Translate this page into:
Green synthesized chitosan modified platinum-doped silver nanocomposite: An investigation for biomedical and environmental applications
⁎Corresponding authors. gannadurai@msuniv.ac.in (Annadurai Gurusamy), mahamed@ksu.edu.sa (Maqusood Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Eco-friendly and inexpensive syntheses of metal-based nanocomposites is now gaining attention for their potential exploitation in biomedical and environmental fields. In this study, green method was applied to synthesize chitosan-modified platinum-doped silver nanocomposites (Ch-Pt-Ag NCs) using leaf extract of Desmostachya bipinnata, a plant with several medicinal values. Synthesis of Ch-Pt-Ag NCs was proven by XRD, SEM, FTIR, and TGA characterizations. Photocatalytic and biological activities of green-prepared Ch-Pt-Ag NCs were further explored. Crystallites of Ch-Pt-Ag NCs exhibited a mean size of 24 nm measured by XRD. SEM analysis indicated the formation of cubic and rod-shaped structures of Ch-Pt-Ag NCs. Functional groups present in plant extract were responsible for stabilizing and capping of Ch-Pt-Ag NCs, which were confirmed by FTIR analysis. Green synthesized Ch-Pt-Ag NCs exhibited dose-dependent anti-inflammatory and larvicidal (anti-dengue) activities. Green prepared Ch-Pt-Ag NCs exerted very low toxicity in Brine shrimp lethality assay indicated the biocompatible nature of synthesized nanostructures. The Ch-Pt-Ag NCs were also examined as a photocatalytic agent, resulting in 98% degradation of Rhodamine dye. Green synthesized Ch-Pt-Ag NCs with great biological and photocatalytic activities warrants further research on its biomedical and environmental fields.
Keywords
Nanocomposite
Biosynthesis
Desmostachya bipinnata
Photocatalysis
Anti-inflammatory activity
Larvicidal activity
Biocompatibility
1 Introduction
Nanostructures are very interesting materials from scientific and practical points of view because of their numerous applications in electronics, catalysis, sensor, biomedicine, and the removal of environmental contaminants (Ahamed et al., 2021a; Kumar et al., 2019). Chemical and physical routes for the preparation of nanostructures are not worthwhile for their application in biomedicine because of use of high pressure, high energy, and hazardous chemicals (Smitha et al., 2018). Green methods for production of nanostructures have been reckoned safe and environmentally friendly (Ahamed et al., 2022a). Moreover, green synthesis has also proven a cost-effective approach and led to complete elimination of toxic chemicals (Ahamed et al., 2022b). Green methods now attract researchers to produce multi-metallic nanostructures. Moreover, green-prepared multi-component metal nanoparticles (nanocomposites) have shown higher and cumulative properties than those of individual materials (Gholami-Shabani et al., 2022). Preparation of platinum (Pt)-doped silver (Ag) nanocomposites (NCs) has received considerable attention due to their unique physicochemical characteristics such as optical polarizability, electrical conductivity, catalysis, and surface-enhanced Raman scattering (SERS) (Ibrahim, 2015; Siddiqi and Husen, 2016). The Pt-doped Ag NCs with controlled morphology (due to easy synthesis) is crucial for uncovering their specific properties to attain their biological and environmental applications (Olajire et al., 2017; Unuofin et al., 2020).
Chitosan, a natural biodegradable polymer, is obtained by deacetylation of chitin (Abbasi, 2017). This linear polysaccharide has been widely used in pharmaceutical nanotechnology as a surface coating polymer (Abdel Aziz et al., 2015). It is well known that the coating of nanoparticles with chitosan can offer many advantages. Chitosan is a good stabilizer for Ag nanoparticles, which have high efficiency in kill of bacterial, and fungal pathogens (Ahamed et al., 2014; Chen et al., 2020). It is expected that chitosan-coated Pt-doped Ag NCs might have superior biological and photocatalytic activities than those of their individual components (Ahamed et al., 2021b).
Desmostachya bipinnata is a medicinally vital herb commonly known as dharba, belongs to the Poaceae family (BehlolAyaz et al., 2014). It is considered as a holy grass and comprehensively used in Indian Vedic practices during rituals (Guntur et al., 2018). It is well known for its medicinal values and is being utilized as traditional Indian medicine (Ayurveda) to treat several diseases such as bladder infections, jaundice, menorrhagia, hemorrhoids, diarrhea, urinary calculi, and asthma (Ibrahim et al., 2018). Leaf extract of Desmostachya bipinnata has shown analgesic, antibacterial, and anti-inflammatory activities (Guntur et al., 2018). Due to presence of several phytochemicals, Desmo the stachya bipinnata can be used for preparation of nanostructures. Identification of new green routes for synthesis of nanostructures for potential treatment of persistent global diseases is the need of hour.
There is limited information on green synthesis and its biological and photocatalytic activity of chitosan, Pt, and Ag based nanocomposites. In this present work, chitosan modified platinum-doped silver were nanocomposites (Ch-Pt-Ag NCs) was synthesized using the leaf extract of Desmostachya bipinnata. Phytochemicals present in the leaf extract Desmostachya bipinnata served as natural reducing and stabilizing agents for the preparation of Ch-Pt-Ag NCs. Green prepared Ch-Pt-Ag NCs was further examined for anti-inflammatory activity, larvicidal activity, and Brine shrimp lethality assay. Photocatalytic activity of Ch-Pt-Ag NCs was also examined by monitoring the degradation of Rhodamine dye under UV light irradiation.
2 Materials and methods
2.1 Preparation of leaf extract
Desmostachya bipinnata leaves were purchased from a local area of Alwarkurichi, India. Leaves were dried in shadow and ground into a fine powder by an electric grinder. Leaf powder (50 g) was steeped in 500 ml distilled water for 20 min before being boiled at 150 °C for 2 h. The soaked powder was maintained in an incubator at 37 °C overnight (around 12 h) for optimum extraction. The solvent was evaporated through a rotatory evaporator at 40 °C after the extract was filtered through a filter paper (Whatman no. 1823). The resulting leaf extract was kept at 4 °C for further application.
2.2 Synthesis and characterization of chitosan coated Pt-doped Ag nanocomposites
Firstly, Pt-doped Ag nanoparticles was synthesized. Briefly, 1 mM of chloroplatinate and 1 Mm of silver nitrate was dissolved in 80 ml of distilled water. Then, 20 ml of leaf extract was added and kept the mixture on a magnetic stirrer at 60 °C for 2 h. After the reaction was completed, the liquid was allowed to cool at 25 °C before being centrifuged for 20 min at 4000 rpm. The obtained pellet was washed with distilled water and dried in an oven for 1 h at 90 °C. The dried Pt-doped Ag nanoparticles was then ground into fine powder in a pestle and mortar.
Secondly, 1 g of the raw chitosan powder and 1 g of Pt-doped Ag nanoparticles were suspended in 10 ml of distilled water. Then, 5 ml of acetic acid was added to this mixture and stirred at room temperature for 24 h. After that obtained turbid suspension filtered and dried under vacuum to get fine powder Ch-Pt-Ag NCs.
Green synthesized Ch-Pt-Ag NCs was characterized by UV–vis spectroscopy, fluorescence spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDX), Fourier-transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and dynamic light scattering (DLS).
2.3 Photocatalytic activity
The photocatalytic performance of Ch-Pt-Ag NCs was evaluated by monitoring the degradation of Rhodamine dye under UV irradiation. In this procedure, 0.1 g of NCs was added to 100 ml of aqueous solution of Rhodamine dye with an initial concentration of 1 ppm. Prior to irradiation, the suspension containing NCs and dye was stirred in dark for 30 min to achieve adsorption/desorption equilibrium. Then, suspension was irradiated with UV light for different time intervals. During irradiation around 2 ml of the suspension was taken from the mixture at regular intervals (30 min) and centrifuged to separate the photocatalyst particles. Then the supernatant was analyzed by UV–vis spectrophotometer to measure the concentration of Rhodamine solution which exhibits characteristic absorption at λmax of 550 nm.
The degradation efficiency was calculated using following formula. where Co = initial Rhodamine dye concentration, and C = concentration of the Rhodamine dye solution after the degradation time ‘t’.
2.4 Anti-inflammatory activity
Green prepared Ch-Pt-Ag NCs was applied to see inhibition of denaturation of bovine serum albumin (BSA) as reported elsewhere (Gunathilake et al., 2018). The diclofenac sodium was used as a standard reagent. Ch-Pt-Ag NCs was dissolved in dimethylformamide (DMF) and diluted with 0.2 M of phosphate buffer (pH 7.4). The obtained DMF concentration in all the solutions was maintained by less than 2.5%. The 4 ml containing the different concentrations (10–50 µl/ml) of NCs was mixed in 1 ml of BSA (1 mM) incubated for 15 min at 37 °C and then heated to 50 °C for 15 min. After cooling samples to room temperature the turbidity was estimated at 660 nm by UV–vis spectrophotometer. Percentage of protein denaturation was determined utilizing the following equation,
2.5 Brine shrimp lethality assay
Brine shrimp assay was applied to see the toxicity/biocompatibility of Ch-Pt-Ag NCs as described earlier (Ali and Mohammed, 2021). Different concentrations of green prepared NCs (20–100 µl/ml) were added to the pre-labelled vials containing 10 live brine shrimp nauplii in 5 ml simulated seawater. After 24 h, the vials were examined by magnifying glass and the number of survived nauplii in each vial was counted. The mortality endpoint of this bioassay was defined as the absence of controlled forward motion during 30 s of observation.
2.6 Larvicidal assay
Larvicidal potential of biogenic Ch-Pt-Ag NCs was assessed as described by World Health Organization (WHO, 2005). Four experimental groups and one control group were created for this assay. Experimental groups used plastic well holding 25 third instar larvae and 200 ml of the tested suspension, whereas the control group used distilled water. Four technical replicates were designed for each group containing 500 larvae per Larvicidal assay simplified as (100 larvae per concentration × 4 concentrations) + (100 larvae per control group). We provided standard insectary condition 28 ± 1 °C temperature, 80 ± 10% relative humidity, and 12 h light/12 h dark period. The purpose of the condition was to maintain larvae while performing the bioassay. The goal of not feeding the larvae for 24 h after starting the bioassay was to record the death rate of the larvae during the test. The mortality was determined using a typical procedure in which the larvae were given a stimulation and if they did not respond were counted dead.
3 Results and discussion
3.1 UV–vis and florescence spectroscopy study
The UV–vis spectra were recorded to study optical property of Ch-Pt-Ag NCs. The absorption spectrum shows a characteristic surface plasmon resonance (SPR) band of Ch-Pt-Ag NCs between 340 and 450 nm, with a peak around 360 nm (Fig. 1a). Visibly Ch-Pt-Ag NCs were well disseminated in suspension and relatively stable. As shown in Fig. 1b, the fluorescence spectra of Ch-Pt-Ag NCs were recorded at 350–800 nm wavelength range by fluorescence spectrophotometer. Ch-Pt-Ag NCs have one distinct peaks at 647 nm, which was in agreement with previous work (Rehan et al., 2018).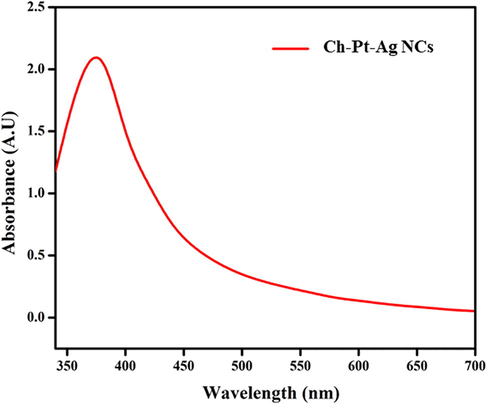
Optical spectra of green prepared Ch-Pt-Ag NCs.
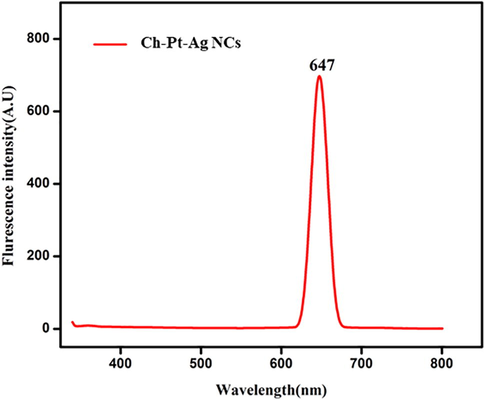
Fluorescence spectrum of green prepared Ch-Pt-Ag NCs.
3.2 X-ray diffraction study
Fig. 2 shows the XRD spectra green synthesized Ch-Pt-Ag NCs. Diffraction peaks were at 2θ values of 32°, 45°, 54°, 68°, and 76° corresponding to crystallographic planes of (1 1 1), (2 0 0), (1 1 0), (2 2 0), and (3 1 1), respectively. These diffraction peaks are characteristic of a face centered cubic structure of Ag (JCPDS Card No. 04–0783) (Unuofin et al., 2020). Presence of one unknown peak indicates some impurity from plant extract. The crystal size of Ch-Pt-Ag NCs was determined using Scherer’s formula. The calculated crystalline size was approximately 25 nm. XRD data of present study was according to previous reports (Shah et al., 2018).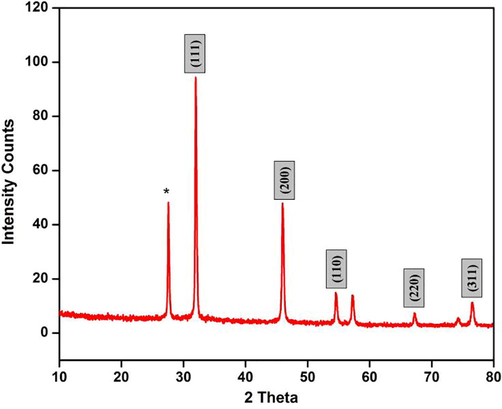
XRD spectra of green prepared Ch-Pt-Ag NCs.
3.3 Dynamic light scattering study
Particle size distribution in aqueous medium (Hydrodynamic size) of green prepared Ch-Pt-Ag NCs was examined by dynamic light scattering (DLS). Prepared sample was suspended in distilled water, sonicate in water bath for 30 min, DLS experiment was performed. Fig. 3 showed that hydrodynamic size of Ch-Pt-Ag NCs in distilled water was around 55 nm. Hydrodynamic size of Ch-Pt-Ag NCs was almost two-times higher than particle size of nanopowder calculated from XRD and SEM. Higher hydrodynamic size of Ch-Pt-Ag NCs could be due agglomeration of nanocomposites in aqueous medium (Ahamed et al., 2017).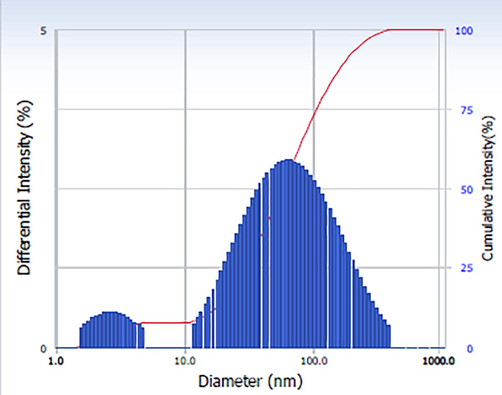
DLS study of green prepared Ch-Pt-Ag NCs.
3.4 Fourier transform infrared (FTIR) spectroscopy
FTIR analysis of green prepared Ch-Pt-Ag NCs was carried out at the wave number range from 450/cm to 4000/cm. As we can see in Fig. 4 the prominent bands of absorbance were observed at around 3122, 2866, 2130, 1878, 1633, 1529, 1058, 883 and 496 cm−1. The observed peaks denote N–H stretch amines, amides, C–H stretch alkanes, nitrile C⚌N stretch, C⚌O (saturated aldehyde) Aldehydes and Ketones, C⚌C stretch (conjugated) alkenes, C-F stretch alkyl halides, C-N Amines, C–H bend (mono) aromatics, C–Br stretch alkyl halides respectively (Table 1). These bands denote stretching vibrational bands of compounds e.g. flavonoids and terpenoids, which might be responsible for efficient capping and stabilization of Ch-Pt-Ag NCs (Kanakarajan et al., 2018).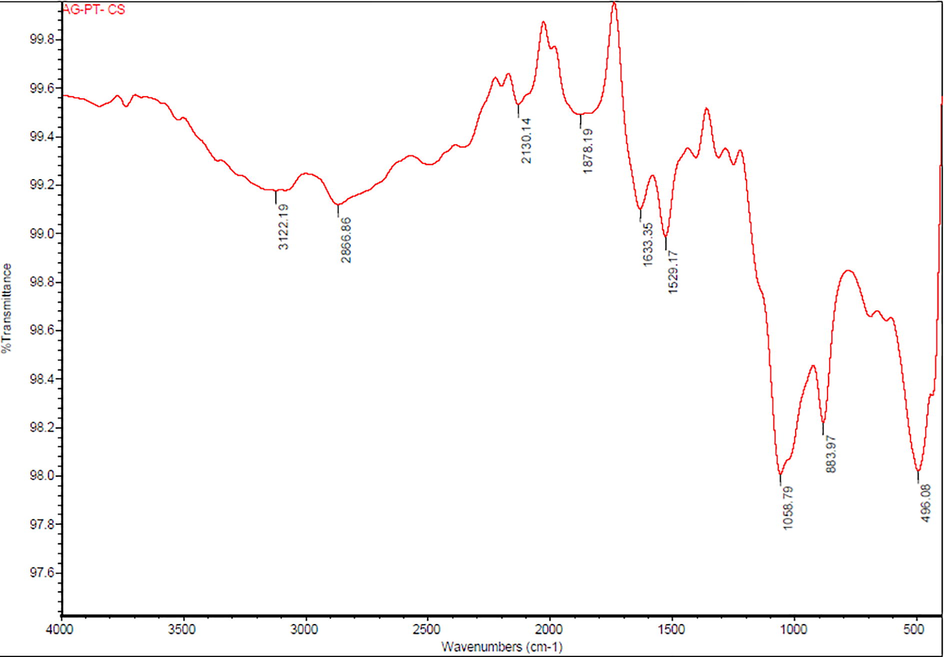
FTIR spectrum of green prepared Ch-Pt-Ag NCs.
S.No
Peak (cm−1)
Functional Group
1
3122
N–H stretch 1°, 2° amines, amides
2
2866
C–H stretch alkanes
3
2130
Nitrile C⚌N Stretch
4
1878
C⚌O (saturated aldehyde) Aldehydes & Ketones
5
1633
C⚌C stretch (conjugated) alkenes
6
1529
C–F stretch alkyl halides
7
1058
C–N Amines
8
883
C–H bend (mono) aromatics
9
496
C–Br stretch alkyl halides
3.5 SEM and EDX study
Typical SEM images of green synthesized Ch-Pt-Ag NCs are shown in Fig. 5a. These images suggested the irregular shape of prepared sample. This could be due to presence different types of phytochemicals present in leaf extract utilized in the formation of Ch-Pt-Ag NC. Our results are supported by others work (Banerjee et al., 2014). Phytochemicals present in plant extract plays crucial role in synthesis and physicochemical properties of prepared nanostructures. This is also supported by the shifts and difference in areas of the peaks obtained in the FTIR analysis. EDX analysis was carried out to understand elemental composition of green synthesized Ch-Pt-Ag NCs. Fig. 5b showed that Ag and Pt were major elements in green prepared sample.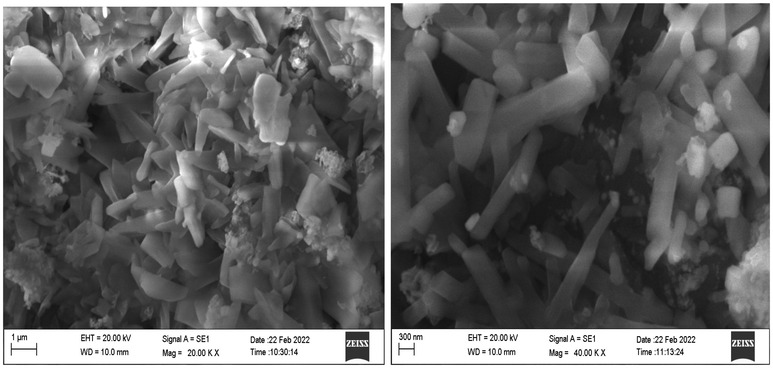
SEM images of green prepared Ch-Pt-Ag NCs.
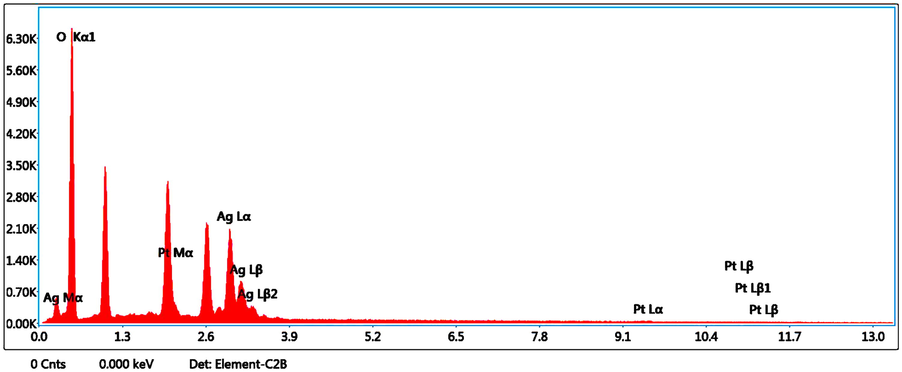
EDX spectrum of green prepared Ch-Pt-Ag NCs.
3.6 TGA
Fig. 6 shows the thermogravimetric analysis (TGA) of green synthesized Ch-Pt-Ag NCs. The temperature ranges were from 50⁰ to 700⁰ ⁰C. At 75 ⁰C, the initial weight loss correlates to the loss of nitrate compounds. After 235 ⁰C, the peak seen corresponds to the decomposition of covalently bound organic material, primarily nitrate, which was transformed to oxide during production. Exothermic peak can be seen in DTA curves of Ch-Pt-Ag NCs between 100 ⁰C and 300 ⁰C due to the desorption and breakdown of nitrate compounds. The initial weight loss detected in TGA was below 75 ⁰C, indicating the loss of water evaporated on the surface of sample. Adsorption due to the breakdown of covalently bonded organic material causes the peak rise above this temperature (Azhary et al., 2019). The weight loss calculated for the Ch-Pt-Ag NCs produced was 24.5%.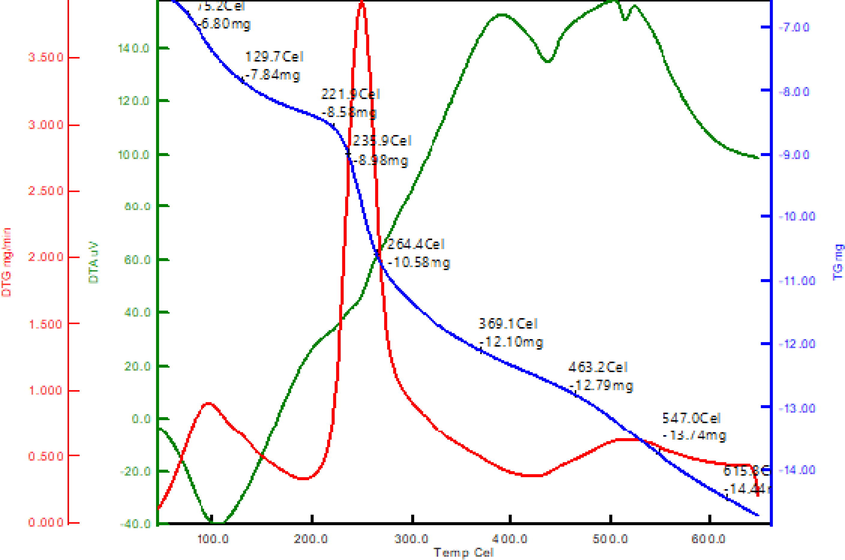
TGA analysis of green prepared Ch-Pt-Ag NCs.
3.7 Photocatalytic activity
Ch-Pt-Ag NCs demonstrated a time-dependent degradation of rodamine dye under UV irradiation. Figures 7a and b showed that with an increase of UV light illumination in the initial 5 min, the degradation was observed at 5.6%, while with a further increase in time, the degradation of dye also increased and the maximum degradation was observed at 240 min, which was 98%. Possible mechanisms of Rhodamine dye degradation is shown in Scheme1. The dye is first adsorbed on the catalyst's surface (in this case Ch-Pt-Ag NCs), after which it is subjected to ultraviolet light to excite valence electrons and allow them to transfer from the valence band to the conduction band. During this process, holes (h+) is lifted inside the valence band. The holes and free electrons will react on the photocatalyst's surface with adsorbed water molecules, producing •OH radicals while the free electrons convert the dissolved oxygen to superoxide anion O2• radicals. The dye molecules are degraded into simple molecules such as H2O by these light-generated radicals (El Mragui et al., 2021; Taha et al., 2020, Fig. 7.).
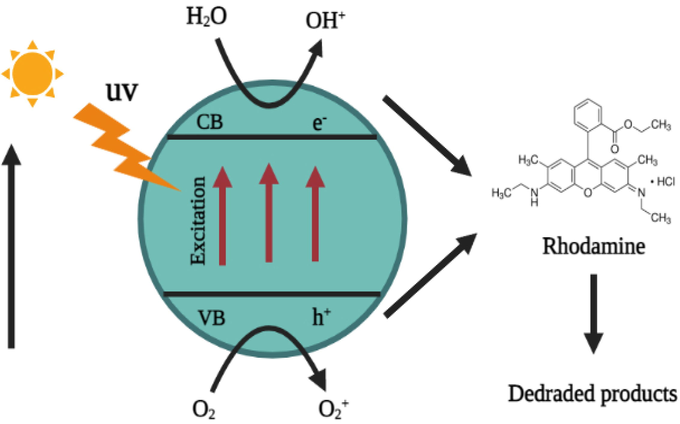
Reaction mechanism of Rhodamine degradation.
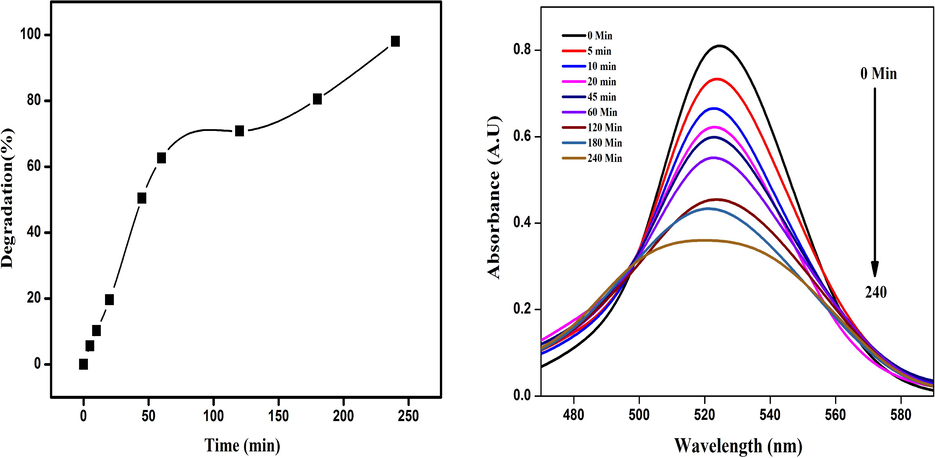
(a) Percent degradation of Rhodamine dye with Ch-Pt-Ag NCs and (b) UV–visible spectra.
3.8 Anti-inflammatory activity
Anti-inflammatory activity (inhibition of denaturation of BSA) of Ch-Pt-Ag NCs was examined at five different concentrations (10 μg/ml, 20 μg/ml, 30 μg/ml, 40 μg/ml, and 50 μg/ml). Result showed that anti-inflammatory efficacy of green prepared Ch-Pt-Ag NCs was dose-dependent (Fig. 8). There was a gradual increase in the anti-inflammatory activity of Ch-Pt-Ag NCs with increasing the concentration of Ch-Pt-Ag NCs and was greater when compared to the standard diclofenac sodium at a higher concentration. The Ch-Pt-Ag NCs, at the concentration of 50 µg/ml showed 85% inhibition of BSA denaturation while standard (diclofenac sodium) exhibited 82% inhibition at same concentration. These results are comparable to earlier reports (Lava et al., 2020).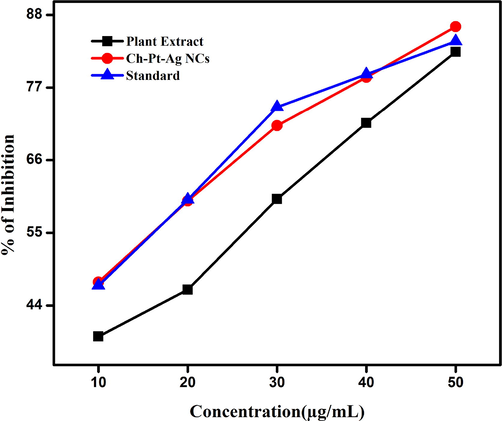
Anti-inflammatory activity of green prepared Ch-Pt-Ag NCs.
3.9 Cytotoxicity study
It is crucial to examine the biocompatibility/toxicity of green prepared nanostructures before their applications in biomedical and environmental fields (Ahamed et al., 2019; Siddiqui et al., 2013; Chaturvedi et al., 2020). Cytotoxic activity of green prepared Ch-Pt-Ag NCs was examined by Brine shrimp lethality assay. This assay is considered a useful technique for preliminary toxicity/biocompatibility assessment of new compounds or nanostructures (Ali and Mohammed, 2021). Five different dosages were taken to examine the toxicity of green synthesized Ch-Pt-Ag NCs (20, 40, 60, 80, and 100 μg/ml). Ten live nauplii have been taken for each concentration and compared with non-treated control group. Results indicated that green prepared Ch-Pt-Ag NCs did not exert toxicity to nauplii at lower concentrations (20–60 μg/ml). As the concentration of Ch-Pt-Ag NCs increases from 80 μg/ml to 100 μg/ml there was indication of slight toxicity (Fig. 9). These results indicated that green synthesized Ch-Pt-Ag NCs exert fewer toxicity.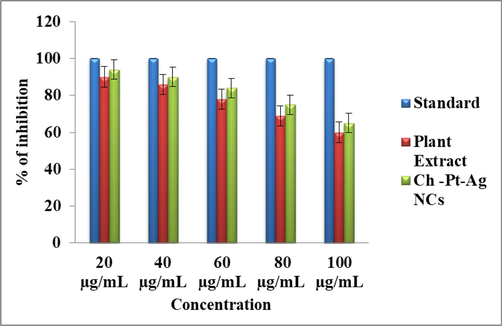
Brine shrimp lethality assay of green prepared Ch-Pt-Ag NCs.
3.10 Larvicidal activity
Dengue fever has become much more common in recent decades all across the world. Dengue fever is estimated to cause 50 million infections worldwide each year, according to the World Health Organization (Velayutham and Ramanibai, 2016). In recent years, Aedes aegypti L., a dengue vector transmitting the dengue arbovirus, has become widely distributed in tropical and subtropical areas. The greatest strategy to prevent the spread of the dengue virus is to combat disease-carrying mosquitos (Morejón et al., 2018). In recent years, material scientists have been more focused on finding better alternatives to the proposed vector employing plant extracts mediated nanostructures (Sutthanont et al., 2019). In the present study, a range of concentrations of green synthesized Ch-Pt-Ag NCs (1–5 µg/ml) were tested against the second and fourth instars of A. aegypti. We observed a dose-dependent larvicidal activity of green synthesized Ch-Pt-Ag NCs against dengue vector A. aegypti. As we can see in Fig. 10, approximately 15% mortality observed at 1 µg/ml concentration of Ch-Pt-Ag NCs while 79% mortality was found at maximum selected concentration (5 µg/ml).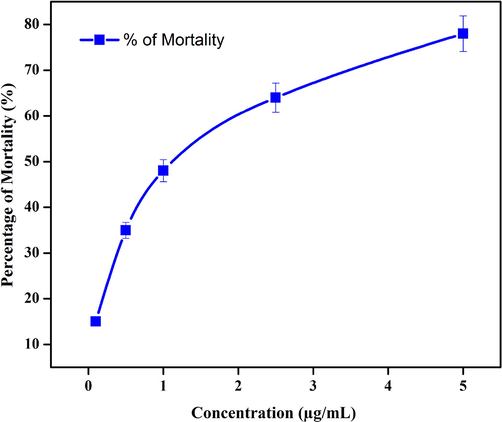
Larvicidal activity of green prepared Ch-Pt-Ag NCs.
4 Conclusion
The goal of this study was to develop a facile, eco-friendly, and inexpensive method for the synthesis of biomedically relevant chitosan modified Pt-doped Ag nanocomposite (Ch-Pt-Ag NCs) utilizing leaf extract of medicinal plant Desmostachya bipinna. The crystallineline structure of prepared NCs was indicated by XRD. The presence of phytochemicals involved in reduction and capping during NCs synthesis was confirmed by FTIR. Morphology and elemental compositions were examined by SEM and EDX, respectively. Size distribution in aqueous state and thermal stability were assessed by DLS and TGA, respectively. Green synthesized Ch-Pt-Ag NCs were effectively degraded the Rhodamine dye under UV light irradiation. Green Ch-Pt-Ag NCs also showed significant anti-inflammatory and larvicidal activities. In Brine shrimp lethality assay, Ch-Pt-Ag NCs showed low cytotoxicity, which is an important feature of nanostructures. This work underlines the significance of plant-based synthesis of metal-based nanocomposites for biomedical and environmental research.
Acknowledgments
Rajaduraipandian Subramanian (Register No: 20111232031009) acknowledges the research center Sri Paramakalyani College, Manonmaniam Sundaranar University, Alwarkurichi for providing the support for this research work. The authors extend their sincere appreciation to the Researchers Supporting Project (RSP-2021/129) at the King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and characterization of magnetic nanocomposite of chitosan/SiO2/carbon nanotubes and its application for dyes removal. J. Clean. Prod.. 2017;145:105-113.
- [CrossRef] [Google Scholar]
- Nanocomposites based on chitosan-graft-poly(N-vinyl-2-pyrrolidone): synthesis, characterization, and biological activity. Int. J. Polym. Mater. Polym. Biomater.. 2015;64:578-586.
- [CrossRef] [Google Scholar]
- Preventive effect of TiO2 nanoparticles on heavy metal Pb-induced toxicity in human lung epithelial (A549) cells. Toxicol. in Vitro. 2019;57:18-27.
- [CrossRef] [Google Scholar]
- Enhanced anticancer performance of eco-friendly-prepared Mo-ZnO/RGO nanocomposites: Role of oxidative stress and apoptosis. ACS Omega. 2022;7(8):7103-7115.
- [CrossRef] [Google Scholar]
- SnO2-doped ZnO/reduced graphene oxide nanocomposites: synthesis, characterization, and improved anticancer activity via oxidative stress pathway. Int. J. Nanomed.. 2021;16:89-104.
- [CrossRef] [Google Scholar]
- Ag-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cells. Sci. Rep.. 2017;7:17662.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and antimicrobial activity of copper oxide nanoparticles. J. Nanomater.. 2014;2014:1-4.
- [Google Scholar]
- Facile green synthesis of ZnO-RGO nanocomposites with enhanced anticancer efficacy. Methods. 2022;199:28-36.
- [CrossRef] [Google Scholar]
- A novel green preparation of Ag/RGO nanocomposites with highly effective anticancer performance. Polymers. 2021;13(19):3350.
- [CrossRef] [Google Scholar]
- Biosynthesis and characterization of platinum nanoparticles using Iraqi Zahidi dates and evaluation of their biological applications. Biotech. Rep.. 2021;30:e00635.
- [Google Scholar]
- Synthesis and characterization of chitosan: SiO2 nanocomposite by ultrasonic spray drying. IOP Conf. Ser.: Mater. Sci. Eng.. 2019;550(1):012037.
- [Google Scholar]
- Facile synthesis of Ag nanoparticles-loaded chitosan antibacterial nanocomposite and its application in polypropylene. Int. J. Biol. Macromol.. 2020;161:1286-1295.
- [CrossRef] [Google Scholar]
- Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014;1:3.
- [CrossRef] [Google Scholar]
- β-Sitosterol-D-glucopyranoside isolated from Desmostachya bipinnata mediates photoinduced rapid green synthesis of silver nanoparticles. RSC Adv.. 2014;4
- [CrossRef] [Google Scholar]
- Rapid eco-friendly synthesis, characterization, and cytotoxic study of trimetallic stable nanomedicine: A potential material for biomedical applications. Biochem. Biophys. Rep.. 2020;24:100812
- [CrossRef] [Google Scholar]
- Elucidation of the photocatalytic degradation mechanism of an azo dye under visible light in the presence of cobalt doped TiO2 nanomaterials. Chemosphere. 2021;266:128931
- [CrossRef] [Google Scholar]
- Platinum nanoparticles as potent anticancer and antimicrobial agent: Green synthesis, physical characterization, and in-vitro biological activity. J. Clust. Sci. 2022
- [CrossRef] [Google Scholar]
- Influence of boiling, steaming and frying of selected leafy vegetables on the in vitro anti-inflammation associated biological activities. Plants. 2018;7:22.
- [CrossRef] [Google Scholar]
- Guntur, S., Kumar, N., Hegde, M., Dirisala, V., 2018. In vitro studies of the antimicrobial and free-radical scavenging potentials of silver nanoparticles biosynthesized from the extract of Desmostachya bipinnata. Anal. Chem. Insights 13, 117739011878287. https://doi.org/10.1177/1177390118782877.
- Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci.. 2015;8:265-275.
- [CrossRef] [Google Scholar]
- In-Vitro activity of Desmostachya bipinnata (L.) Stapf successive extracts against Helicobacter pylori clinical isolates. Saudi Pharm. J.. 2018;26:535-540.
- [CrossRef] [Google Scholar]
- Achyranthes aspera mediated green synthesis of silver nanoparticles. 2018;05:64-73.
- [CrossRef]
- Plant based synthesis of silver nanoparticles from Ougeinia oojeinensis leaves extract and their membrane stabilizing, antioxidant and antimicrobial activities. Mater. Today: Proc.. 2019;17:313-320.
- [CrossRef] [Google Scholar]
- Characterization, anticancer, antibacterial, anti-diabetic and anti-inflammatory activities of green synthesized silver nanoparticles using Justica wynaadensis leaves extract. Mater. Today: Proc.. 2020;46:5942-5947.
- [CrossRef] [Google Scholar]
- Larvicidal activity of silver nanoparticles synthesized using extracts of Ambrosia arborescens (Asteraceae) to control Aedes aegypti L. (Diptera: Culicidae) J. Nanotechnol.. 2018;2018:1-8.
- [Google Scholar]
- Green synthesis of bimetallic Pt@Cu nanostructures for catalytic oxidative desulfurization of model oil. J Nanostruct. Chem.. 2017;7:159-170.
- [CrossRef] [Google Scholar]
- Nanocomposites based on chitosan/silver/clay for durable multi-functional properties of cotton fabrics. Carbohydr. Polym.. 2018;182:29-41.
- [CrossRef] [Google Scholar]
- Chemical synthesis and characterization of chitosan/silver nanocomposites films and their potential antibacterial activity. Int. J. Biol. Macromol.. 2018;16:520-529.
- [CrossRef] [Google Scholar]
- Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Res. Lett.. 2016;11:422.
- [CrossRef] [Google Scholar]
- Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocellular carcinoma cells. PLOS ONE. 2013;8(8):e69534.
- [Google Scholar]
- Stability of biosynthesised silver nanoparticles using Achyranthes aspera roots and its characterization. Int. J. Curr. Microbiol. App. Sci. 2018;7:1566-1575.
- [Google Scholar]
- Larvicidal activity of synthesized silver nanoparticles from curcuma zedoaria essential oil against Culex quinquefasciatus. Insects. 2019;10:27.
- [CrossRef] [Google Scholar]
- Green synthesis of an activated carbon-supported Ag and ZnO zanocomposite for photocatalytic degradation and its antibacterial activities. Molecules. 2020;25:1586.
- [CrossRef] [Google Scholar]
- Novel silver-platinum bimetallic nanoalloy synthesized from Vernonia mespilifolia extract: Antioxidant, antimicrobial, and cytotoxic activities. Arab. J. Chem.. 2020;13:6639-6648.
- [CrossRef] [Google Scholar]
- Larvicidal activity of synthesized silver nanoparticles using isoamyl acetate identified in Annona squamosa leaves against Aedes aegypti and Culex quinquefasciatus. J. Basic Appl. Zool.. 2016;74:16-22.
- [CrossRef] [Google Scholar]
- WHO, 2005. Guidelines for Laboratory and Field Testing of Mosquito Larvicides. World Health Organization; Geneva, Switzerland: 2005. p. 39. WHO/CDS/WHOPES/GCDPP/2005.13.







