Translate this page into:
Green synthesized silver nanoparticles using carrot extract exhibited strong antibacterial activity against multidrug resistant bacteria
⁎Corresponding authors. sobia@uoh.edu.pk (Sobia Nisa), aqayyum@uoh.edu.pk (Abdul Qayyum)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Antimicrobial resistance is a worldwide problem. Pathogenic microorganisms develop antibiotic resistance as a result of exposure to inappropriate quantity of antibiotics and accumulation of mutations. Bio-friendly nanomaterial scan be developed as antimicrobial agents. Present study aimed to investigate the activity of biosynthesized silver nanoparticles (AgNPs) obtained from carrot extract of three different regions namely Gilgit, Haripur and Sheikhupura from Pakistan, having significant difference in climate and altitude. UV–visible spectroscopy, Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) analysis were carried out for the morphological characterization of synthesized nanoparticles. Analysis of XRD revealed crystalline nature of AgNPs and average size calculated was about 22, 17 and 10 nm from Gilgit, Haripur and Sheikhupura samples respectively. The antibacterial efficacy of AgNPs was carried out against 7 American type culture collection strains of pathogenic bacteria and three macrolide resistant clinical isolates with promising results. Moreover, antioxidant activity indicated maximum DPPH radical scavenging effects as 47 % using a concentration of 250 μg/mL. Hemolysis assays indicated biocompatibility of AgNPs at lower concentration like 7 μg/mL and 15 μg/mL. Comparative analysis of bioactivity from different sites of sampling had indicated minute differences which may be due to the change in altitude, soil texture and other local environmental changes.
Keywords
Drug resistance
Silver nanoparticles
Antioxidant
Carrot extract
XRD
1 Introduction
Emergence of antimicrobial resistance is a substantial risk to human health (Nelson et al., 2019). Infections caused by antibiotic resistant bacteria leads to prolonged stay in hospital thus increasing the cost of treatment. Antibiotic resistant bacteria harbor resistant genes and can spread them through horizontal gene transfer. Due to extensive and inappropriate use of antibiotics, number of resistant pathogens has been increased resulting in complication of treatment (Keenan et al., 2015). Erythromycin, clarithromycin and azithromycin are bacteriostatic drugs that belong to class macrolides and are used to treat respiratory tract infections. They have a complex macrocyclic structure composed of a 14-, 15-, or 16-membered lactone ring replaced with neutral or amino sugar groups. They interfere with ribosomal subunits hence, inhibiting protein synthesis (Schroeder and Stephens, 2016). Macrolide-resistant S. pneumoniae strains have emerged as a result of robust selective pressure exerted due to the widespread use of macrolide, consequent in treatment failure (Keenan et al., 2015). According to a study by Gupta et al., (2021) 39 % of S. pneumoniae isolates were found to be resistant to macrolides and this frequency was increased to 47 % in case of respiratory isolates. They also indicated that macrolide resistance was higher in outpatient department signifying that macrolide resistance is widespread in outpatients. The genes responsible for macrolide resistance are drug efflux genes (acr, car, mac, amr, mef, mdt, mex, ole, opr, tor, srm, tlr and msr), genes responsible for ribosomal methylation (erm), and esterification or drug phosphorylation genes (Suzuki et al., 2022). These genes can spread through horizontal transfer among bacterial species hence there is a requirement to acquire alternative drugs and antimicrobial agents so that the rising issue of antibiotic resistance can be managed.
Silver (Ag) has the potential to inhibit growth of bacterial cell, disrupt bacterial cell walls, and interfere with cell metabolic pathways as a result of the interaction between Ag ions and cellular macromolecules. The Ag ions thus decrease membrane permeability, prevent protein synthesis and finally lead to loss of cell viability. The AgNPs are more reactive than Ag in their bulk and had indicated stronger antibacterial ability (Ankegowda et al., 2020; Singhal et al., 2021; Baker et al., 2021). In the past decade, silver nanoparticles (AgNPs) have gained significant attention and are extensive studied nanomaterials (Rao and Tang, 2017). Due to unique physical, chemical, biological and optical properties they have vast uses in drug delivery, biomedicine, topical ointments and creams, catalysis, electronics, food, agriculture and textile industry and in treatment of water (Mittal et al., 2013). Reports are available regarding anti-fungal, anti-angiogenesis, anti-inflammatory, anti-platelet anti-viral activity of AgNPs and they have been proved as effective antimicrobial agent against various pathogenic microorganisms (Bindhu and Umadevi, 2013). Physical, biological and chemical methods are being employed for NPs synthesis (Chen et al., 2008). These methods have their own advantages and disadvantages depending on the purpose of their utilization (Kholoud et al., 2010). Physical methods require high pressure and temperature thus specialized equipment is required (Iravani and Zolfaghari, 2013). Chemical methods had advantage of synthesizing large quantities of NPs in short duration but need capping agents to stabilize NPs and leads to non-friendly and toxic byproducts (Krithiga et al., 2015). Biological approaches of NPs synthesis do not require utilization of toxic chemicals as biological molecules themselves can act as capping agents, thus there is a need to develop green nanotechnology (Liu et al., 2015). Plants and microorganisms including both fungi and bacteria are used for green synthesis of NPs using intracellular and extracellular synthesis methods (Mujaddidi et al., 2021). However, it is advantageous to use plants for NPs synthesis as it does not require specialized and complex processes like isolation, maintenance of cultures and multiple steps of purification (Garibo et al., 2020). Different plant parts like leaf, stem, flower, peel, fruit and roots have been utilized for NPs synthesis. Many important phytochemicals like flavonoids, tannins, polyphenols, ascorbic acids, terpenoids and proteins are present in plant extract and contribute in reduction of metal salts, uptake of metal ions, and as capping agents for stabilizing NPs and inheritance of antimicrobial properties (Aritonang et al., 2019).
Carrots are rich in biotin, vitamin K (phylloquinone), potassium, vitamin B6 and beta-carotene (antioxidant) with significant biological impact. These biomolecules can serve as stabilizing and capping agents for synthesis of NPs. Although silver nanoparticle synthesis and their antibacterial activities are reported but no data is available regarding the effect of altitude or soil conditions on synthesis and bioactivities of nanoparticles. Thus the present study was aimed to synthesize biogenic silver nanoparticles using carrot extract from three different areas of Pakistan (Gilgit, Haripur and Sheikhupura) based on significant difference in environmental conditions and to determine their antioxidant and antibacterial potential especially against macrolide resistant bacteria.
2 Materials and methods
2.1 Collection of vegetable
Carrots were collected from local markets of Gilgit, Haripur and Sheikhupura, Pakistan. These areas were selected on the basis of difference in altitudes and climatic conditions. The altitudes of Gilgit, Haripur and Sheikhupura are 1500 m, 520 m and 236 m respectively.
2.2 Preparation of carrot extract
Tap water was used to wash the carrots and then rinsed with double distilled water. 250 g of carrot was separately boiled in 500 mL distilled water for 2 h and then obtained extracts were strained in the beginning through a muslin cloth which was followed by filtration through Whatmann filter paper No. 1. Lastly, filtration of the extracts was done through 0.45 μm filters (Millipore). Till further use, extracts were preserved and stored at 4 °C (Tamileswari et al., 2015).
2.3 Biosynthesis of silver nanoparticles
Silver nitrate was selected as a precursor metal salt for preparation of nanoparticles. The AgNPs were made by the reaction of carrot extract and 10 mM aqueous solution of AgNO3 (Merck). Silver nitrate solution (10 mM) was mixed with the aqueous extract of carrot extract at a ratio of 1:1 (v/v) to a volume of 100 mL in a flask. Flasks were covered in aluminum foil to prevent photo-activity. Flasks were incubated for different durations i.e. 1–8 hrs duration at different pH of reaction mixture that is (3, 5, 7 and 9) to optimize synthesis of biogenic NPs. UV–vis spectrophotometer (Cecil, 7000 series) was used for initial confirmation of NPs synthesis. The centrifugation of the solution was carried out at 14,000 rpm for 10 min. The resultant pellet was washed three times using distilled water after discarding the supernatant to discard any remaining contamination. The pellet was collected in a Petri plate and kept in drying oven between 60 °C and 70 °C until drying. Dried nanoparticles were scratched from Petri plate and grounded with pistol mortar and the resultant blackish fine powder was kept in falcon tubes at room temperature which was then used for further characterization and bioactivities.
2.4 Characterization of silver nanoparticles
Various analytical techniques such as a UV–visible spectrophotometer, Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD) analysis and Scanning electron microscopy (SEM) were used to characterize silver nanoparticles. FTIR, XRD and SEM were used to analyze quality, structure and composition of biosynthesized AgNPs by following method of Zia et al. (2017).
During UV–visible spectrophotometry full scan was obtained in a range of 200 nm to 800 nm with a resolution of 1 nm at a scan speed of 300 nm/min. Fourier transform infrared spectroscopy (Schimadzu Fourier Transform Infrared Spectrophotometer, Model 270) was used to identify biomolecules allied with the prepared AgNPs. Potassium bromide was added at the ratio of 1:100 to infrared AgNPs for Fourier Transform. Absorbance was recorded from 400 to 4000 cm−1. Different functional groups were indicated in different modes of vibration. The XRD diffractometer (X’pert PRO of PANalytical) was used to determine size of nanoparticles at 40 KV and 30 mA at 37 °C. X-ray grid was coated with AgNPs for analysis. Diameter and size of silver nanoparticles were calculated using peaks’ height and width. Results were analyzed using Debye Scherrer equation.
For performing scanning electron microscopy, a drop of the freshly biosynthesized AgNPs was poured on glass slide and allowed to dry. It was observed under SEM for analysis. Results of SEM and XRD were compared to determine the morphological nature and size of nanoparticles.
2.5 Biological activities of silver nanoparticles
2.5.1 Antibacterial assay
For the determination of antibacterial activity agar well-diffusion method was used against seven ATCC bacterial strains namely Staphylococcus aureus (ATCC 292013), Listeria monocytogenes (ATCC 35152), Acinetobacter baumannii (ATCC 19606), Bacillus spizizenni (ATCC 6633), Escherichia coli (ATCC 25922), Salmonella typhimurium (ATCC 14028), Klebsiella pneumoniae (ATCC 13883) and three macrolide resistant clinical isolates (Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli). Bacterial strains were incubated overnight in nutrient broth at 37 °C the turbidity of the 24 h fresh cultures were adjusted to 0.5 McFarland reagent. Bacterial lawn was made over the media plates by dipping sterilized cotton swabs in the diluted cultures. Sterilized cork borer was used to make wells (8 mm). Different concentrations of biosynthesized AgNPs that is 500 and 1000 µg/mL were used to determine antibacterial activity. Incubation of inoculated plates was done at 37 °C for 24 h, where clear region around the inoculated wells were considered as positive results for antibacterial effects. Antibacterial effects were determined by measuring zone of clearance (mm) using Vernier caliper (Mujaddidi et al., 2021). The assay was taken place in triplicate for each pathogen and the results were represented as mean.
2.5.2 Determination of IC50 value of silver nanoparticles
For determination of IC50 broth dilution technique was performed. Each microtiterplate was inoculated with bacterial suspension of 0.5 McFarland. Different concentrations (250 µg/mL, 125 µg/mL, 62.5 µg/mL, 31.25 µg/mL, 15.6 µg/mL and 7 µg/mL) of silver nanoparticles were used to counter the designated bacterial pathogens. Bacterial suspensions without nanoparticles were used as negative control. Microplates were incubated at 37 °C for 24 h and susceptibility of the verified pathogen was calculated by measuring optical density at 600 nm. The results were calculated in percentage inhibition and Table curve software 2D, Ver.4 was used to calculate IC50.
2.5.3 Antioxidant activity of silver nanoparticles
For the determination of antioxidant activity of AgNPs the DPPH free radical scavenging assay was performed following method of Nisa et al. (2020). Different concentrations of green synthesized silver nanoparticles (250 µg/mL, 125 µg/mL, 62 µg/mL, 31 µg/mL, 15.6 µg/mL and 7 µg/mL) were prepared by dissolving AgNPs in DMSO (Merck). Then 0.15 mL of prepared dilutions were mixed with 2.85 mL of DPPH reagent (1 mg of DPPH reagent per 25 mL of dimethyl sulfoxide) and incubated for 30 min in the dark. To find out the reduction of DPPH free radical the absorbance was recorded at 517 nm using UV–visible Spectrophotometer. Different concentrations (250 µg/mL, 125 µg/mL, 62 µg/mL, 31 µg/mL, 15.6 µg/mL and 7 µg/mL) of ascorbic acid (Sd Fine chem) in dimethylsulfoxide were used as positive control while for negative control solution containing reagents and solvents only was used. Following formula was used to calculate the DPPH free radical-scavenging activity: DPPH radical scavenging activity (%) = [(control OD − sample OD) / control OD)] × 100
Where:
Control OD = Absorbance without nanoparticles.
Sample OD = Absorbance in presence of the nanoparticles.
2.5.4 Hemolytic assay of green synthesized silver nanoparticles
Evaluation of the hemolytic activity was carried out using method described by Andra et al. (2008) with little modification. Fresh human blood was taken and then washed with phosphate buffered saline (PBS: 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 135 mM NaCl, pH-7.4, 2.7 mM KCl). After washing with buffered saline centrifugation was done at 1000 rpm for 10 min and rewashing with PBS was done repeatedly until the supernatant became colorless. Washed red blood cells were further diluted with PBS at the ratio of 1:10. Several concentrations (7–250 mg/mL) of silver nanoparticles were prepared by dissolving in DMSO and mixing them with 1 mL of red blood cells. The solution was then kept in incubation at 37 °C for 1 h and centrifugation was done at 1000 rpm for 5 min. The supernatant was taken to determine absorbance at 540 nm. Triton × 100 and PBS were used as positive and negative control respectively (Dobrovolskaia et al., 2008).
3 Results
3.1 UV–Visible spectrophotometer analysis
Synthesis of AgNPs was initially established by visual observation of change in color. After the exposure of carrot extract to Ag + in silver nitrate solution the change in color of the mixture appeared as dark brown and blackish due to reduction of Ag + ion and synthesis of AgNPs (Fig. 1).
Synthesis of silver nanoparticles from carrot extract along with control.
The AgNPs synthesized in each extract solution were analyzed using UV–vis spectroscopy to find out the peak spectrum of prepared Ag nanoparticles at different pH and time intervals. While moving from pH 3–9, initially no characteristic peak was observed at pH 3 and later at pH 5, 7 and 9 maximum absorbance was recorded for pH 9, followed by 7 and 5 indicating maximum nanoparticle synthesis at alkaline pH (Fig. 2). It has been reported in literature that alkaline pH favors the reduction of ion to NPs. The conversion of silver ion into silver nanoparticles obeys first order reaction and presence of NaOH or other agents that makes the pH of the medium alkaline favor the reaction (Yaseen et al., 2022). Furthermore, the alkaline pH also favors stability of the AgNPs (Khan and Talib, 2010).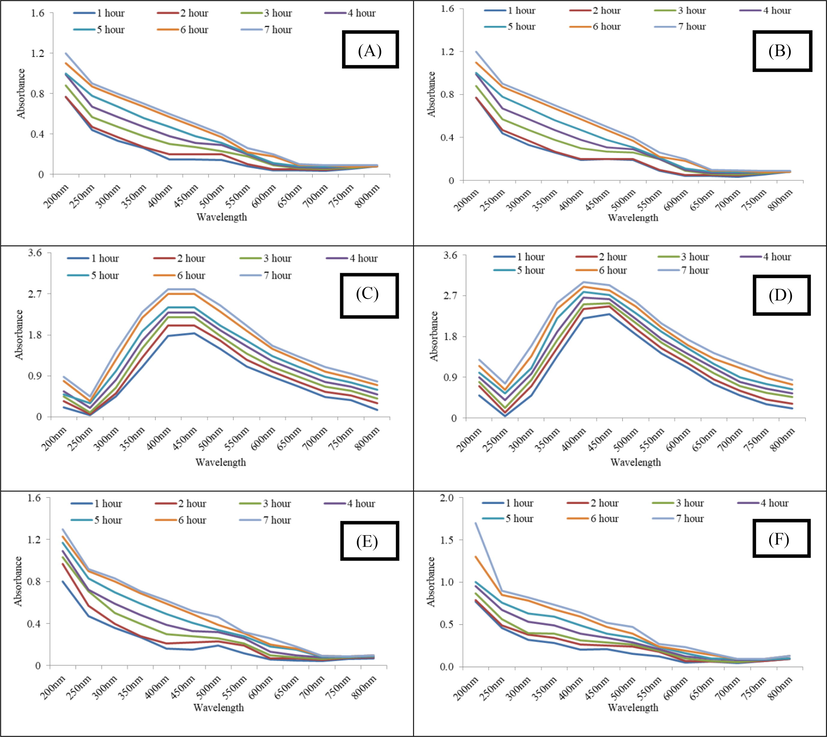
(A-D) UV–Vis spectrum of carrot extract AgNPs (Gilgit) synthesized at pH 3, 5, 7 and 9 (E-H) UV–Vis Spectrum of carrot extract AgNPs (Haripur) synthesized at pH 3, 5, 7 and 9 and (I-L) UV–Vis Spectrum of carrot extract AgNPs (Sheikhupura) synthesized at pH 3, 5, 7 and 9.
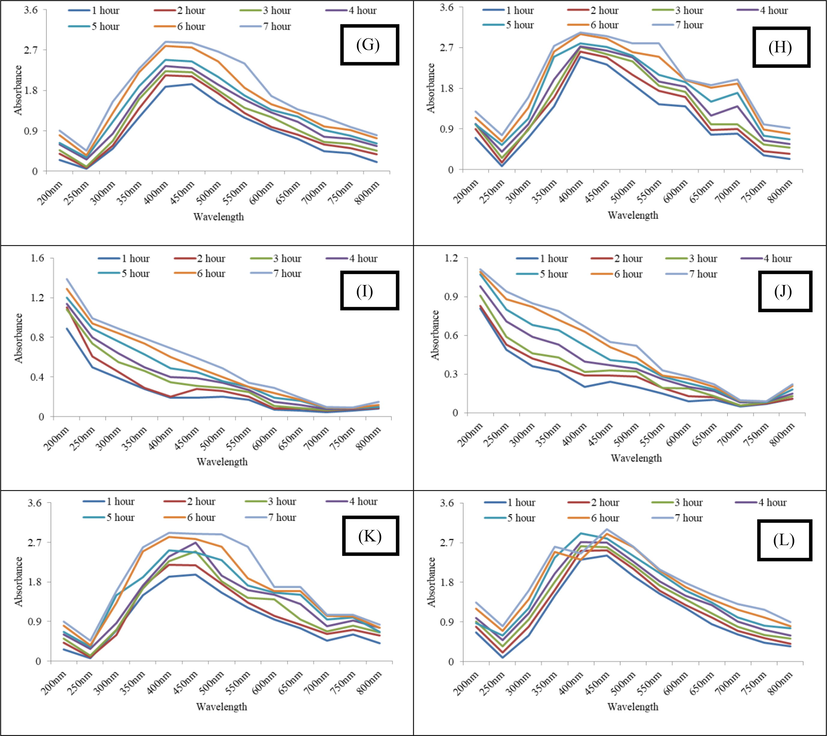
(A-D) UV–Vis spectrum of carrot extract AgNPs (Gilgit) synthesized at pH 3, 5, 7 and 9 (E-H) UV–Vis Spectrum of carrot extract AgNPs (Haripur) synthesized at pH 3, 5, 7 and 9 and (I-L) UV–Vis Spectrum of carrot extract AgNPs (Sheikhupura) synthesized at pH 3, 5, 7 and 9.
3.2 Fourier transform infrared spectroscopy (FTIR)
FTIR spectrum of AgNPs synthesized by carrot extract collected from Gilgit region revealed major peaks at 3242 cm−1 corresponding to –OH stretching, 1335.08 cm−1 corresponding to C-F and NO2, 572.63 and 531.14, 559.10, 547.05, 522.86 cm−1 relates to C-Br (Fig. 3A). Spectra of AgNPs synthesized from carrot extract collected from Haripur region revealed major peaks at 3203.24 cm−1 corresponds to –OH stretch which is due to the vitamin C, 1547.48 cm−1 determines C⚌O amide, 1336.04 cm−1 represents NO2 (N from silver nitrate) and 587.35 cm−1, 567.15 cm−1, 560.30 cm−1, 546.78 cm−1, 602.40 cm−1 indicates C-Br (Fig. 3B). In Sheikhupura samples FTIR spectrum showed absorption bands at 3195.80, 1391.69, 1004.11, 586.75, 546.85, 522.70 cm−1 which indicates the presence of capping agent with the nanoparticles (Fig. 3C). The band at 3195.80 cm−1 corresponds to –OH stretching vibration indicating presence of alcohol and phenol. Band at 1391.69 cm−1 denotes NO2 stretch (due to silver nitrate) and 1004.11 cm−1 corresponds to C-F and bands at 586.75, 546.85, 522.70 cm−1 represent C-Br which is characteristic of alkyl halides.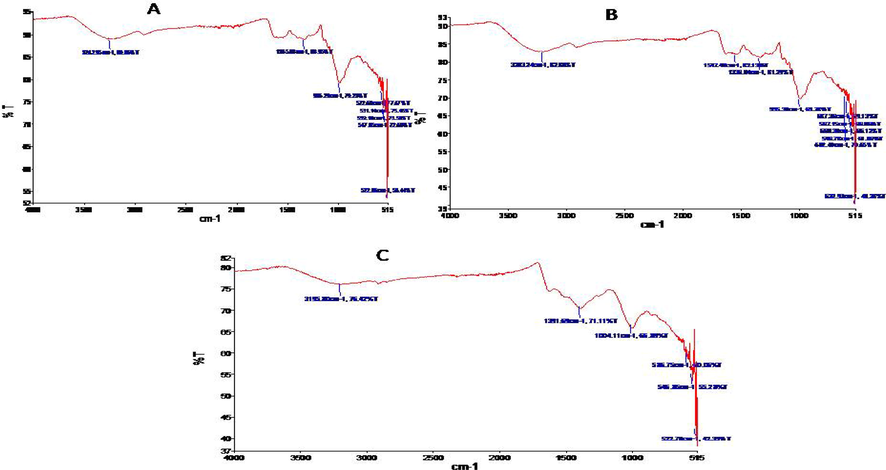
(A) FTIR spectra of carrot extract mediated silver nanoparticles collected from Gilgit, (B) FTIR spectra of carrot extract mediated silver nanoparticles collected from Haripur and (C) FTIR spectra of carrot extract mediated silver nanoparticles collected from Sheikhupura.
3.3 X-ray diffraction analysis
The biosynthesized silver nanoparticles from carrot extract of Gilgit region illustrated distinct diffraction peaks at 35° to 65° i.e. at 38.18°, 44.18°, 46.45° and 64.56° corresponding to planes of (1 1 1), (2 0 0) and (2 2 0) (Fig. 4A). The XRD peaks indicated crystalline nature of AgNPs with average size of 22.75 nm. The biosynthesized silver nanoparticles using carrot extract from Haripur, illustrated three peaks at 30° to 65° i.e. at 38.2°, 46.34° and 64.85° indexed to the (1 1 1), (2 0 0) and (2 2 0) planes (Fig. 4B). The XRD peaks indicated crystalline nature of AgNPs with average size of 17.12 nm. Similarly, nanoparticles synthesized from carrot extract collected from Sheikhupura region indicated peaks at 35° to 65° i.e. at 38.15°, 44.23° and 64.56°. The peaks were characterized and indexed to the (1 1 1), (2 0 0) and (2 2 0) which also confirmed the phase formation (Fig. 4C). The XRD peaks indicated crystalline nature of AgNPs with average size of 10.06 nm. The XRD peaks observed by all the three green synthesized AgNPs are in accordance to JCPDS data base (file no. 84–0713). The SEM analysis of biosynthesized AgNPs from Sheikhupura region (Fig. 5) further confirmed spherical shape and size of the nanoparticles ranging from 17 to 30 nm.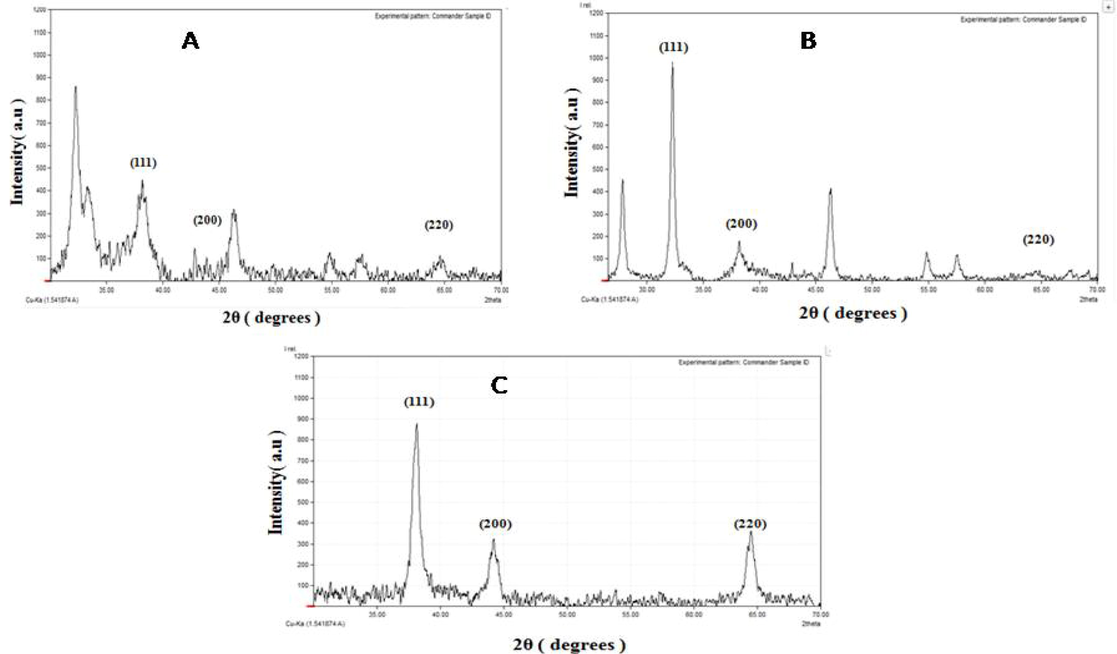
(A)XRD analysis of carrot extract’s silver nanoparticles collected from Gilgit, (B) XRD analysis of biosynthesized NPs collected from Haripur and (C) XRD analysis of carrot extract AgNPs collected from Sheikhupura.

SEM analysis of carrot extract AgNPs collected from Sheikhupura.
3.4 Biological assays of silver nanoparticles
3.4.1 Antibacterial activity
Antibacterial activity of AgNPs was determined against ATCC bacterial strains and macrolide resistant bacterial strains at a concentration of 500 and 1000 µg/mL. Results indicated dose dependent antibacterial effects of AgNPs. Maximum antibacterial activity exhibited by AgNPs synthesized with carrot extracts of Sheikhupura region with zone of clearance of 23 mm against S. typhimurium and S. aureus followed by Haripur and Gilgit region with zone of clearance of 20 and 19 mm against S. typhimurium at a concentration of 1000 µg/mL (Table 1). Similar trend of antibacterial activity was also observed against macrolide resistant bacterial strains but with reduced zones of clearance. The maximum activity (16 mm) was observed against E. coli and P. aeruginosa at a concentration of 1000 µg/mL by carrot extract mediated nanoparticles of Sheikhupura region followed by 15 and 10 mm of zone of clearance against E. coli by AgNPs mediated by carrots collected from Haripur and Gilgit region respectively (Table 2).
S. No
Bacterial Strains
Zones of clearance of Biosynthesized AgNPs at different concentrations
Gilgit
Haripur
Sheikhupura
500 µg/mL
1000 µg/mL
500 µg/mL
1000 µg/mL
500 µg/mL
1000 µg/mL
1
Staphylococcus aureus
9 ± 0.5
11 ± 0.8
18 ± 0.6
19 ± 0.56
15 ± 0.9
23 ± 1.5
2
Listeria monocytogenes
12 ± 1.2
17 ± 0.8
13 ± 1.6
18 ± 0.67
13 ± 1.5
19 ± 1.7
3
Bacillus spizizenni
5.5 ± 0.4
14.5 ± 0.9
11 ± 0.89
15 ± 1.1
13 ± 0.75
21 ± 0.87
4
Escherichia coli
10 ± 0.45
16 ± 1.3
12 ± 0.78
17 ± 0.55
16 ± 0.37
20 ± 1.56
5
Klebsiella pneumonia
12 ± 0.67
17 ± 1.23
13 ± 0.86
18 ± 0.65
14 ± 1.12
22 ± 1.45
6
Salmonella typhimurium
14.5 ± 0.5
19 ± 1.13
16 ± 1.5
20 ± 0.75
17 ± 0.53
23 ± 0.98
7
Acinetobacter baumanni
5 ± 0.53
5 ± 0.89
7 ± 1.54
11 ± 0.76
9 ± 0.66
12 ± 0.53
S. No
Bacterial Strains
Zones of clearance of Biosynthesized AgNPs at different concentrations
Gilgit
Haripur
Sheikhupura
500 µg/mL
1000 µg/mL
500 µg/mL
1000 µg/mL
500 µg/mL
1000 µg/mL
1
Staphylococcus aureus
0
5 ± 0.56
17 ± 1.1
11 ± 0.87
9 ± 0.65
13 ± 1.2
2
Escherichia coli
10 ± 1.65
10 ± 0.45
10 ± 0.78
15 ± 0.96
11 ± 0.75
16 ± 0.65
3
Pseudomonas aeruginosa
0
9 ± 0.98
12 ± 0.49
15 ± 1.4
14 ± 0.88
26 ± 1.43
Various concentrations of AgNPs synthesized by carrot extract of Gilgit, Haripur and Sheikhupura samples were used to determined IC50 value against macrolide resistant bacterial strains. Results indicated lowest IC50 values for AgNPs of Sheikhupura region with best activity against S. aureus (IC50 83.6 µg/mL) followed by E. coli (IC50 110.97 µg/mL) and P. aeruginosa (IC50 119.16 µg/mL). Nanoparticles synthesized by vegetable collected from Gilgit region indicated lowest efficacy against bacterial strains in terms of IC50 (Table 3).
Strains
IC50 (µg/mL)
AgNPs Haripur
AgNPs Sheikhupura
AgNPs Gilgit
Macrolide resistant Staphylococcus aureus
94.07
83.6
174.79
Macrolide resistant Escherichia coli
123.99
110.97
203.65
Macrolide resistant Pseudomonas aeruginosa
116.06
119.16
217.47
3.4.2 Antioxidant activity of biogenic silver nanoparticles
The nanoparticles synthesized from carrot extract exhibited different antioxidant activity depending on the concentration as well as source of nanoparticles. Best antioxidant potential was exhibited by nanoparticles mediated through carrot extract of Sheikhupura with 47 % free radical scavenging activity at a concentration of 250 µg/mL. While 42 % radical scavenging effects of silver nanoparticles synthesized by carrot extract of Haripur region was recorded at the same concentration. Biogenic nanoparticles exhibited concentration dependent free radical scavenging activity (Fig. 6).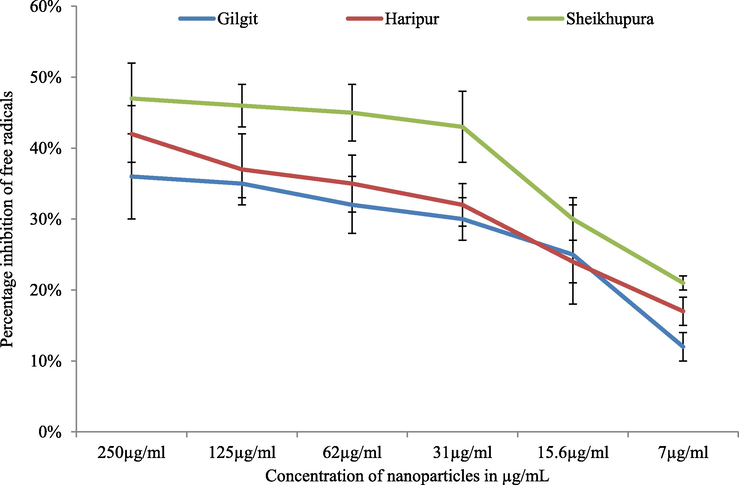
Free radical scavenging activity of biosynthesized AgNPs.
3.4.3 Hemolytic activity of green silver nanoparticles
Results of hemolytic assay indicated greater hemolytic activity at higher concentrations indicating toxicity of AgNPs at higher concentrations. Maximum hemolysis was recorded as 60 % at a concentration of 250 µg/mL for nanoparticles synthesized using carrot extract of nanoparticles from Sheikhupura followed by Haripur and Gilgit region. At 7 µg/mL, no hemolysis was recorded while less than 10 % activity was exhibited at 15.6 µg/mL, indicating their safe utilization at low concentration (Fig. 7).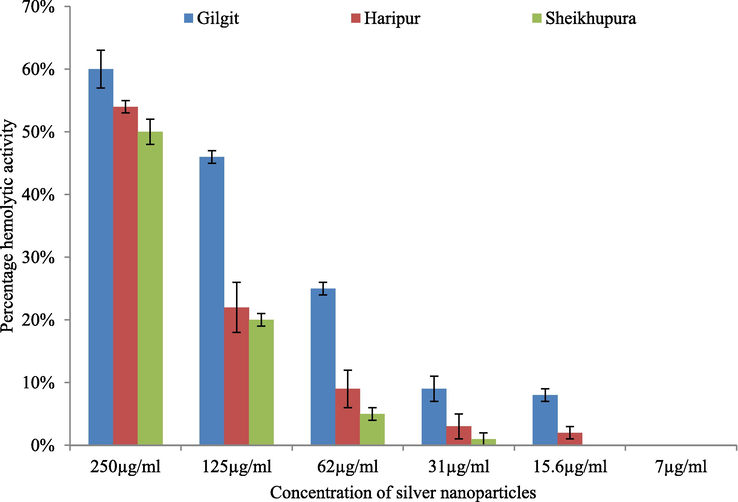
Hemolysis assay of AgNPs synthesized from carrot extract.
4 Discussion
In current era, interest in nanoparticles and their applications has been increased. Advanced area of nano-biotechnology involving living entities like algae, bacteria, viruses, fungi, and plants have been evolved. Use of toxic chemicals to prevent the agglomeration of nanoparticles limits their utilization in biomedical applications thus biologically synthesize nanoparticles have proved to be an attractive alternative as living things might transform chemical nature of the toxic metals by making them non-toxic or decreasing their toxicity (Sharma et al., 2001). The present study was objected to use carrot extract from carrots cultivated in different geographical locations of Pakistan to synthesize silver nanoparticles and to assess their efficacy against multidrug-resistant bacteria.
Confirmation of biosynthesis of silver nanoparticles was initially done by color changed from yellowish to dark brown after treating with silver nitrate. The color change appears as a result of excitation of surface plasmon resonance in the NPs (Ishnava and Shah, 2014). Further a strong peak at 430 nm confirmed extracellular synthesis of AgNPs by using UV visible spectroscopy as a result of excitation of electrons in the conductive band around the surface of nanoparticles (Busi et al., 2014).
pH is an important factor that affects synthesis of nanoparticles. Our results of effect of pH on synthesis of nanoparticles indicated that alkaline pH favors biosynthesis of AgNPs. Similar findings are also reported by Ibrahim (2015). FTIR spectra confirmed the involvement of bio-molecules in synthesis of AgNPs. Major peaks of FTIR spectra indicated the involvement of phytochemicals in capping of AgNPs. Important phytochemicals were amino and carboxylic groups belonging to proteins, alkaloids, hydrocarbons and some peaks corresponding to vitamin C. Results of our study are in accordance with work of Bagherzade et al. (2017). These XRD analyses revealed major peaks corresponding to the planes of 111, 200 and 220 in accordance to Pattanayak et al. (2014) who determined green synthesis of gold nanoparticles using aqueous extract of Daucus carota. The peaks of (1 1 1) and (2 0 0) planes are in accordance to green synthesized AgNPs using Convolvulus arvensis extract (Hamedi et al., 2017). Similarly peaks corresponding to planes of (1 1 1), (2 0 0) and (2 2 0) were examined by Benakashani et al. (2016) that could be attributed to the crystalline planes of the crystalline structure of metallic silver. Results of XRD and SEM were used to find out the size of bio-mediated nanoparticles and indicated smallest size for nanoparticles from Sheikhupura (10.06 nm) followed by Haripur (17.12 nm) and Gilgit (22.75 nm).
Results of antibacterial activity indicated rise in antibacterial activity by increasing the concentration of nanoparticles. Our results of dose dependent response are in accordance with previously published reports indicating larger zones of inhibition at higher concentration of nanoparticles (Gomathi et al., 2017). Antibacterial activities of AgNPs may be due to the release of silver ions, which can interact with microorganisms by getting attached to bacterial cell membrane, penetrating into the bacterial cells, and thus affecting membrane permeability and respiration (Bindhu and Umadevi, 2015). Response of antibiotic resistant bacteria differs from normal strains as they may modify their transport channels. Silver nanoparticles can also interact with DNA and result in lethal impairment of bacteria (Panacek et al., 2006). Silver nanoparticles mediated by carrot extract of Sheikhupura region exhibited highest antibacterial activity followed by Haripur and Gilgit region. The difference in the antibacterial activity can be elaborated by the fact that these regions differ in soil texture and local climatic conditions that can affect the phytochemical composition of plants (Logeswari et al., 2015). This difference in antibacterial activity can also be linked with the difference in average size of AgNPs as smaller size nanoparticles may easily target the bacterial nuclear content due to greatest surface area (Thiel et al., 2007). Similarly, enhanced antibacterial activity was observed in broth dilution method as compared to agar well diffusion method probably due to proper distribution of nanoparticles in broth rather than in agar. Thus it is suggested that silver nanoparticles in broth were in continuous interaction with bacterial cells therefore more activity was observed. Biosynthesized AgNPs also exhibited considerable antioxidant activity with maximum 47 % free radical scavenging capacity at a concentration of 250 µg/mL. These findings are in accordance to Gecer et al. (2021) who reported antioxidant activity of AgNPs mediated by dragon tongue bean outer peel extract and Echinacea purpurea (L.) respectively. The test on the hemolytic activity is a necessary component for the development of new pharmaceutical preparations together with determination of their antibacterial activity as membrane damage can be caused by interacting few peptide antibiotics with the erythrocyte membrane as a result of which hemoglobin can be released from the cell. The structural integrity of the lipid layer can also be lost due to severe damage caused by specific concentration of an antibiotic (Haug et al., 2016). Our results of biocompatibility experiments indicated cytotoxic effects at higher concentration while at lower dosage these effects were minimized. High hemolytic activity at effective concentrations limit wide use of a number of antibacterial preparations. The results of hemolytic activity are in line with Mujaddadi et al. (2021) who also reported hemolytic activity at higher concentrations of biosynthesized nanoparticles.
5 Conclusion
The phytochemical differences of vegetables grown in different regions effected the synthesis and dispersion of nanoparticles and hence properties. Synthesized silver nanoparticles revealed good antibacterial activity against the selected pathogenic bacteria. These biosynthesized AgNPs displayed antioxidant activity as well. However, at higher concentration the nanoparticles were found to be toxic thus it is concluded that higher concentration of nanoparticles cannot be used for biological application. However, these bioactive nanoparticles can be efficiently used for coating of surgical equipments and different surfaces being used in intensive care units.
Acknowledgment
The authors extend their appreciation to the Researchers supporting Project number (RSP-2021/367), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ankegowda, V.M.; Kollur, S.P.; Prasad, S.K.; Pradeep, S.; Dhramashekara, C.; Jain, A.S.; Prasad, A.; Srinivasa, C.; Sridhara Setty, P.B.; Gopinath, S.M.; S., R.P.; Bahkali, A.H.; Syed, A.; Shivamallu, C. Phyto-Mediated Synthesis of Silver Nanoparticles Using Terminalia chebula Fruit Extract and Evaluation of Its Cytotoxic and Antimicrobial Potential. Molecules 2020, 25, 5042. https://doi.org/10.3390/molecules25215042
- Structure and mode of action of the antimicrobial peptide arenicin. Biochem. J. 2008;410(1):113-122.
- [Google Scholar]
- Baker A, Iram S, Syed A, Elgorban AM, Bahkali AH, Ahmad K, Khan Sajid M, Kim J. Fruit Derived Potentially Bioactive Bioengineered Silver Nanoparticles. Int J Nanomedicine. 2021;16:7711-7726 --- https://doi.org/10.2147/IJN.S330763
- Biosynthesis of silver nanoparticles using Capparisspinosa L. leaf extract and their antibacterial activity. Karbala International Journal of Modern. Science. 2016;2(4):251-258.
- [Google Scholar]
- Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2013;101:184-190.
- [Google Scholar]
- Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2015;135:373-378.
- [Google Scholar]
- Green rapid biogenic synthesis of bioactive silver nanoparticles (AgNPs) using Pseudomonas aeruginosa. IET Nanobiotechnol.. 2014;8(4):267-274.
- [Google Scholar]
- Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett.. 2008;8(8):2180-2187.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Lysilomaacapulcensis exhibit high-antimicrobial activity. Sci. Rep.. 2020;10(1)
- [Google Scholar]
- Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with antioxidant profile. Particulate Science and Technology; 2021. p. :1-8.
- Green synthesis of silver nanoparticles using Daturastramonium leaf extract and assessment of their antibacterial activity. Resour.-Effic. Technol.. 2017;3(3):280-284.
- [Google Scholar]
- A Multicenter Evaluation of the US Prevalence and Regional Variation in Macrolide-Resistant S. pneumoniae in Ambulatory and Hospitalized Adult Patients in the United States. Open Forum Infect. Dis.. 2021;8(7)
- [CrossRef] [Google Scholar]
- Evaluation of the catalytic, antibacterial and anti-biofilm activities of the Convolvulus arvensis extract functionalized silver nanoparticles. J. Photochem. Photobiol. B Biol.. 2017;167:36-44.
- [Google Scholar]
- Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci.. 2015;8(3):265-275.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Pinuseldarica bark extract. Biomed Res. Int.. 2013;2013:1-5.
- [Google Scholar]
- Anticariogenic and hemolytic activity of selected seed protein extracts in vitro conditions. Journal of Dentistry (Tehran, Iran). 2014;11(5):576.
- [Google Scholar]
- Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv. Nutr.. 2015;6(2):198-205.
- [Google Scholar]
- Growth of different morphologies (quantum dots to nanorod) of Ag-nanoparticles: Role of cysteine concentrations. Colloids Surf.. 2010;76(1):164-169.
- [Google Scholar]
- Krithiga, N., Rajalakshmi, A., &Jayachitra, A. 2015. Green synthesis of silver nanoparticles using leaf extracts of Clitoriaternatea and Solanumnigrum and study of its antibacterial effect against common nosocomial pathogens. Journal of Nanoscience, 2015.
- Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc.. 2015;19(3):311-317.
- [Google Scholar]
- Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv.. 2013;31(2):346-356.
- [Google Scholar]
- Pharmacological properties of biogenically synthesized silver nanoparticles using endophyte Bacillus cereus extract of Berberis lyceum against oxidative stress and pathogenic multidrug-resistant bacteria. Saudi journal of biological sciences. 2021;28(11):6432-6440.
- [Google Scholar]
- Rapid molecular detection of macrolide resistance. BMC Infect. Dis.. 2019;19(1):1-12.
- [Google Scholar]
- Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B. 2006;110(33):16248-16253.
- [Google Scholar]
- Green Synthesis of gold nanoparticles using Daucuscarota (carrot) aqueous extract. World J Nano SciTechnol. 2014;3:52-58.
- [Google Scholar]
- Green synthesis of silver nanoparticles with antibacterial activities using aqueous Eriobotrya japonica leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol.. 2017;8(1):015014
- [Google Scholar]
- Macrolide resistance in Streptococcus pneumoniae. Front. Cell. Infect. Microbiol.. 2016;6:98.
- [Google Scholar]
- Influence of sodium ion on heavy metal-induced inhibition of light-regulatd proton efflux and active carbon uptake in the cyanobacterium Anabaena flos-aquae. World J. Microbiol. Biotechnol.. 2001;17(7):707-711.
- [Google Scholar]
- Singhal, M.; Chatterjee, S.; Kumar, A.; Syed, A.; Bahkali, A.H.; Gupta, N.; Nimesh, S. Exploring the Antibacterial and Antibiofilm Efficacy of Silver Nanoparticles Biosynthesized Using Punica granatum Leaves. Molecules 2021, 26, 5762. https://doi.org/10.3390/molecules26195762
- Macrolide resistance genes and mobile genetic elements in waterways from pig farms to the sea in Taiwan. Journal of global antimicrobial resistance.. 2022;29:360-370.
- [Google Scholar]
- Synthesis of silver nanoparticles using the vegetable extract of raphanussativus (radish) and assessment of their antibacterial activity. International Journal of Advanced Technology in Engineering and Science.. 2015;03(5):2348-7550.
- [Google Scholar]
- Detailed Kinetic and Mechanistic Study for the Preparation of Silver Nanoparticles by a Chemical Reduction Method in the Presence of a Neuroleptic Agent (Gabapentin) at an Alkaline pH and its Characterization. ACS Omega. 2022;7(7):5739-5750.
- [Google Scholar]
- Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET Nanobiotechnol.. 2017;11(2):193-199.
- [Google Scholar]







