Translate this page into:
Green synthesis of Terminalia Glaucescens Planch (Udi plant roots) extracts as green inhibitor for aluminum (6063) alloy in acidic and marine environment
⁎Corresponding author. owoeyeseun@gmail.com (Seun S. Owoeye) owoeyeseun_s@fedpolyado.edu.ng (Seun S. Owoeye)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Green synthesis of Terminalia Glaucescens Planch extracts for potential application as green inhibitor for aluminum (6063) alloy immersed in acidic (0.3 M H2SO4) and marine (3.5% NaCl) solution respectively was investigated. The phytochemical examination of the extract was conducted to confirm its bioactive constituents while the inhibition potential of the extract at varied concentration on corrosion of Al (6063) alloy in acid and salt solutions was evaluated using potentiodynamic polarization studies and electrochemical impedance spectroscopy respectively (EIS). Scanning electron microscopy (SEM) was used to examine the morphology of the alloy in the absence and presence of inhibitor while FT-IR was used to determine functional group present in the inhibitor extract. The results showed considerable amount of bioactive agents such as Flavanoids, Tannis and Keller killani present in the Terminalia Glaucescens Planch extracts which is indication of its inhibition potential. The corrosion rates obtained from the polarization curves indicate significant decrease as the concentration of the extracts increases both in acid and salt solutions which is in agreement with the EIS measurements. The surface morphology of the uninhibited alloys in acid and salt showed more pronounced deterioration due to aggressive attack from corrosive media unlike the inhibited alloys.
Keywords
Terminalia Glaucescens Planch extract
Corrosion inhibition
Acid solution
SALT solution
Bioactive agents
1 Introduction
Terminalia Glaucescens Planch is a multipurpose and perennial species of trees from the genus Terminalia which grows across Africa but largely in Nigeria (Mangoyi et al., 2012). Over the past few decades, this plant leaves and roots extracts have been used for several medicinal preparations in treatment of some diseases and infections (Okpekon et al., 2004) but never has it been used as green inhibitor in corrosion prevention of metals and alloys until recently which is largely attributed to bioactive compounds known to be present in the plant especially the roots. These bioactive compounds have been identified to possess inhibition potential when adsorbed on metal surface (El-Etre et al., 2004; Raja, 2010).
Aluminum and its alloys remain the most utilized metallic alloys and as a base matrix material in the development of aluminum matrix composites (AMCs) and material of choice for most applications in engineering such as aerospace, automotive, electronics and devices and food packaging; which is attributed to their lightweight, low cost, ratio of strength to weight and high electrical capacity (Soyoboyejo, 2003; Miracle 2005; Senthilvelan et al., 2012). Although, aluminum alloys possess considerable resistance to atmospheric condition but suffer poor mechanical properties when in contact with aggressive or corrosive environments like acids (H2SO4, HCl) and salt solutions (Pai et al., 1995; Ambat and Dwarakadasa, 1994).
Corrosion is an unavoidable menace confronting almost all engineering materials and has been considered as one of the worst phenomenon of our time causing technical damages (Stansbury and Buchanan, 2000). The overall cost commitment and environmental implications associated with the problem of corrosion in most engineering industries have led to several useful efforts for protecting metals and their alloys under service conditions in corrosive media in order to retard corrosion reaction and in the process corrosion rate is minimized (Emeka, 2008; Potgieter et al., 2012). In this regard, one of the prominent methods employed to militate against excessive dissolution of metals and alloys due to action of corrosion is the use of corrosion inhibitors. These inhibitors have capacity of slowing down the rate of corrosion attack (Orubite and Oforka, 2004).
In previous times, the use of inorganic inhibitors had been adopted by most industries in combating corrosion but these inhibitors such as chromates, phosphates and arsenates are known to be costly and contain compounds which are toxic and not environmental friendly, thus their usage has been discouraged and criticized (Orubite and Oforka, 2004; El-Etre et al., 2004). In view of this, several efforts have been geared towards the usage of non-toxic, biodegradable, eco-friendly and cost efficient corrosion inhibitors which are generally known as green inhibitors from plant extracts (Orubite and Oforka, 2004; El-Etre et al., 2004; Raja, 2010). Despite several investigated studies on inhibitive potential of plant extracts as corrosion inhibitors (Emeka, 2008; El-Etre et al., 2004; Alaneme and Olusegun, 2012; Odewunmi et al., 2015; Loto et al., 2011; Nnanna et al., 2016), these studies were focused mainly on mild steels mostly in HCl solutions with little or no attempts on the inhibitive potential of plant extracts as inhibitors on corrosion of aluminum alloys in aggressive media such as sulfuric acid and salt solutions.
In this regard, this study attempt to extend investigation to green synthesis of Terminalia Glaucescens Planch (Udi plant roots) extracts as green inhibitor for corrosion of aluminum (6063) alloy immersed in 0.3 M H2SO4 and 3.5 wt% NaCl respectively. The selection of Terminalia Glaucescens Planch as green inhibitor in this study is based on its phytochemical constituents indicating the presence of bioactive agents like Flavanoids, Tannis, Keller killani etc. which are known to possess inhibition properties.
2 Materials and methods
2.1 Material preparation
Aluminum (6063) was used to serve as a base Al-alloy matrix for this work. The Al-alloy was commercially obtained in its as-received state in billet form. The Al (6063) billets were first charged inside a gas-fired crucible furnace with a fitted temperature probe to monitor temperature and heated to a temperature of 700 ± 30 °C to obtain a completely molten Al-alloy which was then poured into a prepared sand moulds. The as-cast Al-alloy was machined into samples of 10 mm diameter and 10 mm height. The samples were then polished and prepared for corrosion test following standard procedures. The chemical composition of the aluminum (6063) as determined by the spark spectrometric analysis is shown in Table 1.
Si
Fe
Cu
Mg
Mn
Ti
Zn
Cr
Pb
Al
0.43
0.20
0.001
0.48
0.016
0.015
0.008
0.003
0.001
Bal.
2.2 Synthesis of Terminalia Glaucescens Planch extracts (Udi plant roots)
The synthesis of the extract adopted in this study was based on the procedures stated by Alaneme et al. (2016). The Udi roots were sourced from Ado-Ekiti, Nigeria. The obtained root samples were first washed; oven dried at 90 °C for 48 h and thoroughly pulverized using electrically powered blender. 20 g of the powdered roots was weighed followed by soaking in 100 ml of ethanol for 72 h. The mixture was then filtered to obtain the filtrate of the extract, then heat to evaporation at 80 °C to obtain the extract free of ethanol. Varied amount of 0.1, 0.2, 0.3 and 0.4 g of the extract were weighed respectively and dissolved in one liter of 0.3 M H2SO4 and 3.5 wt% NaCl respectively which were used as test solutions.
2.3 Phytochemical determination
Phytochemical screening was carried out to determine the presence of bioactive compounds such as Saponins, Tannins, Flavonoids, Keller Killani etc. present in the Terminalia Glaucescens Planch extracts. This screening was carried out using adopted methods by previous works (Bao et al., 2005; Brunner, 2005; Makkar et al., 1993).
2.4 Characterization of the extract
The characterization of the Terminalia Glaucescens Planch (Udi plant roots) extract was conducted using Perkin-Elmer- Fourier transform infra-red spectrophotomer (FT-IR). This was done to determine the functional groups responsible for the inhibitive potentials of the synthesized extract.
2.5 Corrosion analysis (Electrochemical measurements)
Corrosion analysis was investigated using electrochemical methods as stated by ASTM G59-97 (2014) standard. Corrosion of the Al-alloy samples were investigated in 0.3 M H2SO4 and 3.5 wt% NaCl solutions at 298 K. Corrosion experiments were conducted using a three electrode corrosion cell set-up comprising the Al-alloy samples as working electrode, saturated silver/silver chloride as reference electrode while platinum rod serves as counter electrode. The Al-alloy samples (working electrode) were immersed in test solutions (0.3 M H2SO4 and 3.5 wt% NaCl respectively) with and without Terminalia Glaucescens Planch extract. Open-circuit corrosion potential measurements were conducted in a separate cell for 2 h while on the other hand polarization measurements were carried out using a potentiodynamic scan rate of 0.25 mV/s. The electrolytes and the samples were replaced after each experiment. Corrosion potential (Ecorr) and corrosion current density (Icorr) was then obtained from the linear Tafel segments of the anodic and cathodic curves extrapolation. The electrochemical impedance spectroscopy (EIS) measurement was carried out according to ASTM standard procedures with the use of EIS300 soft ware packages developed by Gamry. The experiments were conducted in triplicates to substantiate the results obtained. It was observed that there were no substantial differences between results gotten from the triplicates.
3 Results and discussion
3.1 Phytochemical determination
Table 2 presents the result of the phytochemical constituents of the Terminalia Glaucescens Planch (Udi plant roots) extract. It can be observed that the extract consist of Tannis, Flavonoids, Keller Killani in moderate amounts respectively. These constituents are referred to as bioactive organic compounds which have been reported to contain functional groups and molecular structures which promote adsorption, thus enhancing the inhibitory potential of this plant extract (Nnanna et al., 2016). Previous studies had attributed the inhibitory performance of several green inhibitors to the availability of these bioactive organic agents (Ajayi et al., 2014; Alaneme and Olusegun, 2012). + = Present in moderate amount. − = absent.
Saponins
Tannins
Flavonoids
Keller Killani
–
+
+
+
3.2 Characterization of Terminalia Glaucescens Planch extracts
The IR spectrum of the extract was recorded by FT-IR at wavenumber ranging from 500 to 4000 cm−1 is shown in Fig. 1. The spectrum revealed several adsorption peaks of heteroatoms of N, O and group like OH– which indicate the corrosion inhibition ability of the extract. The peak at 3201.8 cm−1 indicates the presence of OH– group while the peaks 1028–1174 cm−1 indicate C–O stretching; the peaks 1599–1718 indicate C⚌O stretching. The adsorption of these inhibitor molecules on the metal surface initiate barrier to corrosion (Chauhan and Gunasekaran, 2007).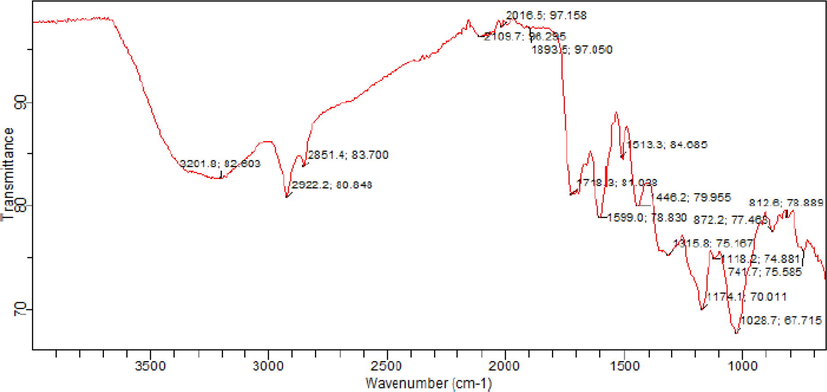
FT-IR transmittance spectra of Terminalia Glaucescens Planch extracts.
3.3 Electrochemical measurement in acid medium
Fig. 2 shows the corrosion results obtained from the Tafel plots electrochemical studies in 0.3 M H2SO4 while Table 3 presents the Tafel extrapolations of the corrosion current density (Icorr), corrosion potential (Ecorr), corrosion rate (C.R.), inhibitor efficiency (%I.E) and surface coverage (ɵ). The Table indicates clear distinct corrosion behavior in the absence (blank) and presence of varied amounts of Udi roots extracts. It is observed that as the amount of the extract increases from 0.1 g/L to 0.4 g/L there is significant increment in the efficiency of the inhibitor (% I.E). The highest inhibition efficiency of 98.6% was obtained at 0.4 g/L concentration. This shows several particles of the extract were adsorbed on the surface of the Al-alloy to enhance a larger surface coverage and thereby acting as adsorption inhibitor (Mokhtari et al., 2013). It can also be observed that as the concentration of the inhibitor increases there seems to be significant drop in corrosion rate (C.R) with the least corrosion rate of 0.0465 mmpy observed in the presence of 0.4 g/L udi root extracts. This is an indication of the inhibitive efficacy of this extract at influencing the corrosion of Al-alloy in sulphuric acid (acidic medium). Also from Table 3, Icorr decreases significantly with increase in amount of the extract which might be attributed to the increase in covered fraction of the Al-alloy surface through adsorption (Fouda et al., 2014). It is also observed from Table 3 that as the amount of extract increases, a significant shift towards more negative potential occurs to indicate the efficacy of the Udi root extracts as suitable corrosion inhibitor. Also, it can be observed that as the amount of the extracts increases the Icorr decreases, Ecorr increases and the % I.E increases which imply that the extracts impede both cathode and anode reaction sites owing to coverage by the extracts molecule (El-Shafei et al., 2004).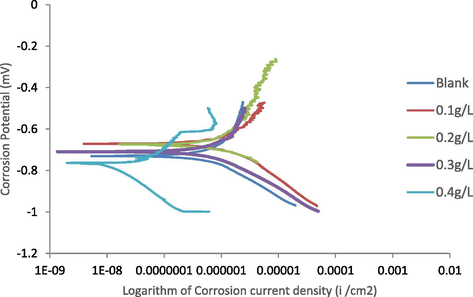
Polarization curves of Al (6063) alloys without (blank) and with (inhibited) varied amounts of Terminalia Glaucescens Planch (Udi plant roots) extracts in acid.
Conc.
βa (mvdec-1)
βa (mvdec-1)
Icorr (µAcm−2)
Ecorr (mV)
C.R (mmpy)
% I.E
Surface coverage (ɵ)
Blank
2.49E + 30
352.393
−288.045
−730.283
3.3424
–
–
0.1 g/L
467.773
241.25
−220.50
−670.888
2.5586
23.5
0.23
0.2 g/L
655.229
130.26
−146.728
−676.043
1.7026
49.1
0.49
0.3 g/L
567.023
193.046
−131.308
−709.407
1.5237
54.4
0.54
0.4 g/L
227.228
389.046
−4.272
−787.859
0.0465
98.6
0.99
3.4 Electrochemical measurement in salt solution
Fig. 3 presents the corrosion results from the Tafel plots electrochemical studies in 3.5 wt% NaCl while Table 4 shows the Tafel extrapolations of the Icorr, Ecorr, corrosion rate (C.R.), inhibitor efficiency (%I.E) and surface coverage (ɵ). The Table indicates clear distinct corrosion behavior without and with varied amounts of Udi roots extract. It can be observed that the inhibition efficiency improves as the extract concentrations increase from 0.1 g/L to 0.2 g/L but slightly dropped from 0.3 g/L to 0.4 g/L in contrast to what was observed in acidic environment (Table 3). The highest inhibition efficiency of 91.5% obtained at 0.2 g/L concentration shows better adsorption of the inhibitor on the Al-alloy surface thereby promoting a larger surface coverage acting as adsorption inhibitor (Mokhtari et al., 2013). The extracts adsorption on the Al-alloy surface produce barriers to the deterioration of the Al-alloy in the corrosive medium (Oguzie, 2007). The inhibition efficiency of 88.5% and 85.0% obtained at 0.3 g/L and 0.4 g/L extract concentration though in decreasing order can still be considered high enough in relation to previous works (Jamiu et al., 2016; El-Shafei et al., 2004). The decrease in inhibition efficiency might be attributed to surface coverage of the inhibitor obtained on the Al-alloy at that level and which is supported by most works. It is also observed that the corrosion rate displayed same trend as inhibition efficiency which is expected, as the higher the inhibition efficiency, the lower the corrosion rate. The least corrosion rate of 0.036908 mmpy was obtained at extract concentration of 0.2 g/L having the highest inhibitive efficiency. It is also observed from Table 4 that the Icorr decreases with increase in the extract concentration except for 0.3 g/L and 0.4 g/L with slight increment which might be attributed to the increase in sealed fraction of the Al-alloy surface through adsorption (Fouda et al., 2014). It can also be observed that there is a shift towards more negative potential (Ecorr) as the amount of Udi plant roots extract increase, indicating that the Udi plant roots extract is a suitable corrosion inhibitor also in salt solution.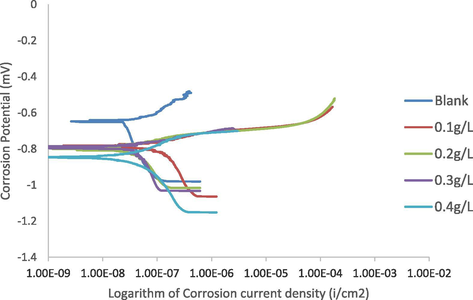
Polarization curves of Al (6063) alloys without (blank) and with (inhibited) varied amounts of Terminalia Glaucescens Planch (Udi plant roots) extracts in salt solution.
Conc.
Ba (mvdec-1)
βa (mvdec-1)
Icorr (µAcm−2)
Ecorr (mV)
C.R (mmpy)
% I.E
Surface coverage (ɵ)
Blank
3.43
3.136
−39.993
−773.928
0.43531
–
–
0.1 g/L
177.143
5.42E + 33
−32.485
−788.885
0.35359
18.8
0.18
0.2 g/L
59.78
357.079
−3.39
−785.113
0.03691
91.5
0.92
0.3 g/L
65.729
662.804
−4.595
−788.866
0.05002
88.5
0.88
0.4 g/L
157.257
437.699
−6.007
−842.718
0.06538
85.0
0.85
3.5 Electrochemical impedance measurements (EIS)
Figs. 4 and 5 (a – e) illustrate the EIS measurements of the samples in the absence and presence of inhibitor both in acid and salt medium respectively. Fig. 4 illustrates the Nyquist plot of Al-alloy without (blank) and with inhibitor at different concentrations at 298 K in acid medium. It will be observed from Fig. 4 that semicircular-like curves which are distinctive of each inhibitor concentration are obtained. This semicircular-like diameter curve has been reported to be associated with the rate of corrosion inhibition (Ahmed et al., 2014). It can also be observed from the plot that the semicircular curves increase as the concentration of the corrosion inhibitor increases, which are indication of decrease in corrosion rate as the concentration of the inhibitors increase. This implies that the inhibition efficiency increases as the semicircular-like curves increase as stated by Ahmed et al. (2014). It can also be observed from the impedance plot that the semicircular curves are complete which might indicate that charge transfer process is controlling the corrosion of the Al-alloy (Birshi et al., 2018).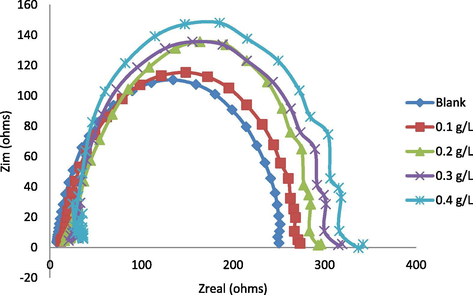
Nyquist curves for Al (6063) alloys without (blank) and with (inhibited) varied amounts of Terminalia Glaucescens Planch (Udi plant roots) extracts in acid solution.
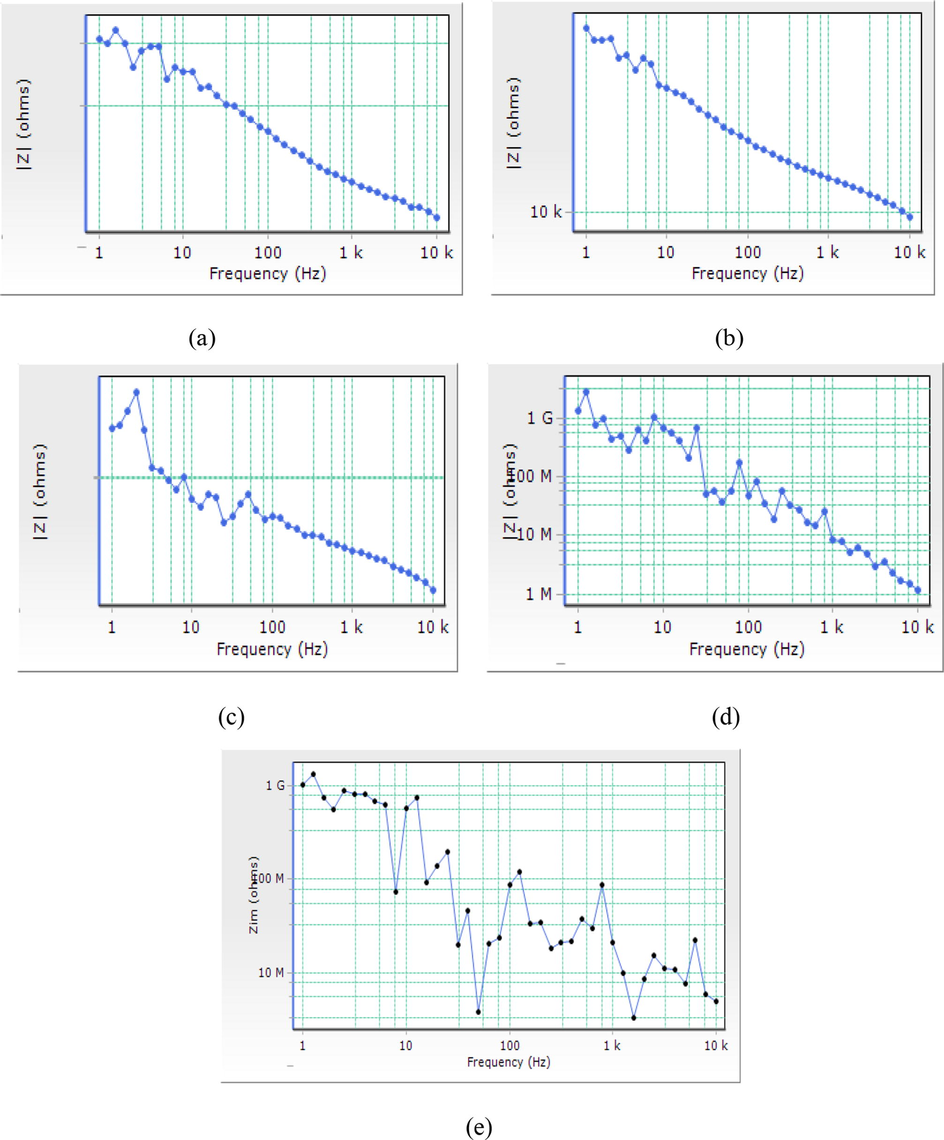
Bode plots of impedance against frequency for of Al (6063) alloys in salt solution (a) blank (b) 0.1 g/L (c) 0.2 g/L (d) 0.3 g/L (e) 0.4 g/L of varying inhibitor.
In Fig. 5(a – e), Bode plots of impedance against frequency is used to illustrate the EIS measurement of the samples without (blank) and with inhibitor at varying concentrations at 298 K in salt solution respectively. It can be observed that there is significant difference in the shape of the impedance modulation which increase as the inhibitor increases indicating that as the inhibitor increases a protective layer is formed on the Al-alloy surface preventing corrosion.
3.6 SEM investigations
The results of the corroded surface of the aluminum (6063) alloys in the absence (uninhibited) and presence (inhibited) of inhibitor both in acid and salt are presented in Figs. 6–8 respectively. Fig. 6 represents the SE images of the uninhibited Al-alloy (blank) in acid and salt respectively. It is observed that the Al-alloy surface seems to be under aggressive attacks from the corrosive media which is indicated by several pits (black-like portions) and cracks observed on the alloy while the somewhat white-like lines on the uninhibited Al-alloy might be due to polishing. However, the attack is more pronounced in acid medium than in salt. In Figs. 7 and 8 which illustrate the SE images of the inhibited Al-alloys in acid and salt at 0.2 g/L and 0.4 g/L concentration of extract respectively; it can be observed that a somewhat protective film (white-like coverage unlike the white-like lines observed in uninhibited samples) with minimal corroded portions can be seen in the microstructure which can be attributed to the adsorption of Terminalia Glaucescens Planch extracts on the surface of the Al-alloys creating a tenacious barrier to the aggressive attack of the corrosive media thus making the surface of the Al-alloys to be less damaged.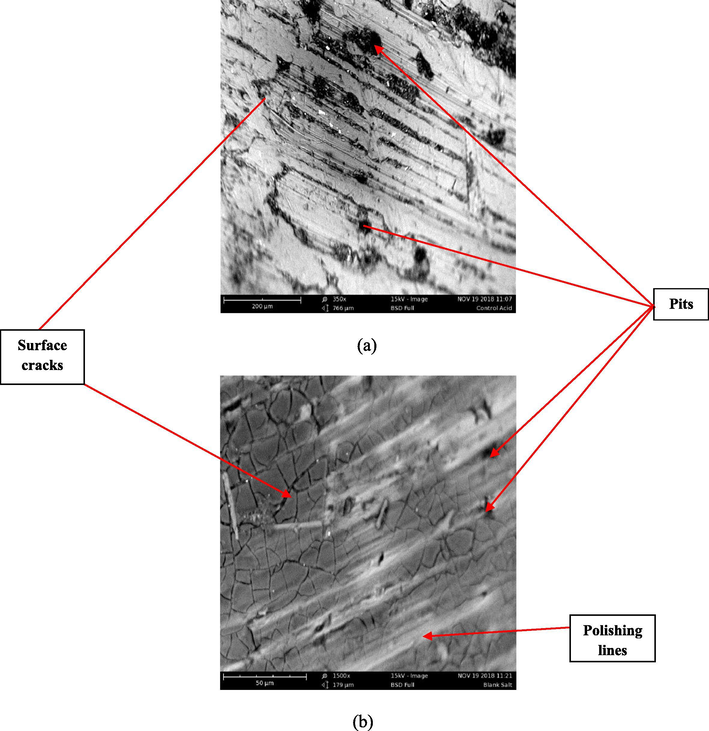
Representative SE images of uninhibited Al-alloys in (a) 0.3 M H2SO4 (b) 3.5% NaCl respectively.
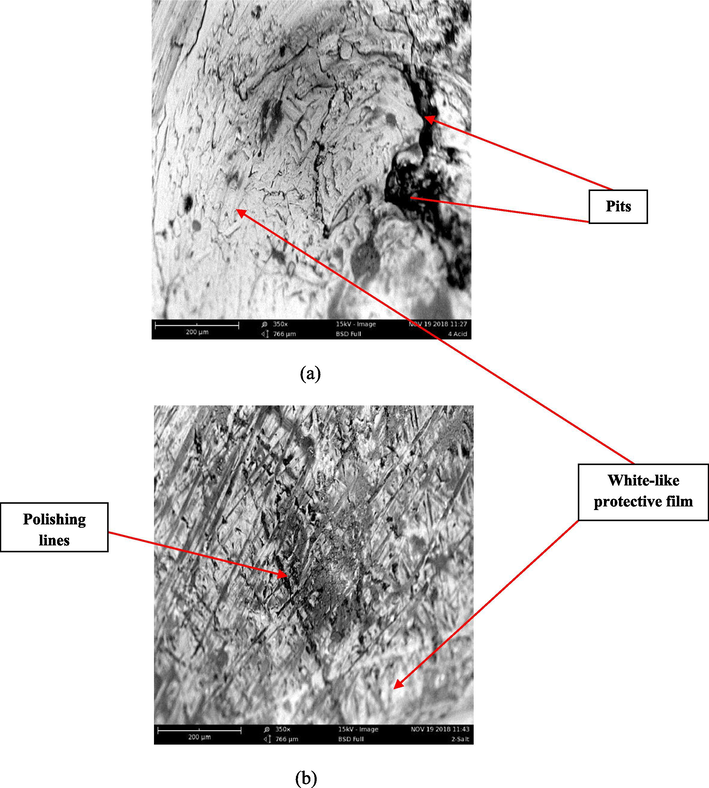
Representative SE images of inhibited Al-alloys containing 0.2 g/L inhibitor extract in (a) 0.3 M H2SO4 (b) 3.5% NaCl respectively.
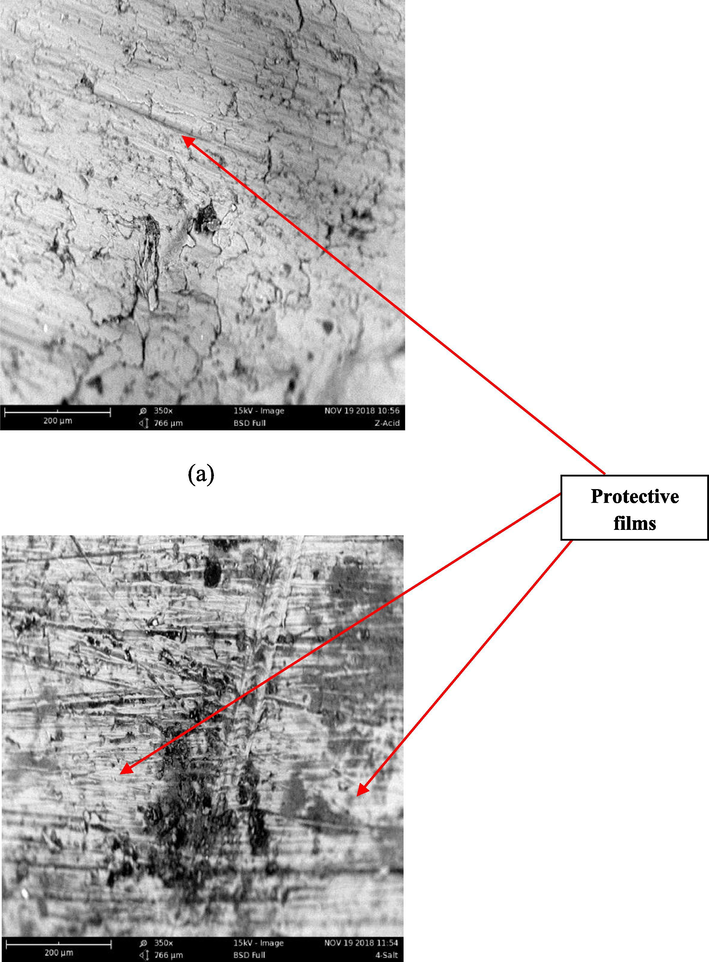
Representative SE images of inhibited Al-alloys containing 0.4 g/L inhibitor extract in (a) 0.3 M H2SO4 (b) 3.5% NaCl respectively.
4 Conclusions
Corrosion inhibition performance of Terminalia Glaucescens Planch (Udi plant roots) extract on Al-Mg-Si alloy immersed in acidic (0.3 M H2SO4) and marine (3.5 wt% NaCl) environments have been studied using potentiodynamic polarization technique. The following conclusions are drawn based on the results of the investigation:
-
The Terminalia Glaucescens Planch extract contain bioactive compounds such as Tannins, Flavonoids and Keller Killani which enhance corrosion inhibition through their adsorption on the surface of the Aluminum alloy.
-
The inhibition efficiency of the Terminalia Glaucescens Planch extract is a function of the amount of the extract, it increased with increasing amount of extract in acid and salt solutions respectively except at 0.3 g/L and 0.4 g/L in salt.
-
The corrosion rate of the Al-alloy decreased significantly in 0.3 M H2SO4 as the extract increases while same trend is observed in 3.5 wt% NaCl except at concentration of 0.3 g/L and 0.4 g/L extract.
-
The Terminalia Glaucescens Planch extract was found to be efficient and can serve as effective corrosion inhibitor in both sulfuric and salt solutions
-
The Terminalia Glaucescens Planch extract was also found to be eco friendly and low cost green inhibitor.
Acknowledgements
The authors wish to acknowledge the support of Corrosion Laboratory of the Department of Metallurgical and Materials Engineering, FUTA, Nigeria.
I therefore make a simple declaration as directed that the submitted paper has not been published previously and that it is not under consideration for publication elsewhere. I also declare that there are no potential conflicts as regard any part of the paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Inhibition of mild steel corrosion in sulfuric acid solution by New Schiff Base. Materials. 2014;7:787-804.
- [Google Scholar]
- Inhibition of mild steel corrosion using Jatropha Curcas leaf extract. J. Electrochem. Sci. Eng.. 2014;4(2):67-74.
- [Google Scholar]
- Corrosion inhibition performance of lignin extract of sunflower (Tithonia Diversifolia) on medium carbon low alloy steel immersed in H2SO4 solution. Leonardo J. Sci.. 2012;20:59-70.
- [Google Scholar]
- Corrosion inhibition behavior of biden pilosa extract on aluminum matrix composites in 1M HCl solution. J. Assoc. Prof. Eng. Trinidad Tobago. 2016;44(2):35-42.
- [Google Scholar]
- Studies on the influence of chloride ion and PH on the electrochemical behavior of aluminum alloys 8090 and 2014. J. Appl Electrochem.. 1994;24(9):911-916.
- [Google Scholar]
- Anthocyanins, flavonoids and free radical scavenging activity of myrialrubia extracts and their color properties and stability. J. Agric. Food Chem.. 2005;53:2327-2332.
- [Google Scholar]
- Evaluation of 2-thiophene carbonitrile as corrosion inhibitor on mild steel in acidic media. J. Mater. Environ. Sci.. 2018;9(9):2678-2685.
- [Google Scholar]
- Direct spectrophotometric determination of saponins. Anal. Chem.. 2005;34:1314-1326.
- [Google Scholar]
- Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corrosion Sci.. 2007;49:1143-1161.
- [Google Scholar]
- Corrosion inhibition of some metals using lawsonia extract. Corrosion Sci.. 2004;47:385-395.
- [Google Scholar]
- The role of indole and its derivatives in the pitting corrosion of aluminum in neutral chloride solution. Corrosion Sci.. 2004;46(3):579-590.
- [Google Scholar]
- Evaluation of inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corrosion Sci.. 2008;50:2993-2998.
- [Google Scholar]
- Moringa Oleifera leaves extract as green corrosion inhibitor for zinc in polluted sodium chloride solutions. Int. J. Adv. Res.. 2014;2(7):1158-1170.
- [Google Scholar]
- Inhibition efficiency of Moringa Oleifera leaf extract on the corrosion of reinforced steel bar in HCl solution. West Ind. J. Eng.. 2016;38(2):64-70.
- [Google Scholar]
- Effect of neem leaf (Azadirachita indica) extract on the corrosion inhibition of mild steel in dilute acids. Int. J. Phys. Sci.. 2011;6(9):2249-2257.
- [Google Scholar]
- Gravimetric determination of tannis and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric.. 1993;61:161-165.
- [Google Scholar]
- Antifungal activities and preliminary phytochemical investigation of Combretum species from Zimbabwe. J. Microbial Biochem. Technol.. 2012;4:037-044.
- [Google Scholar]
- Metal Matrix Composite-from science to technological significance. Compos. Sci. Technol.. 2005;65(15–16):2526-2540.
- [Google Scholar]
- Inhibition of steel corrosion in 1 M HCl by Jatropha Curcas oil. J. Mater. Environ. Sci.. 2013;5(1):310-319.
- [Google Scholar]
- Aqueous extracts of pentaclethra macrophylla bentham roots as eco-friendly corrosion inhibition for mild steel in 0.5M KOH medium. Int. J. Mat. Chem.. 2016;6(1):12-18.
- [Google Scholar]
- Watermelon waste products as green inhibitors for mild steel in HCl solution. J. Environ. Chem. Eng.. 2015;3:286-296.
- [Google Scholar]
- Corrosion inhibition of aluminum in acidic and alkaline media by sansevieria trifasciata extract. Corrosion Sci.. 2007;49(3):1527-1539.
- [Google Scholar]
- Antiparasitic activities of medicinal plants used in Ivory Coast. J. Ethnophamcol.. 2004;90:91-97.
- [Google Scholar]
- Inhibition of the corrosion of mild steel in hydrochloric acid solutions by the extracts of leaves of Nypafrutican Wurms. Mater. Lett.. 2004;58:1768-1772.
- [Google Scholar]
- Role of magnesium in cast aluminum alloy matrix composites. J. Mater. Sci.. 1995;30(8):1903-1911.
- [Google Scholar]
- Investigation of the potential of some plant extracts to inhibit the corrosion of duplex stainless steels in acidic media. J. Metal. Eng.. 2012;1(2):41-47.
- [Google Scholar]
- Natural products as green corrosion inhibitor for metals in corrosive media. LAP Lambert Academic Publishing; 2010.
- Fabrication and characterization of SiC, Al2O3 and B4C reinforced Al-Zn-Mg-Cu alloy (AA 7075) metal matrix composites. Adv. Mat. Res.. 2012;622–623:1295-1299.
- [Google Scholar]
- Mechanical Properties of Engineering Materials. New York, USA: Marcel Dekker Inc.; 2003. p. :1-608.
- Fundamentals of electrochemical corrosion. USA: ASM International; 2000. p. :2-4.







