Translate this page into:
Green synthesized zinc oxide/neodymium nanocomposites from Avaram Senna flower extract induces apoptosis in gastric cancer AGS cell line through inhibition of the PI3K/AKT/mTOR signaling pathway
⁎Corresponding author. drvishnupriyav@gmail.com (Vishnu Priya Veeraraghavan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Gastric cancer (GC) is one of the common cancers that have a direct impact on the numerous facets of life. GC still remains a life threatening with one million mortalities per year worldwide.
Objective
In this work, we planned to formulate the Neodymium/Zinc oxide nanocomposites by using the Avaram Senna flower extract (AS-Nd/ZnONCs) and examine its anticancer potential against the GC AGS cells via inhibition of PI3K/AKT/mTOR signaling pathway.
Methodology
The developed AS-Nd/ZnONCs were characterized by various techniques like UV–vis spectroscopy, XRD, FE-SEM, EDAX, FT-IR, and photoluminescence studies. Antibacterial assay was performed by well diffusion method. The anticancer potential of AS-Nd/ZnONCs were investigated against AGS cells by MTT, ROS and MMP measurement, and apoptotic cell death using respective fluorescence staining techniques. The cell adhesion and antioxidants status were detected by standard method. The mRNA expression of PI3K/AKT/mTOR signaling molecules was studied via RT-PCR technique.
Results
The formulated AS-Nd/ZnONCs exhibited the hexagonal wurtzite structure with 33.56 nm size. The outcomes of antibacterial assay proved that the AS-Nd/ZnONCs has remarkable antibacterial activity. The MTT assay evidenced that the AS-Nd/ZnONCs dose-dependently suppressed the AGS cell viability. Fluorescence staining results demonstrated that the AS-Nd/ZnONCs provoked the apoptotic cell death in AGS cells. AS-Nd/ZnONCs treatment effectively inhibited the PI3K/AKT/mTOR signaling cascade and triggered the apoptotic pathway in the AGS cells.
Conclusion
In conclusion, our findings confirmed that the formulated AS-Nd/ZnONCs could be a hopeful chemopreventive agent in the future to treat the GC.
Keywords
Gastric cancer
Zinc oxide nanoparticles
Cassia auriculata
Neodymium
AGS cells
1 Introduction
Gastric cancer (GC) is an extremely heterogeneous disease that can emerge at any part of the stomach. Due to this feature, it is the fourth major cancer among oncological ailments in males and fifth in females. GC still remains an extremely life threatening and the 5-year survival rate of GC victims are <20%, along with one million mortalities per year worldwide (Parkin et al., 2000; Xiong et al., 2016). PI3K/AKT/mTOR signaling pathway controls a numerous cellular activities in diverse ailments and cancer development. This cascade contributes a major functions in the survival, autophagy, and apoptosis in most of the cancer cells including GC. mTOR performs a crucial function in the tumor progression. Previous studies demonstrated that the tumors generally over-trigger the AKT/mTOR signaling cascade. AKT is a serine–threonine kinase downstream of the phosphatidylinositol 3-kinase (PI3K) signaling cascade that controls manifold cellular events like survival, multiplication, differentiation, metabolism and cytoskeletal re-organization (Fruman et al., 2017). AKT functions as an imperative effector protein in the PI3K cascade and regulates the usual cellular physiology like growth, movement, multiplication, and metabolism. AKT is also regarded as one of the most hyper-activated kinases in the various human cancers, prompting numerous biological events, which are unswervingly participated in tumourigenesis. Recurrent stimulation of AKT was also highlighted in nearly 78% of GC (Nam et al., 2003).
Apoptosis in numerous cancers were blocked by the PI3K/AKT cascade. The expression of PI3K/AKT was found to be greater in cancer cells and is regarded that it could instruct resistance towards anti-cancer agents. Targeting the PI3K/AKT cascade could leads to apoptosis stimulation in chemo-drug resistant cancer cells. Chemoresistance is a major problem that leads to the treatment failures. It was well-known that the abnormal stimulation of PI3K/AKT signaling event could results in the chemoresistance via apoptosis evasion and improved cell survival (Terakawa et al., 2003). The typical treatment options for the GC include chemotherapy, surgery, and chemoradiation was extensively executed. At present, the successful prognosis, potential therapeutic options, and prior diagnosis were still lack. Likewise, the diagnosis of GC in earliest phase leftovers challenging, and in many incidences, surgery is the only option to treat the GC. The successful therapy to the GC is still a huge task even with many advancements in the medical field (Tabernero et al., 2018). It is essential to find out the multi-potent nanocomposites with efficient and targeted cytotoxicity to overwhelm the adverse effects of non-targeted drugs. Nanotechnology is a hopeful field for the cancer therapy.
Avaram senna (Cassia auriculata L.) is an imperative herbal plant of Cesalpinaceae family. It is extensively utilized to treat numerous ailments in Asia. It was highlighted that the C. auriculata demonstrated potent anti-diabetic activity (Mishra et al., 2017), controls blood glucose (Surana et al., 2008), dyslipidemia and reduces cardiovascular risks (Javekar and Halade, 2006), anti-rheumatism (Joshi, 2000). C. auriculata also demonstrated the hepatoprotective (Rajagopal et al., 2003) and anticancer activity (Prasanna et al., 2009). The five different parts of Avaram Senna i.e. flowers, leaves, roots, bark and fruits were used to prepare the herbal decoction also known as Kalpa herbal tea (Avarai panchaga chooranam). In this study, we formulated the Neodymium/Zinc oxide nanocomposites by using the Avaram Senna flower extract (AS-Nd/ZnONCs) by the green process and scrutinized its anticancer potential against the human gastric cancer AGS cells through the inhibition of PI3K/AKT/mTOR signaling axis.
2 Materials and methods
2.1 Chemicals
Zinc (II) nitrate hexahydrate (Zn (NO3)2·6H2O), Neodymium nitrate (Nd (NO3)36H2O), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Dulbecco’s Modified Eagles Medium (DMEM), Antibiotics, and other chemicals was attained from Sigma Aldrich, St. Louis, USA.
2.2 Synthesis of AS-Nd/ZnONCs
Initially, the Avaram Senna flower was cleaned thrice with distilled water and dried at shade. Then, 10 g of fresh Avaram Senna flower was mixed with 100 ml of distilled water and heat-macerated at 80 °C for 20 min. Resultant extract was filtered through filter paper. For the preparation of AS-Nd/ZnONCs: 0.010 M of Nd (NO3)36H2O salt and 0.090 M Zn (NO3)2·6H2O salt was mixed with 100 ml of Avaram Senna flower extract solution. Then this yellow color homogeneous mixture was agitated continually for 6 h at 80˚C. Finally the resulting AS-Nd/ZnONCs were gathered and utilized for further studies.
2.3 Characterization techniques of AS-Nd/ZnONCs
The formulated AS-Nd/ZnONCs were characterized by an X-ray diffractometer (model: X’PERT PRO PANalytical). The size of the crystalline was determined through Scherrer formula, where D-is the size (nm), λ is a radiation wavelength (1.5406 Å for CuKα), k is a constant (0.94), βD – is the peak width at half-maximum in radian along (1 0 1) plane and is Bragg’s diffraction angle.
The AS-Nd/ZnONCs was examined via Field Emission-Scanning Electron Microscopy (Carl Zeiss Ultra 55 FESEM) with EDAX (model: Inca).
UV–Visible spectroscopy investigation was executed on a spectrophotometer (Perkin Elmer Instrument, USA). The optical energy band gap of AS-Nd/ZnONCs is determined through classical Tauc relation (Tauc, 1974). where Eg is the optical bandgap. A is a constant and the exponent n depends on the transition. The value of n = 1/2, 3/2, 2, or 3 depends on the nature of the electronic transition (1/2 for allowed direct transition, 2 for allowed in-direct transition, 3/2 and 3 for forbidden direct and forbidden indirect transition, respectively). Extrapolation of the linear region of these plots to (αhυ)2 = 0 gives the corresponding direct energy bandgap. The bandgap of 3.34 eV is obtained for NdZnO nanocomposite.
The functional group of the formulated AS-Nd/ZnONCs was scrutinized by Fourier transform infrared (FT-IR) analysis in the wavenumber 500–4000 cm−1. Photoluminescence spectra were measured using the Cary Eclipse spectrometer.
2.4 Antibacterial assay
The green synthesized AS-Nd/ZnONCs (40 mg) was dispersed into 1 ml of sterile 5% Dimethyl sulfoxide (DMSO) and utilized as a stock. The antibacterial activity was executed via well diffusion technique. AS-Nd/ZnONCs was investigated against gram-positive (S. pneumonia and B. subtilis) and gram-negative (K. pneumonia and P. vulgaris) bacterial strains on Mueller Hinton Agar (MHA), as reported by the Clinical and Laboratory Standards Institute (Wright, 2000). AS-Nd/ZnONCs was investigated at a doses of 1, 1.5, and 2 mg/ml of the AS-Nd/ZnONCs was loaded on the bacterial plates and sustained at 37 °C for 24 h. The inhibition zones were monitored and recorded. For positive control, standard antibiotic Amoxicillin (30 µg disc) was used.
2.5 Cell culture collection and maintenance
GC AGS and BGC-823 cells were acquired from the ATCC, USA. The normal human gastric epithelial GES-1 cells were procured from the Beijing Institute of Cancer Research, Beijing, China. All cells were sustained in a DMEM along with FBS (10%) and Streptomycin/Penicillin (1%) at 37 °C in a CO2 (5%) chamber for additional studies.
2.6 MTT cytotoxicity assay
Cytotoxic effects of formulated AS-Nd/ZnONCs against the AGS, BGC-823, and normal GES-1 cells were studied by MTT assay. Briefly, cells at 5 × 103 density were loaded in a separate microtiter plates and stand overnight for the adhesion in DMEM medium. Then, cells were supplemented with the fabricated AS-Nd/ZnONCs at 1, 2.5, 5, 7.5, 10, 12.5, 15 μg for 24 h. After that, 100 µl of DMEM with 20 μl of MTT was mixed with every well and maintained for another 4 h. Then, the developed formazan crystals were dissolved with 100 μl of DMSO. Finally, absorbance was taken at 570 nm using microplate reader.
2.7 Detection of reactive oxygen species (ROS) generation
The fabricated AS-Nd/ZnONCs provoked ROS accumulation in AGS cells was scrutinized by DCFH-DA staining. Cells were loaded at 1 × 106 density in 24-well plate with DMEM and sustained at 37 °C for 24 h. Then cells were challenged with 7.5 μg of AS-Nd/ZnONCs and 2 μg of standard drug doxorubicin (DOX) and maintained for 24 h. After that, 10 μl of DCFH-DA dye was mixed to every well and sustained for additional 1 h. Lastly, status of ROS was scrutinized beneath the fluorescence microscope.
2.8 Analysis of mitochondrial membrane potential (MMP)
MMP status in the control and AS-Nd/ZnONCs challenged AGS cells were observed by Rhodamine-123 (Rh-123) staining. Shortly, cells were loaded at 1 × 106 density in 24-well plate along with DMEM medium and maintained for 24 h at 37 °C. Then cells were supplemented with 7.5 μg of AS-Nd/ZnONCs and 2 μg of DOX and sustained for another 24 h. After that, cells were stained with the 10 μg/ml of Rh-123 for 30 min. Cells were observed beneath the fluorescence microscope to detect the MMP status.
2.9 Dual staining assay
The AS-Nd/ZnONCs triggered apoptotic cell death in the AGS cells was scrutinized by acridine orange/ethidium bromide (AO/EB) staining. Shortly, AGS cell at the 5 × 105 cells population was loaded in a 24-well plate with DMEM medium and maintained for 24 h at 37 °C. Cells were then administered with 7.5 μg of AS-Nd/ZnONCs and 2 μg of DOX for 24 h then the cells were stained with 100 μg/ml of AO/EB (1:1) stain for 5 min at 37 °C. Finally cells were monitored beneath fluorescence microscope to identify the apoptotic cell death.
2.10 Propidium iodide (PI) staining
The morphology of apoptotic cells were detected by PI staining. Cells at the 1 × 106 density were placed on the 24-well plate with DMEM and sustained for 24 h at 37 °C. Then 7.5 μg of AS-Nd/ZnONCs and 2 μg of DOX were administered to the cells for 24 h. Then, 5 µl of PI dye was placed on the cells for 20 min in a dark. Lastly, the morphology of apoptotic cells was monitored under fluorescence microscope.
2.11 Cell adhesion assay
AGS cells were seeded on a gelatin-coated plate with the DMEM then challenged with 7.5 µg AS-Nd/ZnONCs and 2 μg of DOX and sustained for 24 h at 37 °C. Then cells were cleansed with buffered saline and trypan blue dye was mixed to each well to identify and differentiate the viable and non-viable cells. The trypan blue could be taken up by only dead cells, and viable cells remain without stained. Cells were then observed beneath the microscope to detect the viable and dead cells.
2.12 Estimation of lipid peroxidation (LPO) and antioxidants level
The AS-Nd/ZnONCs challenged AGS cells were gathered and homogenated with the saline for the biochemical examinations. The LPO status in the AS-Nd/ZnONCs challenged AGS cells were scrutinized by the Niehius and Samuelson (1968) approach. The superoxide dismutase (SOD) activity was examined by Marklund and Marklund (1974) approach. Sinha (1972) method was applied to determine the catalase (CAT) activity. The status of glutathione (GSH) was tested by the Aykac et al. (1985) technique.
2.13 Statistical analysis
Results were scrutinized using SPSS software version 17. Data were described as mean ± SD of triplicates. One-way ANOVA successively DMRT test was implemented to scrutinize the statistical variations between groups and the significance was set at p < 0.05.
3 Results
3.1 Characterization studies of formulated AS-Nd/ZnONCs
The formation of AS-Nd/ZnONCs was evidenced by UV–Vis Spectrophotometer. The outcomes of UV visible spectra of the fabricated AS-Nd/ZnONCs were depicted in a Fig. 1. The AS-Nd/ZnONCs absorbance edge peak is observed around 372 nm (Fig. 1).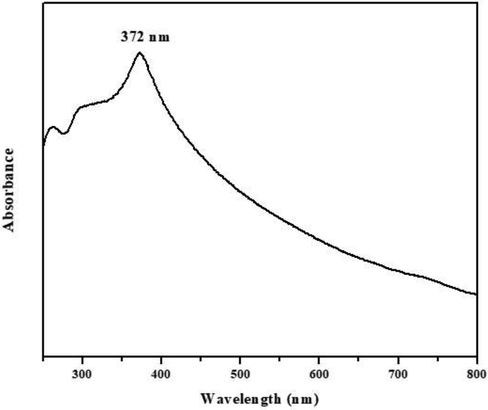
UV–Vis spectrum of the formulated AS-Nd/ZnONCs. The UV absorbance was confirmed the presence of formulated AS-Nd/ZnONCs and its absorbance edge peak is observed around 372 nm.
The X-ray diffraction pattern of AS-Nd/ZnONCs is depicted in the Fig. 2. The XRD peaks are located at angles (2θ) of 31.70, 34.35, 36.18, 47.56, 56.53, 62.80, 66.25, 67.87, 69.01 and 77.03 corresponding to (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2), (2 0 1) and (2 0 2) hkl planes of the AS-Nd/ZnONCs (Fig. 2). The standard diffraction peaks show the hexagonal wurtzite structure of AS-Nd/ZnONCs with space group p63mc (JCPDS Card No: 36–1451). However the AS-Nd/ZnONCs, the ionic radii of the Nd (III) ions 99.5 pm is higher than that of Zn (II) ions 74 pm, so the substitution of Nd (III) should make the cell parameter higher than of Zn and there is small change in the Zn-O lattice site. The NdZnO nanocomposite average crystallite size is 33.56 nm.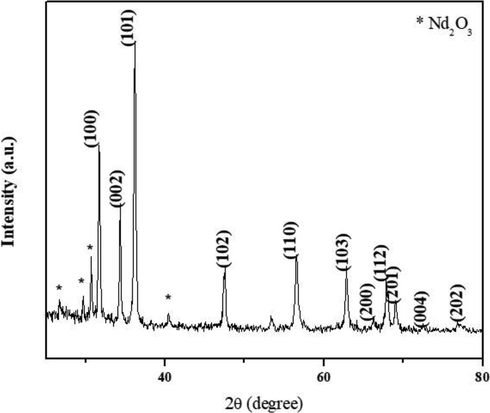
XRD patterns of formulated AS-Nd/ZnONCs. The XRD peaks are located at angles (2θ) of 31.70, 34.35, 36.18, 47.56, 56.53, 62.80, 66.25, 67.87, 69.01 and 77.03 corresponding to (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2), (2 0 1) and (2 0 2) hkl planes of the AS-Nd/ZnONCs.
The morphological image of green synthesized AS-Nd/ZnONCs is illustrated in the Fig. 3(a-b). In the FE-SEM image, the AS-Nd/ZnONCs is exhibit a hexagonal structure with uniform grain boundaries, and the average size is 43 nm (Fig. 3).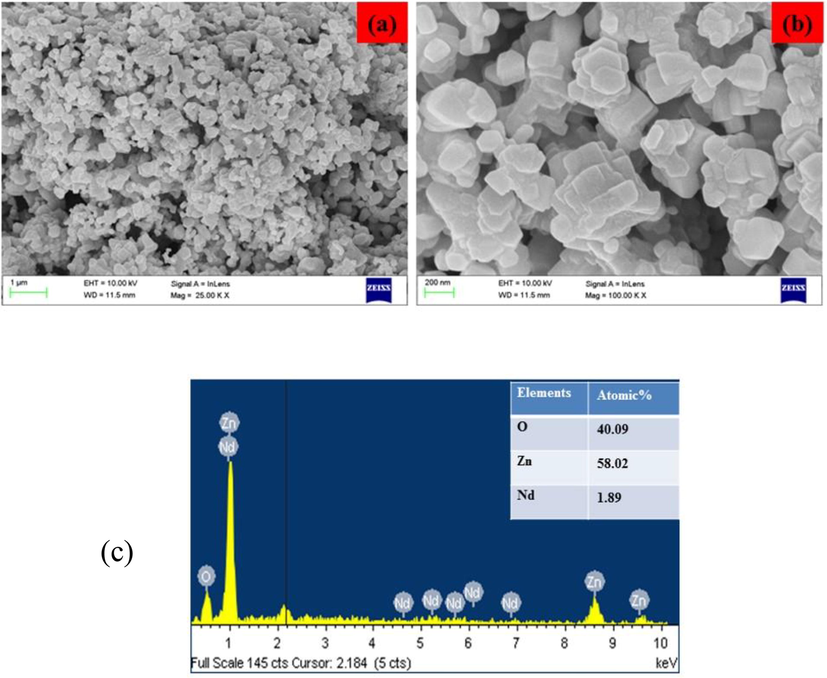
(a-b): FE-SEM images and EDAX analysis of the formulated AS-Nd/ZnONCs. The formulated AS-Nd/ZnONCs were studied using FE-SEM (a&b) and EDAX analysis (c), which confirmed the hexagonal structure with uniform grain boundaries of AS-Nd/ZnONCs with 43 nm average size.
The typical EDAX spectrum of the AS-Nd/ZnONCs is depicted in the Fig. 4. From the EDAX analysis, the AS-Nd/ZnONCs, the atomic percentage of the Nd atom is found to be 1.89%. The chemical compositions of Zn and O are monitored at 58.2 % and 40.09 % respectively (Fig. 3).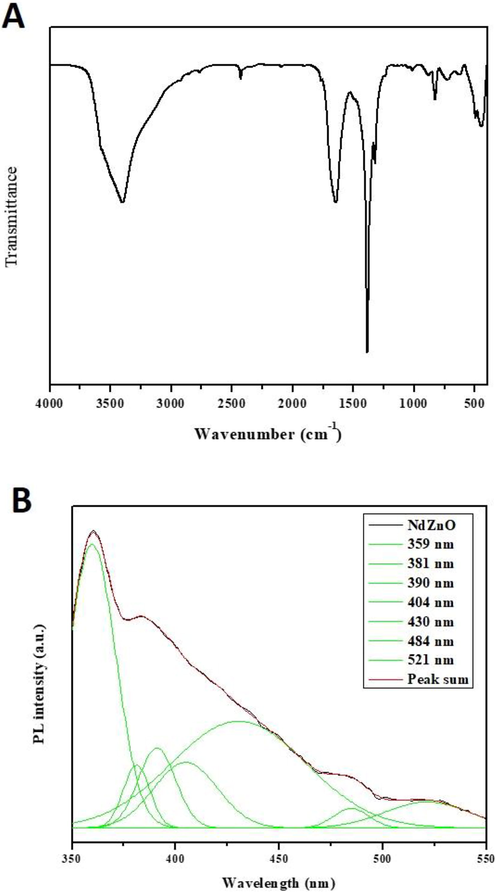
(a-b): FTIR spectra and PL spectrum of the formulated AS-Nd/ZnONCs. The FTIR spectra analysis (A) shows the different peaks that resembles the presence of various functional groups. (B) shows the spectrum analysis of formulated AS-Nd/ZnONCs.
The FT-IR spectrum of the AS-Nd/ZnONCs is displayed in the Fig. 4A. The absorption in the band 3750–3000 cm−1 are assigned to O–H stretching from residual alcohols, a water molecule. From the result, the O–H stretching band is observed at 3419 cm−1 for AS-Nd/ZnONCs (Fig. 4A). The symmetric and asymmetric C–H bonds are located at 2855 cm−1 and 2971 cm−1, due to the CH2 groups of AS-Nd/ZnONCs. The C⚌C stretching group around 1629 cm−1, which is responsible for Avaram Senna capping on the AS-Nd/ZnONCs. The C⚌O symmetric stretching band was observed at 1384 cm−1. From this outcomes, the weak Zn-O stretching bands are observed at 876 cm−1 for AS-Nd/ZnONCs. The Zn-O stretching bands appear at 470 cm−1 for the AS-Nd/ZnONCs.
The PL spectrum of the AS-Nd/ZnONCs emission values are observed at 359 nm, 381 nm, 390 nm, 404 nm, 430 nm, 484 nm, and 521 nm, separately (Fig. 4B). The PL spectra of AS-Nd/ZnONCs provide information about the crystal modality, structural and surface defects, etc. The UV radiations (359 nm, 381 nm and 390 nm) for near band edge (NBE) emission that is corresponding to free exciton recombination. The origin of the violet emission is centered at 404 nm and is corresponding to an electron transition from a shallow donor level of the natural zinc interstitials to the top level of the valence band. The blue and blue-green emission bands are observed at (430 nm and 484 nm) attributed to singly ionized Zn vacancies and interstitial oxygen vacancies. The green emission is origin at 521 nm, which is attributed to singly ionized oxygen vacancies in the AS-Nd/ZnONCs (Fig. 4B).
3.2 Antibacterial activity of formulated AS-Nd/ZnONCs
Antibacterial activity of AS-Nd/ZnONCs have tested against S. pneumonia, B. subtilis, K. pneumonia and P. vulgaris bacterial strains are inspected by well diffusion technique and the outcomes were illustrated in the Fig. 5. The results antibacterial activity of AS-Nd/ZnONCs clearly shows the zones of inhibition around the well loaded with various doses (1.0, 1.5, and 2.0 mg/ml) of AS-Nd/ZnONCs. The zone size observed (Fig. 5), ranged from 15 mm to 25 mm, depending on the AS-Nd/ZnONCs concentration and the nature of the test organisms.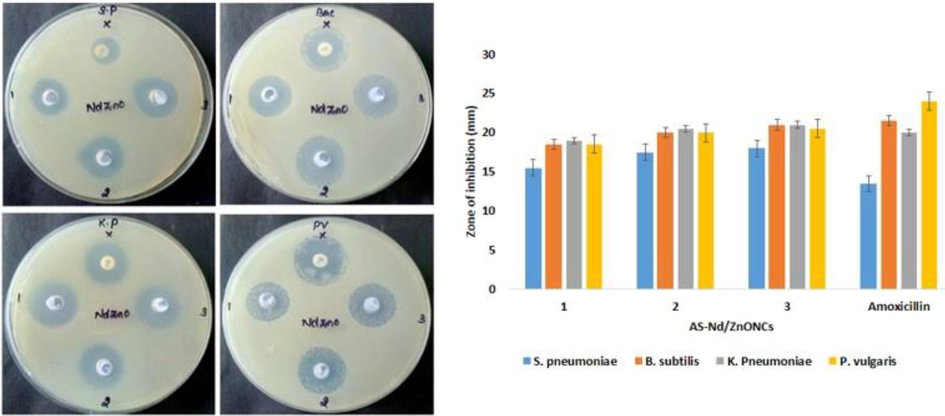
Antibacterial activity of the AS-Nd/ZnONCs. Antibacterial activity of AS-Nd/ZnONCs was demonstrated that all the tested bacterial strains i.e. S. pneumonia, B. subtilis, K. pneumonia and P. vulgaris are sensitive to the AS-Nd/ZnONCs. The inhibition zones were noted around 15 to 25 mm. Data were depicted as mean ± SD of triplicates. Outcomes were are scrutinized by one-way ANOVA consequently DMRT. Note: Data not sharing common superscript and significantly differs at p < 0.05.
3.3 Effect of AS-Nd/ZnONCs on the cell viability of BGC-823, AGS, and normal GES-1 cells
The cell viability of AS-Nd/ZnONCs treated GC AGS, BGC-823, and normal GES-1 cells were scrutinized by MTT assay and the outcome was depicted in the Fig. 6(A–C). The viability of AGS and BGC-823 cells were diminished notably with the treatment of AS-Nd/ZnONCs (1–15 µg) at dose-reliant manner. On the other hand, AS-Nd/ZnONCs treatment did not possessed cytotoxicity to the normal GES-1 cells with the same doses. The AS-Nd/ZnONCs appreciably suppressed the AGS BGC-823 cell viability and the IC50 dose of AS-Nd/ZnONCs were found at 7.5 µg for AGS cells. The remaining doses possessed great cytotoxicity to the AGS cells (Fig. 6A). This outcome clearly proved that the AS-Nd/ZnONCs display the selective cytotoxicity to the GC cells. Hence the 7.5 µg of AS-Nd/ZnONCs were opted for the additional investigations.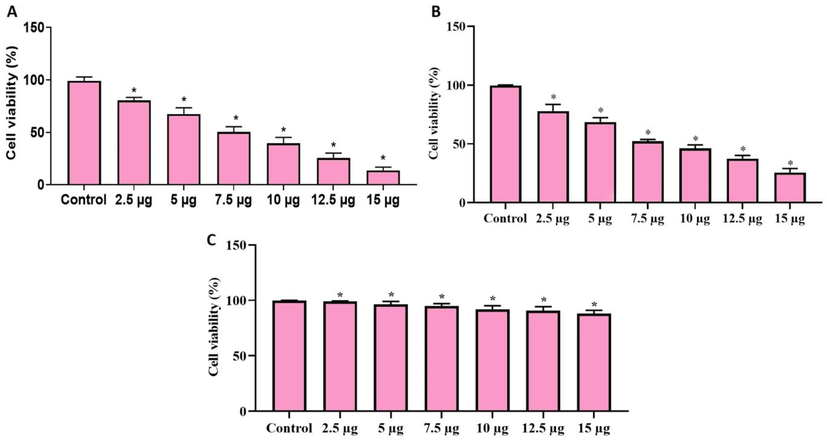
Effect of AS-Nd/ZnONCs on the viability of AGS cells. The AS-Nd/ZnONCs treatment remarkably diminished the AGS (Fig. 6A) and BGC-823 (Fig. 6B) cell viability and the IC50 dose was found at 7.5 µg for AGS cells. However, the viability of normal GES-1 cells was not affected by the AS-Nd/ZnONCs treatment (Fig. 6C). Data were depicted as mean ± SD of triplicates. Outcomes were are scrutinized by one-way ANOVA consequently DMRT. Note: Data sharing common superscript (*) and significantly differs at p < 0.05.
3.4 Effect of AS-Nd/ZnONCs on the apoptotic cell death in AGS cells
The apoptotic cell death in the AS-Nd/ZnONCs challenged AGS cells was studied by the AO/EB staining and the outcomes were illustrated in the Fig. 7A. As Fig. 7A demonstrated, the AS-Nd/ZnONCs treatment was notably enhanced the apoptotic cell death in the AGS cells, while compared with control. The AS-Nd/ZnONCs (7.5 µg) administered cells exhibited the higher orange/yellow fluorescence than control, which confirms the increased number of apoptotic cells. DOX administration also improved the apoptotic cell death in the AGS cells. Outcomes of 7.5 µg of AS-Nd/ZnONCs and DOX administered cells were found more similar with each other.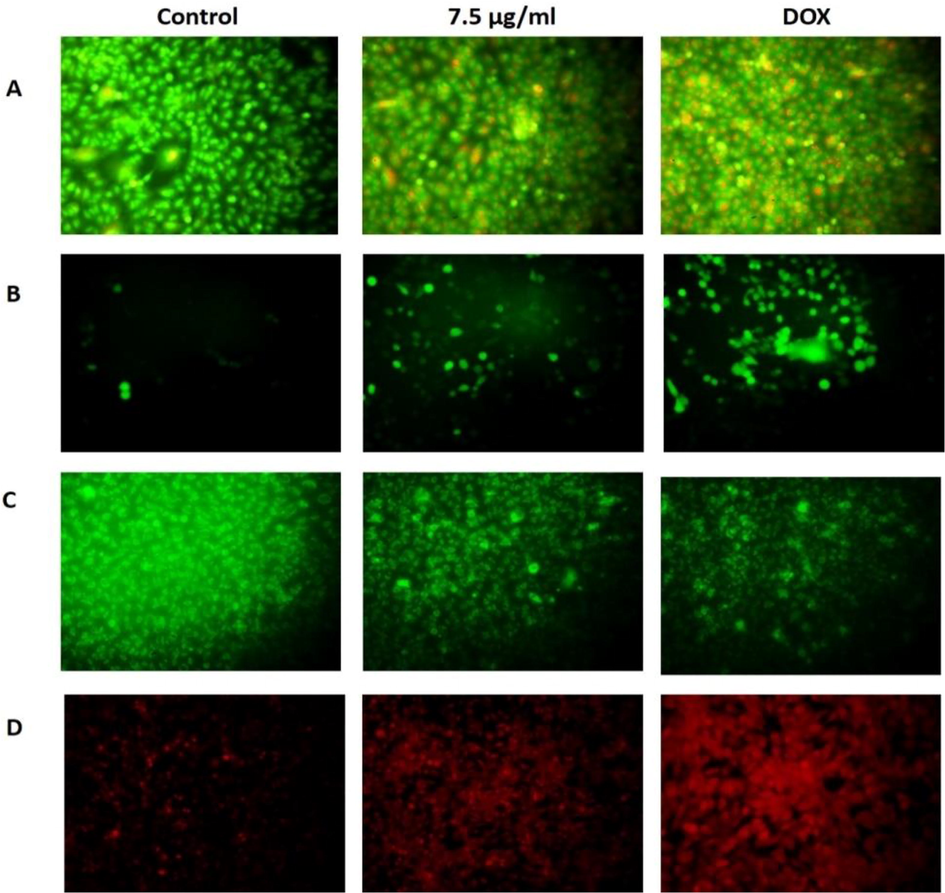
Effect of AS-Nd/ZnONCs on theAO/EtBr, ROS, MMP and PI staining levels in AGS cells. A. The apoptotic cell death in the AS-Nd/ZnONCs treated AGS cells were inspected by the AO/EB staining. Outcomes demonstrated that the AS-Nd/ZnONCs (7.5 µg) administered cells displays improved orange/yellow fluorescence that denotes the elevated number of apoptotic cells. B. The 7.5 µg of AS-Nd/ZnONCs challenged AGS cells demonstrates a bright green fluorescence that proves the enhanced ROS status. The outcomes of AS-Nd/ZnONCs and DOX were found similar with each other. C. The AGS cells administered with 7.5 µg of AS-Nd/ZnONCs exhibited the reduced green fluorescence than control that evidences the diminished MMP status in the AS-Nd/ZnONCs challenged AGS cells. D. The outcomes of PI staining evidenced that the AS-Nd/ZnONCs treated AGS cells displayed the increased signs of apoptosis. 7.5 µg of AS-Nd/ZnONCs administered cells improved that apoptotic cell death in AGS cells.
3.5 Effect of AS-Nd/ZnONCs on the ROS level in AGS cells
Fig. 7B revealed that the AS-Nd/ZnONCs administration notably enhanced the intracellular ROS status in the AGS cells. The 7.5 µg of AS-Nd/ZnONCs administered cells shows a bright green fluorescence that evidences the elevated ROS status in the AGS cells. The standard drug DOX also improved the ROS status of AGS cells. The outcomes of AS-Nd/ZnONCs and DOX were more similar, which is contrast to the control cells.
3.6 Effect of AS-Nd/ZnONCs on the MMP level in AGS cells
As depicted in the Fig. 7C, the MPP status of AGS cells were diminished gradually by the AS-Nd/ZnONCs administration. The 7.5 µg of AS-Nd/ZnONCs treated AGS cells displayed the diminished green fluorescence than control. This outcomes reveals the diminished MMP status in the AS-Nd/ZnONCs challenged AGS cells. As demonstrated by the AS-Nd/ZnONCs treatment, the standard drug DOX also suppressed the MMP status in the AGS cells (Fig. 7C).
3.7 Effect of AS-Nd/ZnONCs on the apoptotic cell morphology of AGS cells
The AS-Nd/ZnONCs provoked apoptosis in the AGS cells were identified by the PI staining and the outcome was depicted in the Fig. 7D. The AS-Nd/ZnONCs treated AGS cells demonstrated the clear signs of apoptosis. The treatment with the 7.5 µg of AS-Nd/ZnONCs were exhibited the chromatin condensation and membrane blebbings that evidences the apoptotic cells. The DOX treatment also improved the apoptosis in the AGS cells. The outcome of AS-Nd/ZnONCs was more comparable with the DOX treatment.
3.8 Effect of AS-Nd/ZnONCs on the cell adhesion in AGS cells
Fig. 8 reveals that the formulated AS-Nd/ZnONCs administered cells were demonstrated the declined cell adhesion, which is contrast to the control. The cell adhesion of AGS cells were effectively suppressed by the AS-Nd/ZnONCs treatment. The DOX treatment also restrained the AGS cell adhesion. The AS-Nd/ZnONCs and DOX was demonstrated almost similar kind of outcomes (Fig. 8).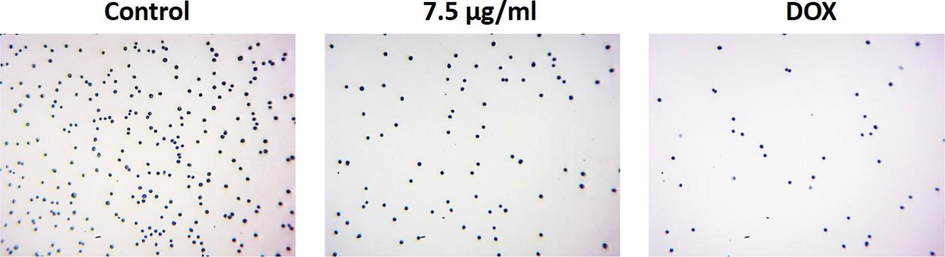
Effect of AS-Nd/ZnONCs on the cell adhesion in AGS cells. The cell adhesive property of AGS cells were effectively suppressed by the AS-Nd/ZnONCs treatment. The DOX treatment also inhibited the AGS cell adhesion.
3.9 Effect of AS-Nd/ZnONCs on LPO and antioxidants level in AGS cells
The status of LPO and antioxidants in the AS-Nd/ZnONCs administered AGS cells were investigated and the outcome was displayed in the Fig. 9. The LPO status was noticeably elevated in the AS-Nd/ZnONCs challenged AGS cells than the control. AS-Nd/ZnONCs also suppressed the antioxidants i.e. CAT, SOD, and GSH in the AGS cells, when compared with control. This finding evidenced that the AS-Nd/ZnONCs diminished the antioxidants and improved the oxidative stress in the AGS cells (Fig. 9). The AS-Nd/ZnONCs and DOX treatment demonstrated the similar kind of results.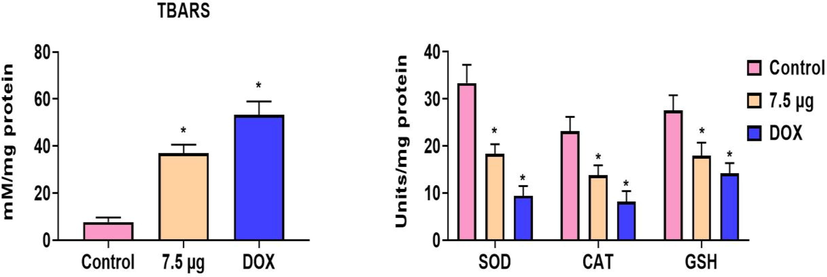
Effect of AS-Nd/ZnONCs on LPO and antioxidants level in AGS cells. The AS-Nd/ZnONCs challenge to the AGS cells demonstrated the improved LPO and diminished the antioxidants i.e. CAT, SOD, and GSH status. Data were presented as mean ± SD of triplicates. Outcomes were are scrutinized by one-way ANOVA consequently DMRT. Note: ‘*’ p < 0.05 compared with control.
3.10 Effect of AS-Nd/ZnONCs on the mRNA expressions of PI3K/AKT/mTOR signaling proteins in the AGS cells
The mRNA expressions of PI3K/AKT/mTOR signaling genes were investigated by RT-PCR and the outcomes were portrayed in the Fig. 10. The 7.5 µg of formulated AS-Nd/ZnONCs administered AGS cells exhibited the suppressed expression of PI3K, AKT, and mTOR genes. The mRNA expressions of PI3K, AKT, and mTOR genes in the AGS cells were down-regulated by the AS-Nd/ZnONCs treatment. The mRNA expressions of antiapoptotic gene Bcl-2 also diminished by the AS-Nd/ZnONCs administration. Contrastingly, the expression of apoptotic genes i.e. Bax and Bad was found up-regulated in the AGS cells. Hence, it was clear that the AS-Nd/ZnONCs appreciably inhibited the PI3K/AKT/mTOR signaling axis and triggered the apoptotic cascade in the AGS cells. The outcomes of AS-Nd/ZnONCs were more comparable with the DOX treatment.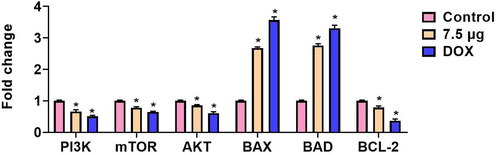
Effect of AS-Nd/ZnONCs on the mRNA expressions of PI3K/AKT/mTOR signaling proteins in the AGS cells. Fig. 10 evidenced that the mRNA expressions of PI3K/AKT/mTOR signaling genes were suppressed and apoptotic genes Bax and Bad expressions were enhanced by the AS-Nd/ZnONCs treatment in AGS cells. Data were presented as mean ± SD of triplicates. Outcomes were are scrutinized by one-way ANOVA consequently DMRT. Note: ‘*’ p < 0.05 compared with control.
4 Discussion
GC is one of the common cancers that have a direct impact on the numerous facets of life. In most incidences, GC was diagnosed at progressed stage that could restrict the lines therapies. The surgical resection along with the chemotherapy is the mainstay option for the GC based on the phases of the disease (Joharatnam-Hogan et al., 2020). Besides, the incidences with the far metastasis still have a deprived prognosis. Hence, the exploration for the novel sources of GC treatment essentially required to enhance the prevention and better management of the GC. To address this issues, nanodrugs refers to the utilizations of functionalized nano-carriers in the medical field exhibits a novel option for enhanced therapeutic approach providing the route for more personalized cancer treatments (Harris et al., 2002).
In this work, we formulated the AS-Nd/ZnONCs by the green process using Avaram Senna flower extract and investigated its anticancer potential against the AGS cells. The outcomes of characterizations studies revealed the formation of AS-Nd/ZnONCs. UV spectra revealed the absorbance edge peak of AS-Nd/ZnONCs at 376 nm (Fig. 1). The absorbance spectra of the fabricated AS-Nd/ZnONCs were is dependent on several parameters like bandgap, oxygen deficiency, surface roughness, and impurity centers (Azam et al., 2010). FT-IR study revealed the symmetric and asymmetric C–H bonds due to the CH2 groups of AS-Nd/ZnONCs (Munoz-Hernández et al., 2008). The C⚌C stretching group at 1629 cm−1 is responsible for Avaram Senna capping on the AS-Nd/ZnONCs (Perkins, 1987) From the FT-IR result, the weak Zn-O stretching bands are observed at 876 cm−1 for AS-Nd/ZnONCs (Devi and Velu, 2016). The PL spectra of AS-Nd/ZnONCs provide information about the crystal modality, structural defects, Zn interstitials etc. (Hameed et al., 2013).
The antibacterial efficacy of AS-Nd/ZnONCs generally depends on the generation of ROS that primarily related to the size, larger surface area, and Zn2+ ions release (Karthikeyan et al., 2020). From the antibacterial activity of AS-Nd/ZnONCs; it showed a similar antibacterial effect as compared to the standard antibiotic amoxicillin. The smaller sized NPs indeed have more activity as antibacterial. The XRD pattern showed the particle size of AS-Nd/ZnONCs was 33.56 nm. Nano size particles could effortlessly penetrate into bacterial cells because of their large surface area and the PL study show oxygen vacancies (Ov) at 521 nm of the sample. The elevated ROS production triggered by the AS-Nd/ZnONCs could responsible for their antibacterial potency (Bai et al., 2019).
Apoptosis plays a key function in the instigation and progression of tumors including GC. The apoptosis evasion is a predominant event of cancers. The dysregulation of apoptotic cascades accelerates tumor progression and metastasis. Apoptosis is a crucial event in guarding the organisms against anomalous proliferation via eliminating abnormally proliferating cells. Bcl-2 protein family consists of prime regulators, which are contributed in mitochondria-mediated apoptosis. Bcl-2 binds to mitochondrial membrane to block the deliverance of cytochrome-c to the cytosol. The pro-apoptotic protein Bax are necessary for the permeabilization of mitochondrial membrane during apoptosis (Green and Chipuk, 2008). As expected, AS-Nd/ZnONCs treatment activated the Bax and Bad expressions and inhibited the Bcl-2 expression in the AGS cells (Fig. 10). Our findings provided the evidence that the AS-Nd/ZnONCs treatment triggered the apoptotic pathway in the AGS cells. Oxidative stress is one of the imperative hallmark linked with cancers. This phenomenon was characterized as gene expression alterations, cellular metabolism, and homeostasis due to the excessive accretion of ROS and instabilities in antioxidant systems (Maciejczyk et al., 2019). In this work, we noticed that the AS-Nd/ZnONCs treatment elevated the LPO and suppressed the antioxidants CAT, SOD, and GSH status in the AGS cells (Fig. 9).
Earlier literatures were identified the processes of epigenetic regulation of PI3K/AKT signaling in GC (Yang et al., 2019). PI3K/AKT/mTOR signaling is vital for numerous cellular activities like proliferation, growth, survival, and intracellular trafficking (Schurmann et al., 2000). Overactivation of PI3K/Akt/mTOR signaling was concerned in numerous cancers, which providing a exclusive therapeutic target to design novel drugs against cancers (Ong et al., 2016). AKT performs a crucial function in the invasion and metastasis of various cancers due to its increased expression in distant metastasis than in primary tumors (Mohan et al., 2016). As expected, our findings from this work evidenced that the AS-Nd/ZnONCs treatment appreciably repressed the mRNA expression of AKT in the AGS cells (Fig. 10).
mTOR down-regulation could provoke the autophagy through elevating the numerous autophagy markers (Sohn and Park, 2017). This pathway could be triggered via a stimulation of mTOR and it can enhance the cell multiplication by inhibiting autophagy. mTOR can inhibit the autophagy commencement. As an crucial signaling cascade, targeting and inhibiting the PI3K/AKT/mTOR pathway could be a hopeful target for GC treatment (Khorasani et al., 2021). In this work, we noticed that the mRNA expressions of antiapoptotic gene Bcl-2 were diminished and the expressions of apoptotic genes i.e. Bax and Bad were found up-regulated in the AS-Nd/ZnONCs administered AGS cells. This finding proved that the AS-Nd/ZnONCs effectively inhibited the PI3K/AKT/mTOR signaling axis and triggered the apoptotic pathway in the AGS cells (Fig. 10). The findings from the fluorescence assays also evidenced the apoptosis inducing ability of AS-Nd/ZnONCs in the AGS cells. Altogether, our findings proved that the formulated AS-Nd/ZnONCs has displayed the potent cytotoxicity to the GC cells and triggered the apoptosis through the inhibition of PI3K/AKT/mTOR pathway.
5 Conclusion
In summary, AS-Nd/ZnONCs was prepared through the green process using Avaram Senna flower extract. The characterization studies confirmed that the prepared nanocomposite was exhibited the wurtzite hexagonal structures with average crystalline size was 33.56 nm. The antibacterial activity of the AS-Nd/ZnONCs proved its potent antibacterial activity. The findings from the in vitro anticancer assays evidenced that the AS-Nd/ZnONCs prevented cell growth and induced the apoptosis through the inhibition of PI3K/AKT/mTOR pathway and stimulation and apoptotic pathway in the AGS cells. Hence, AS-Nd/ZnONCs could be a possible chemopreventive agent in the future to treat the GC.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2021/383) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- PRL-3 promotes the peritoneal metastasis of gastric cancer through the PI3K/Akt signaling pathway by regulating PTEN. Oncol. Rep.. 2016;36:1819-1828.
- [Google Scholar]
- Akt/Pkb activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. APMIS. 2003;111:1105-1113.
- [Google Scholar]
- Loss of PTEN expression followed by Akt phosphorylation is a poor prognostic factor for patients with endometrial cancer. Endocr. Relat. Cancer. 2003;10:203-208.
- [Google Scholar]
- Pertuzumab plus trastuzumab and chemotherapy for HER2-positive Metastatic Gastric or Gastro-Oesophageal Junction Cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol.. 2018;19:1372-1384.
- [Google Scholar]
- Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discovery Today. 2017;22(12):1825-1834.
- [Google Scholar]
- Antihyperglycemic activity of various fractions of Cassia auriculata Linn: in Alloxan diabetic rats. Indian J. Pharm. Sci.. 2008;70:227-229.
- [Google Scholar]
- Hypoglycemic activity of Cassia auriculata in neonatal streptozotocin-induced non-insulin dependent diabetes mellitus in rat. J. Nat. Rem.. 2006;1:14-18.
- [Google Scholar]
- Cesalpinaceae -Cassia Auriculata Textbook of Medicinal Plants. IBH Publishing Oxford.. 2000;119
- [Google Scholar]
- Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J. Nutr. Biochem.. 2003;14:452-458.
- [Google Scholar]
- Anti-cancer effect of Cassia auriculata leaf extract in vitro through cell cycle arrest and induction of apoptosis in human breast and larynx cancer cell lines. Cell Biol. Int.. 2009;33:127-134.
- [Google Scholar]
- Amorphous and liquid semiconductor. London and New York: Plenum Press; 1974. p. :159.
- Resisting resistance: new chemical strategies for battling superbugs. Chem. Biol.. 2000;7(6):127-132.
- [Google Scholar]
- Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid per-oxidation. Eur. J. Biochem.. 1968;6:126-130.
- [Google Scholar]
- Involvement of superoxide anion radical in the auto-oxidation of pyrogallol and a constituent assay for superoxide dismutase. Eur. J. Biochem.. 1974;47:469-479.
- [Google Scholar]
- The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology. 1985;36(1):71-76.
- [Google Scholar]
- Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev.. 2020;85:101980
- [Google Scholar]
- Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25-36.
- [Google Scholar]
- Study of electrical properties of nickel doped SnO 2 ceramic nanoparticles. J. Alloys Compd.. 2010;506(1):237-242.
- [Google Scholar]
- Thermolytic growth of ZnO nanocrystals: morphology control and optical properties. Cryst. Growth Des.. 2008;9(1):297-300.
- [Google Scholar]
- Perkins, W.D., 1987. Fourier Transform - Infrared Spectroscopy, Part 2: Advantages of FT-IR, Journal of chemical education. 1987; 269–271.
- Synthesis, structural and optical properties of pure ZnO and Co doped ZnO nanoparticles prepared by the co-precipitation method. J. Theor. Appl. Phys.. 2016;10(3):233-240.
- [Google Scholar]
- Impact of alkaline metal ions Mg2+, Ca2+, Sr2+ and Ba2+ on the structural, optical, thermal and antibacterial properties of ZnO nanoparticles prepared by the co-precipitation method. J. Mater. Chem. B. 2013;1(43):5950-5962.
- [Google Scholar]
- Biomolecule chitosan, curcumin and ZnO-based antibacterial nanomaterial, via a one-pot process. Carbohydr. Polym.. 2020;249:116825
- [Google Scholar]
- PDIA6 modulates apoptosis and autophagy of non-small cell lung cancer cells via the MAP4K1/JNK signaling pathway. eBioMedicine. 2019;42:311-325.
- [Google Scholar]
- Insulin resistance and oxidative stress in the brain: what’s new? Int. J. Mol. Sci.. 2019;20:874.
- [Google Scholar]
- Emerging role of PI3K/AKT in tumor-related epigenetic regulation. Semin. Cancer Biol.. 2019;59:112-124.
- [Google Scholar]
- p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol. Cell Biol.. 2000;20:453-461.
- [Google Scholar]
- Judicious toggling of mTOR activity to combat insulin resistance and cancer: current evidence and perspectives. Front. Pharmacol.. 2016;7:395.
- [Google Scholar]
- Trisubstituted-Imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS One. 2016;11(4):e0153155
- [Google Scholar]
- Natural agents mediated autophagic signal networks in cancer. Cancer Cell Int.. 2017;17:110.
- [Google Scholar]
- The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur. J. Pharmacol.. 2021;898:173983
- [Google Scholar]







