 (Li et al., 2017). Quinone rich PzMH fraction inhibit the cell proliferation in MG-63 with the GI50 value of 53.07 µg/ml. The migration of cancer cells to nearby tissues and infiltration into the bloodstream is the cornerstone of the metastatic cascade (Novikov et al.,2021). Osteosarcoma is a kind of bone cancer that is highly aggressive and has a high proclivity for metastasis (Zhu et al.,2021). MG-63 cells subjected to various concentrations of PzMH in the cell migration experiment showed a concentration-dependent reduction in cell migration as compared to untreated cells, confirming the anti-migratory potential. Nimlamool et. al., (2022) reported similar results wherein a methanolic extract of Mitrephora chulabhornian was found to possess anti-proliferative and anti-migratory activity against HeLa cells (Nimlamool et al.,2022). Natural quinones, plumbagin, juglone, and thymoquinone were shown to effectively suppress the proliferation and migration of PANC-1 cells (Narayanan et al.,2022). MG-63 cell shape changed after they were exposed to varying amounts of PzMH fraction. These alterations were marked by cell shortening, loss of membrane integrity and cell shape by becoming fusiform and spherical, eventually detaching from the culture flask, leading to cell death. The morphologic alterations were accompanied by decreased adhesion to the cell culture plates.
The study conducted by Wan et al. (2016) yielded similar results when examining the effects of an ethyl acetate extract derived from Potentilla chinensis on MG-63 cells (Wan et al.,2016). Furthermore, DAPI nuclear staining showed signs of apoptosis marked by alterations in nuclear morphology, such as shrinkage of a nucleus, chromatin fragmentation and condensation (Yang et al.,2009). Prior work on MG-63 cells treated with Sterculia foetida seed powder extract supports these findings (Jafri et al.,2019).
Additionally, the ability of the PzMH fraction to induce apoptosis was evaluated using AO/EtBr double staining under fluorescent microscope and Annexin V-FITC/PI double labeling using flow cytometry. MG-63 cells labelled with AO/EtBr dye and treated with PzMH fraction demonstrated early and late apoptosis in cells treated at higher concentrations. Flow cytometric investigations confirmed these findings, revealing that 73.8 % of cells were in the early stages of apoptosis at 183.48 µg/ml. Ethanolic extract of Crocus sativus tepals was reported to suppress the proliferation and migration of U87 glioblastoma cells (Baba et al.,2022). 183.48 µg/ml concentration of PzMH fraction caused an arrest of 62 % cell population at G0/G1 stage. Our results align with Rasouliyan et. al., (2021), who demonstrated that lawsone in A549 cells induced apoptosis and caused an arrest in cell cycle at G0/G1 stage (Rasouliyan et al.,2021).
The significance of ROS in cancer is critical. Proliferation of cells, progression of cell cycle, growth and death of cells, cell–cell attachment, cell migration, blood vessel development and tumor pluripotency maintenance are influenced by ROS (Liou & Storz, 2010). The ROS generation can trigger the permeability of mitochondrial membrane, resulting in MMP loss and membrane integrity (Ma et al.,2014). Flow cytometric investigations revealed that PzMH fraction treated MG-63 cells showed rise in ROS and MMP disruption dose-dependently. Fluorescent imaging of MG-63 cells using the Rh-123 dye produced results consistent with the flow cytometric studies. Cajanol, from Cajanus cajan, induced apoptosis via ROS generation and MMP disruption (Luo et al.,2010). Ishteyaque et. al. (2020), showed that a lawsone rich extract of Lawsonia inermis killed cancer cells through apoptosis, ROS generation, and interfering with mitochondrial membrane potential (Ishteyaque et al.,2020).
The p53 gene governs apoptosis, senescence, genetic stability, DNA repair and arrest of cell cycle. It also directly participates in the intrinsic apoptosis cascade by increasing outer mitochondrial membrane permeability through its interaction with multidomain Bcl-2 family proteins (Vaseva & Moll, 2009). The interaction between antiapoptotic (Bcl-2) and apoptosis promoting (Bad, Bax) proteins is a hallmark of p53-mediated apoptosis through intrinsic pathway. These molecules act as molecular switches to regulate apoptosis and are found in the outer mitochondrial membrane. Disruption of mitochondrial membrane potential promotes the release of cytochrome c in cytosol, which in turn activates caspase 3 and 9 and trigger the intrinsic apoptotic cascade (Zhou et al.,2019). In the present investigation as PzMH fraction treated MG-63 cells displayed upregulation of p53, Bad, Bax, caspase 3 and 9, while a significant decrease in expression of Bcl-2 protein was observed in comparison to control cells. Plumbagin from Nepenthes alata increased the Bax/Bcl2 ratio and produced intracellular ROS in MCF-7 cells, resulting in apoptosis (De Laet et al.,2019). The study by Yan et al. (2020), demonstrated that colchicine treatment in Caski and HeLa cells upregulated the expression levels of caspase-3, Bax and cytochrome proteins, while downregulating Bcl-2 protein expression. The outcomes of our investigation demonstrate that the PzMH fraction extracted from P. zeylanica manifests anticancer properties in MG-63 cells by instigating the activation of the p53-dependent intrinsic apoptotic pathway. This mechanism entails the disruption of the mitochondrial membrane potential (ΔΨm), accumulation of ROS, arrest of cell cycle at G0/G1 phase, downregulation in the expression of Bcl-2, and upregulation in the protein expression of p53, Bax, Bad, cleaved-caspase-3 and caspase-9. These impacts are likely attributable to the presence of quinones within the PzMH fraction (Fig. 6).
5 Conclusions
In the current investigation, it was demonstrated that the PzMH fraction derived from P. zeylanica had significant anti-proliferative effects against MG-63 osteosarcoma cell lines in human. The loss of mitochondrial membrane permeability, subsequent formation of ROS, and arrest of cell cycle at the G0/G1 stage initiated the intrinsic mitochondrial-mediated apoptotic pathway in MG-63 cells. The fraction of Plumbago zeylanica (PzMH) was found to exhibit inhibitory effects on migration and induced apoptosis by upregulating the expression levels of proteins like p53, bad, bax, cleaved-caspases 3 and 9, while downregulating Bcl-2 expression. These findings suggest a significant potential for PzMH as an anticancer agent, particularly in the treatment of osteosarcoma. However, further investigation is necessary to fully understand its efficacy and safety profile, ultimately leading to its potential development as a chemotherapeutic drug.
Ethical approval
Not applicable as no animals or humans were involved in this study.
Consent to participate
All authors consent to participate in the publication of the manuscript.
Consent for publication
All authors approve the manuscript to be published.
Funding
Not Applicable.
CRediT authorship contribution statement
Neha Sharma: Writing – original draft, Formal analysis. Rasdeep Kour: Methodology, Investigation, Formal analysis. Shagun Verma: Methodology, Investigation, Formal analysis. Vandana Sharma: Methodology, Data curation. Deepika Singh: Methodology, Investigation. Sumit G. Gandhi: Writing – review & editing, Supervision, Software, Data curation. Vaseem Raja: Writing – review & editing, Writing – original draft, Validation, Conceptualization. Satwinderjeet Kaur: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Conceptualization. Naveen Kumar: Methodology, Investigation. Khalid Mashay Al-Anazi: Writing – review & editing, Resources, Funding acquisition. Mohammad Abul Farah: Writing – review & editing, Resources, Funding acquisition.
Acknowledgments
The invaluable support and resources from the CELS and GNDU, Amritsar, India are highly acknowledged. We also extend their heartfelt thanks to Department of Science and technology for their support through DST-FIST and DST-PURSE. The authors would also like to acknowledge King Saud University in Riyadh, Saudi Arabia, for its invaluable support to this research through Project Number (RSP2024R154).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tumor-suppressor genes, cell cycle regulatory checkpoints, and the skin. N. Am. J. Med. Sci.. 2015;7(5):176-188.
- [CrossRef] [Google Scholar]
- Crocus sativus L. Tepal extract induces apoptosis in human U87 glioblastoma cells. Biomed Res. Int. 20224740246
- [CrossRef] [Google Scholar]
- Standard operating procedure of Purification of Chitraka (Plumbago zeylanica Linn.) along with pharmacognostical and analytical profiles of Plumbagin. Ayu. 2020;41(2):117-122.
- [CrossRef] [Google Scholar]
- Bone, muscle, and metabolic parameters predict survival in patients with synchronous bone metastases from lung cancers. Bone. 2018;108:202-209.
- [CrossRef] [Google Scholar]
- Global cancer statistics 2022: the trends projection analysis. Chemical Biology Lett.. 2022;10:451.
- [Google Scholar]
- Osteosarcoma: a surveillance, epidemiology, and end results program-based analysis from 1975 to 2017. Cancer 2022
- [Google Scholar]
- Eichhornia crassipes: a powerful bio-indicator for water pollution by emerging pollutants. Sci. Rep.. 2019;9(1):7326.
- [CrossRef] [Google Scholar]
- Extraction, identification and quantitative HPLC analysis of flavonoids from sarang semut (Myrmecodia pendan) Ind. Crop. Prod.. 2013;41:392-396.
- [CrossRef] [Google Scholar]
- In vitro: cytotoxicity, apoptosis and ameliorative potential of Lawsonia inermis extract in human lung, colon and liver cancer cell line. Cancer Invest.. 2020;38(8–9):476-485.
- [CrossRef] [Google Scholar]
- Phytochemical screening of Sterculia foetida seed extract for anti-oxidant, anti-microbial activity, and detection of apoptosis through reactive oxygen species (ROS) generation, mitochondrial membrane potential (MMP) decrease, and nuclear fragmentation in human osteosarcoma cells. J. Histotechnol.. 2019;42(2):68-79.
- [CrossRef] [Google Scholar]
- Medicinal plants for treatment of cancer: a brief review. Pharmacognosy J.. 2016;8(2):87-102.
- [CrossRef] [Google Scholar]
- Cytotoxicity of synthesized 1,4-naphthoquinone analogues on selected human cancer cell lines. Bioorg. Med. Chem.. 2014;22(17):5013-5019.
- [CrossRef] [Google Scholar]
- Recent advances in cancer chemoprevention with phytochemicals. J. Food Drug Anal.. 2020;28(1):14-37.
- [CrossRef] [Google Scholar]
- Lawsone inhibits cell growth and improves the efficacy of cisplatin in SKOV-3 ovarian cancer cell lines. Afr. J. Tradit. Complement. Altern. Med.. 2017;14(5):8-17.
- [Google Scholar]
- In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc.. 2007;2(2):329-333.
- [CrossRef] [Google Scholar]
- Reactive oxygen species in cancer. Free Radic. Res.. 2010;44(5):479-496.
- [CrossRef] [Google Scholar]
- Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem. Biol. Interact.. 2010;188(1):151-160.
- [CrossRef] [Google Scholar]
- Photosynthetic responses of wheat (Triticum aestivum L.) to combined effects of drought and exogenous methyl jasmonate. Photosynthetica. 2014;52(3):377-385.
- [CrossRef] [Google Scholar]
- Chemosensitivity testing of primary human renal cell carcinoma by a tetrazolium based microculture assay (MTT) Urol. Res.. 1990;18(2):131-136.
- [CrossRef] [Google Scholar]
- Natural quinones induce ROS-mediated apoptosis and inhibit cell migration in PANC-1 human pancreatic cancer cell line. J. Biochem. Mol. Toxicol.. 2022;36(5)
- [CrossRef] [Google Scholar]
- Anticancer activity of novel plant extracts and compounds from Adenosma bracteosum (Bonati) in human lung and liver cancer cells. Molecules. 2020;25(12):2912.
- [Google Scholar]
- The leaf extract of Mitrephora chulabhorniana suppresses migration and invasion and induces human cervical cancer cell apoptosis through caspase-dependent pathway. Biomed Res. Int. 20222028082
- [CrossRef] [Google Scholar]
- Mutational drivers of cancer cell migration and invasion. Br. J. Cancer. 2021;124(1):102-114.
- [CrossRef] [Google Scholar]
- Use of rhodamine 123 to investigate alterations in mitochondrial activity in isolated mouse liver mitochondria. Biochem. Biophys. Res. Commun.. 1988;151(1):568-573.
- [CrossRef] [Google Scholar]
- From body art to anticancer activities: perspectives on medicinal properties of henna. Curr. Drug Targets. 2012;13(14):1777-1798.
- [Google Scholar]
- The role of dietary polyphenols in osteosarcoma: a possible clue about the molecular mechanisms involved in a process that is just in its infancy. J. Food Biochem.. 2022;46(1):e14026
- [Google Scholar]
- Naphthoquinones and derivatives as potential anticancer agents: an updated review. Chem. Biol. Interact.. 2022;368:110198
- [CrossRef] [Google Scholar]
- Preparation, physicochemical characterization, and anti-proliferative properties of Lawsone-loaded solid lipid nanoparticles. Chem. Phys. Lipids. 2021;239:105123
- [CrossRef] [Google Scholar]
- Phytochemistry and pharmacological studies of Plumbago zeylanica L.: a medicinal plant review. Clin. Phytosci.. 2021;7(1):34.
- [CrossRef] [Google Scholar]
- Can ceylon leadwort (Plumbago zeylanica L.) acclimate to lead toxicity?-studies of photosynthetic apparatus efficiency. Int. J. Mol. Sci.. 2020;21(5)
- [CrossRef] [Google Scholar]
- The mitochondrial p53 pathway. Biochimica et Biophysica Acta (BBA) -. Bioenergetics. 2009;1787(5):414-420.
- [CrossRef] [Google Scholar]
- In vitro antitumor activity of the ethyl acetate extract of Potentilla chinensis in osteosarcoma cancer cells. Mol. Med. Rep.. 2016;14(4):3634-3640.
- [CrossRef] [Google Scholar]
- Involvement of p53-dependent apoptosis signal in antitumor effect of Colchicine on human papilloma virus (HPV)-positive human cervical cancer cells. Biosci. Rep.. 2020;40(3) (BSR20194065)
- [Google Scholar]
- Capsaicin induces apoptosis by generating reactive oxygen species and disrupting mitochondrial transmembrane potential in human colon cancer cell lines. Cell. Mol. Biol. Lett.. 2009;14(3):497-510.
- [CrossRef] [Google Scholar]
- Phytochemical profiles, antioxidant activity and antiproliferative mechanism of Rhodiola rosea L. phenolic extract. Nutrients. 2022;14:3602.
- [Google Scholar]
- Magnolol induces apoptosis in osteosarcoma cells via G0/G1 phase arrest and p53-mediated mitochondrial pathway. J. Cell. Biochem.. 2019;120(10):17067-17079.
- [CrossRef] [Google Scholar]
- Melittin inhibits lung metastasis of human osteosarcoma: evidence of wnt/β-catenin signaling pathway participation. Toxicon. 2021;198:132-142.
- [CrossRef] [Google Scholar]







