Translate this page into:
Green synthesis, characterization and anti microbial activities of ZnO nanoparticles using Euphorbia hirta leaf extract
⁎Corresponding author.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study we proposed a green approach for the synthesis of ZnO nano particles using Euphorbia hirta leaves extract. ZnO nanoparticles have a very broad range of applications especially as antimicrobial agent. There are various methods are available for the synthesis of ZnO nanoparticles but among them the synthesis of ZnO nanoparticles by using plant material is a very good alternative and eco friendly method. Leaves extract was used as a biological reducing agent for the synthesis of ZnO nano particles from the zinc nitrate. The prepared nano particles were characterized by using various analytical and spectroscopic tools such as UV visible spectroscopy, Fourier transform infrared spectroscopy (FT- IR), X-ray diffraction (XRD) and scanning electron microscopy (SEM) analysis. Along with this study we also investigate the antimicrobial activity of bio synthesized nanoparticles by using disc diffusion method against clinical and standard strains of Streptococcus mutans, Streptococcus aureus, Clostridium absonum, Escherichia coli and Arthogrophis cuboida, Aspergillius fumigates and Aspergillius nigar.
Keywords
Green synthesis
Nanoparticles
Euphorbia hirta
ZnO
Antimicrobial activity
1 Introduction
Nanotechnology is the science of the manipulating matter at nanoscale has received much attention in last few years due to its multifaceted beneficial properties including medicinal, electrical, optical, chemical stability and catalytic activity (Prakasham et al., 2014, Nadagouda et al., 2014). The novel properties of nanoparticles are widely deployed for various applications in medicine, cosmetics, biomedical devices and environmental remediation (Jamdagni et al., 2016; Syed, 2014). Nano materials are also called as “wonder of modern medicine “due to their distinctive features such as catalytic, optical, antimicrobial, wound healing and anti-inflammatory properties (Rajiv et al., 2013; Hebbalalu et al., 2013). Among the available large number of nanoparticles, metal oxide nanoparticles are considered to be more promising as they exhibit unique physical, chemical and biological properties (Baruwati et al., 2009). Novel Properties and functions of nano particles are basically dependent on as size, distribution and morphology (Sangeetha et al., 2011, Kou and Varma, 2012). Furthermore for the better antimicrobial and catalytic activity of nano particles there is a certain control over the shape and size of the nano particles which is achieved by using different stabilizer reducing agents and employing different synthesis method (Rouhi et al., 2013, Nadagouda and Varma, 2008). Various physical and chemical methods are available for the synthesis of nano particle, in these methods various hazardous chemicals are used which is very toxic to our environment (Siddiqi et al., 2018). Thus, a better alternative is required which can be attained by green synthesis, Green synthesis of nanoparticle is an eco-friendly approach which is in common practice (Kolekar et al., 2013). The green synthesis is a simple alternative to chemical and physical methods due to low cost and less use of toxic chemicals. There are so many reports are available in the last few years which clearly indicate the considerable antimicrobial activity of inorganic metal oxides like Zinc oxide, TiO2, SiO2, MgO, CaO, and CeO2. Specially, TiO2, ZnO, MgO, and CaO are of particular concern because they are not only stable under harsh process conditions, but also are considered as safe materials to humans (Thovhogi et al., 2016, Amanulla et al.,2018). In the present work we study the Zinc oxide nanoparticles. The reason for selecting ZnO nanoparticles (ZnO nps) for the present study is that ZnO is a metal oxide, which is very much stable and having longer life as compared to the organic based disinfectants and antimicrobial agents (Gunalan et al., 2012, Khalil et al.,2017). There are so many reports are available in literatures which clearly indicate that the biosynthesized nano particle show better antimicrobial activity as compare to chemically synthesized nano particles (Khalil et al 2017, Ismail et al 2017). Antibacterial studies of organic materials are often not suitable particularly at high temperatures and/or pressures compared to inorganic antibacterial agents. Furthermore, zinc oxide appears to effectively resist microorganisms (Dobrucka and Dugaszewska, 2016).
The aim of our present study was to synthesize zinc oxide nanoparticles using Euphorbia hirta leaf extract. Euphorbia hirta is an important plant for medicinal herb. Euphorbia hirta belong to genus Euphorbia and family Euphorbiaceae. The plant has been widely acknowledged for the treatment of cough, coryza, hay asthma, bronchial infections, bowel complaints, worm infestations, kidney stones in traditional medicine (Ahmad et al., 2017). The biosynthesized nano particles was characterized by using different techniques like UV, FT IR SEM and XRD. The antimicrobial activity of synthesized zinc oxide nanoparticle was also investigated.
2 Materials and methods
2.1 Materials
Zinc nitrate (ZnNO3), ethanol and all other chemicals used in the experiment were of analytical grade and purchased from Merck. All glassware were washed with sterile distilled water and dried in an oven before use.
2.2 Plant collection
The leaves of Euphorbia hirta was collected from the garden of Uttaranchal university, Dehradun. The leaves of collected plant material were washed thoroughly 2–3 times with running water and once with sterile distilled water.
2.3 Preparation of extract
Shade-dried plant leaves chopped into small pieces by using mortar and pestle, grinded into powdered form. The powdered plant material was subjected to solvent extraction by soxhlet extraction method. The extraction was done with ethanol solvents. The extract was evaporated using rotary evaporator. Dried extracts were stored in airtight containers for further studies. Concentrated extracts were subjected to various chemical tests in order to detect the various phyto constituents.
2.4 Phytochemical screening test
Preliminary phytochemical screening was used to find the secondary components presence in the leaf extract by using the standard methods.
(i) Test for saponins: 1 ml extract was slowly added to 2–3 ml of double distilled water. Then the mixture was shaken vigorously. Finally the formation of foam confirms the presence of saponin in the leaf extract (Zhang et al., 2007).
(ii) Test for Alkaloids: A 3 ml of concentrated extract was taken in a test tube and 1 ml of hydrochloric (HCl) acid was added to the extract. Then the mixture was heated gently for 20 min and cool down to room temperature. Finally, the obtained mixture was filtered in the filter paper. The filtrate was treated with Hager’s reagent formation of yellow colour indicate the presence of alkaloids (Sirelkhatim et al., 2015).
(iii) Test for Proteins:
2 ml of extract was treated with few drops of concentrated Nitric (HNO3) acid changes the color of the solution into yellow indicates the presence of proteins.
(iv) Test for Flavonoids: 2 ml of the extract was treated with 10% NaOH solution Formation of intense yellow color indicates the presence of Flavonoid (Zhang et al 2007).
(v) Test for Phenol: 2 ml of leaf extract was treated with 4 drops of alcoholic Ferric chloride (FeCl3) solution. Changes in the solution as bluish black confirms the presence of phenolic compound (Zhang et al 2007).
(vi) Test for Carbohydrate: 1 ml of leaf extract was dissolved gradually in 5 ml of double distilled water and filtered. The filtrate was used for the following test.
Benedict’s test: 2 ml of filtrate was treated with Benedict’s reagent and heated gently. Formation of orange red precipitate indicates the presence of carbohydrate
(vii) Test for Terpenoids: 5 ml of extract was mixed with 2 ml of chloroform followed by careful addition of 3 ml of concentrated sulphuric (H2SO4) acid. A layer of reddish brown coloration indicates the positive result for the presence of Terpenoids.
(viii) Test for steroids: 2 ml of leaf extract was taken in a test tube and dissolved with 10 ml of chloroform. Equal volume of concentrated H2SO4 acid was added to the mixture through the side wall of the test tube. Steroid was confirmed by the changes in the upper layer of the solution as red and H2SO4 acid layer as yellow with green fluorescence (Samat and Nor, 2013).
2.5 Synthesis of ZnO nanoparticle using Euphorbia hirta leaf extract
2 g zinc nitrate was dissolved in 50 ml double distilled water in a beaker under mild stirring for 10 min using magnetic stirrer. After being stirred, 5 ml of the isolated leaf extract was added in the prepared zinc nitrate solution and heated at 60 °C to 90 °C. The color of the resulting solution turned from clear white to yellow color paste confirms the formation of ZnO NPs. The obtained paste was transferred into ceramic crucible and kept into muffle furnace heated at 400 °C for 2hr. The resultant powder was used for further characterization.
2.6 Characterization techniques
(i) UV–Vis spectra analysis:
The sample was measured for its maximum absorbance using UV–Vis spectrophotometry. The optical property of ZnO nanoparticles was analyzed via ultraviolet and visible absorption spectroscopy (spectrophotometer Cary E 500) in the range of 200–800 nm
(ii) Fourier transforms infra-red spectroscopy (FT-IR):
The binding properties of ZnO nanoparticles were investigated by FTIR analysis. The characterization involved Fourier transform infrared spectroscopy (FTIR) analysis of the dried powder of the synthesized ZnO nanoparticles by Perkin Elmer Spectrum 1000 spectrum in attenuated total reflection mode, and using the spectral range of 4000–400 cm−1 with the resolution of 4 cm−1.
(iii) Scanning electron microscopy (SEM):
The morphology of ZnO nanoparticles was examined by means of scanning electron microscopy (SU3500, Hitachi with spectral imaging system Thermo Scientific NSS (EDS), the tape of detector (BSE-3D), acceleration voltage (15.0 kV), working distance (11.6 mm), pressure (in the case of variable vacuum conditions) (40 Pa).
(iv) X-ray diffraction (XRD):
Properly washed and dried sample of synthesized ZnO nanoparticles was used for XRD analysis using Ultima IV (Rigaku, Japan) at the wavelength of 1.5406 A ° . XRD was performed in the 2 h range of 20–80°at 40 kV and 40 mA with a divergence slit of 10 mm in 2 h/h continuous scanning mode.
2.7 Bacterial & fungal culture
The human bacteria such as Streptococcus mutans, Streptococcus aureus, Clostridium absonum, and Escherichia coli were obtained from culture collection center department of biotechnology of Uttaranchal University Dehradun and were maintained in Nutrient agar at 4 °C for experiment studies. The different fungus strains such as Arthogrophis cuboida, Aspergillius fumigates and Aspergillius nigar were isolated from potato dextrose agar.
2.8 Preparation of standard culture inoculums of test organism:
The colonies of different bacteria and strains of different fungus were inoculated in the 20 ml nutrient broth and incubated for 24-72 h.
2.9 Assay of anti-bacterial activity:
Anti bacterial activity of synthesized ZnO nanoparticles was elucidated by using disc diffusion method. In this method 20 ml of sterilized Mueller Hinton Agar was poured into sterile petri plates, after solidification, 120 μl of bacterial culture poured on the plates and the culture was spread on plates using spreader. The various concentrations of ZnO NPs were loaded on 6 mm sterile disc. The loaded disc was placed on the surface of medium and the extract was allowed to diffuse for 5 min and the plates were kept for incubation at 37 °C for 24 hrs. Streptomycin was used as a control packed along with the solvents. At the end of incubation, inhibition zones
Formed around the disc were measured with transparent ruler in millimeter.
2.10 Assay of anti-fungal activity
Like the antibacterial activity the antifungal activity of synthesized nano particle was also elaborated by disc diffusion method. In this method 20 ml of sterilized Mueller Hinton Agar was poured into sterile petri plates, after solidification, 120 μl of fungus culture poured on the plates and the culture was spread on plates using spreader after that the various concentrations of ZnO NPs were loaded on 6 mm sterile disc. The loaded disc was placed on the surface of medium and the extract was allowed to diffuse for 5 min and the plates were kept for incubation at 37 °C for 24 hrs. Inhibition zones formed around the disc were measured with transparent ruler in millimeter.
3 Results and discussion
3.1 Phytochemical screening analysis
Plant extract is generally used as a potential substitute of the stabilizing and reducing agent due the presence of various important bio-components such as terpenoids, alkaloids, phenolics, tannins, proteins, amino acids, polysaccharides, enzymes, vitamins and saponins. The phytochemical screening analysis of isolated ethanolic leaf extract of was carried out and the result is given in Table 1. From the Table 1 it is clear that in alkaloid, flavanoid, sponins, carbohydrate and terpenoids are the major chemical constituents of the extracts obtained from Euphorbia hirta leaf extracts.
S. No
Phytochemicals
Result
1
Alkaloids
Positive
2
Terpenoids
positive
3
Phenolics
positive
4
Tannins
Negative
5
Proteins
positive
6
Carbohydrate
positive
7
Cardial Glygosidase
Negative
8
Steroids
Negative
There are so many reports are available in literature which specified that phenols and flavonoids are involved in the bio-reduction, formation and stabilization of metal and metal oxide NPs (Lakshmi et al. 2012). Presence of enormous OH groups in phenol and flavonoids are the responsible for reducing zinc nitrate into ZnO NPs. Earliar it was also reported that that C⚌O, C⚌O–C and C⚌C groups of heterocyclic compounds may act as a stabilizer (Divya et al., 2013).
3.2 UV–Vis spectroscopy
It is a well known fact that UV–Vis spectroscopy is one of the most widely used technique for the structural characterization of synthesized nanoparticles. The optical property of synthesized zinc oxide nanoparticale was studied by UV–Visible spectroscopy and spectra were shown in Fig. 1. A strong absorption peak observed at 370 nm in the UV–Visible spectra of synthesized ZnO nanoparticles. Observation of a absorption peak at 370 nm proposed the biosynthesis of ZnO nanoparticles which further supported by literature. (Diallo et al 2015, Senthilkumar and Sivakumar, 2014).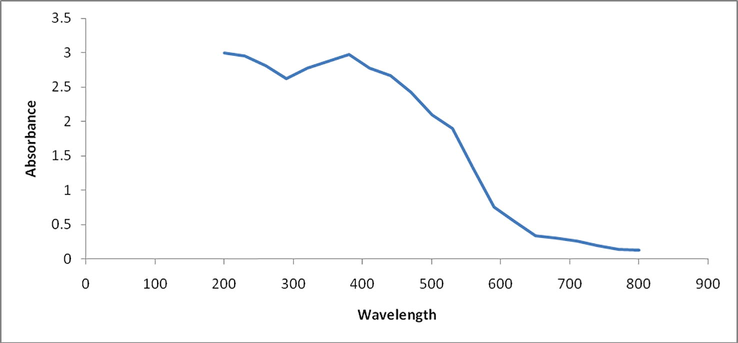
UV–Vis spectrum of ZnO nanoparticle.
3.3 FTIR analysis
The FT IR spectrum of compound is generally used for the determination of functional group present in the compound. Fig. 2 shows the FTIR spectrum of synthesized ZnO NPs. The broad stretch at 3438 cm−1 shows the presence of O–H stretch and hydrogen bonded groups in alcohol or phenolic or water molecules in the extract (Singh et al., 2016). The peaks arises at 2926 cm−1 and 2235 cm−1 were associated with –C⚌C stretching vibration of alkynes. The strong absorption peaks at 1620 cm−1 indicates the stretching vibration of C⚌O hydroxyl (or) carboxyl groups on the surface of the sample. The peak around 1384 cm−1 indicates the asymmetric stretching vibration of nitrate ions (NO3−1) (Yuvakkumar et al., 2015).). The stretching vibration of ZnO NPs peak was observed at 513 cm−1 (Awwad et al., 2013).). The peak in the region between 400 and 600 cm−1 is allotted to Zn–O. The stabilization and capping agent of synthesized ZnO NPs may be due to the coordination of ZnO NPs with –OH and C⚌O groups. It may also conclude that the presence of phenolic and flavonoid group molecules is responsible for the reduction process (Rastogi and Arunachalam, 2011).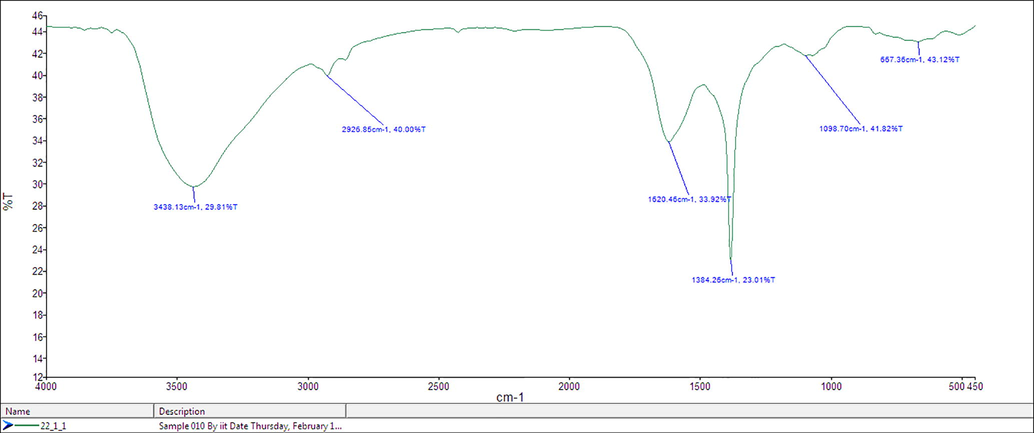
FT-IR spectra of synthesized ZnO nanoparticles using Euphorbia herita leaf Extract.
3.4 XRD analysis
X-ray diffraction pattern of synthesized nanoparticles is used for the calculation of Crystal lattice indices and particle size. Diffraction peaks were observed at 2Θ values of 31.79°, 34.46°, 36.25°, 47.47°, 56.61°, 62.87°, 66.43°, 68.07° and 69.18° corresponding to lattice planes (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2) and (2 0 1) respectively as shown in Fig. 3. The peaks have been attributed to hexagonal phase of ZnO (Khoshhesab et al., 2011; Talam et al., 2012; Zhou et al., 2007).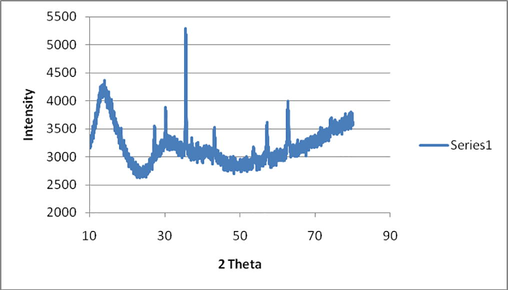
XRD spectra of ZnO nanoparticles.
3.5 Scanning electron microscope (SEM) analysis
Scanning electron microscope is type of electron microscope which is used to capture the images of sample by using high energy electron beam. Heigh energy electron beam interact with the constituent of the compound and give signals reveals important information regarding the composition, surface topography and other properties such as electrical conductivity (Kumar et al., 2013). Therefore the SEM is a useful analytical technique to analyze the surface morphology and size of the synthesized ZnO nanoparticles. SEM image illustrate individual ZnO nanoparticles as well as number of aggregates. Fig. 4 illustrates the particles are predominantly spherical in shape and aggregates into larger particles with no well-defined morphology. The SEM image shows the size of the ZnO nanoparticles ranging from 20 to 25 nm.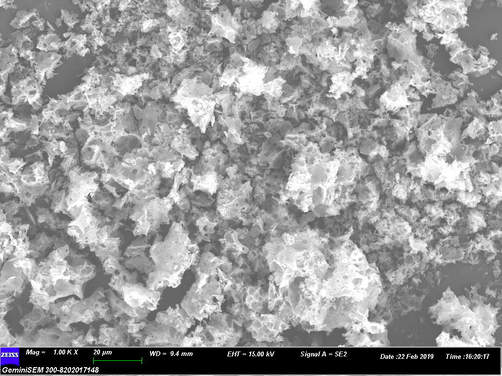
SEM images of ZnO nanoparticles.
3.6 Anti microbial activity
(a) Antibacterial activity
The antibacterial activity of synthesized ZnO nanoparticles was investigated against selected pathogen such as Streptococcus mutans, Streptococcus aureus, Clostridium absonum, Escherichia coli and Proteus mirabilis by disc diffusion method and result was shown in Table 2 and fig. The diameter of zone of inhibition was measured in mm. In the disc diffusion method synthesized ZnO nanoparticles show significant antibacterial activity on all the four bacterial strains.
Name of Micro organism
Zone of inhibition (diameter in mm) at various concentrations
Control
20 mg/ml
40 mg/ml
60 mg/ml
100 mg/ml
Streptococcus mutans,
28
15
17
24
28
Streptococcus aureus
27
13
15
21
29
Clostridium absonum
29
14
18
23
27
Escherichia coli
20
12
16
20
24
The result of antibacterial study clearly indicated that the antibacterial activity (in terms of Zone of inhibition) increases with increasing the conc. of ZnO nanoparticales (20, 40, 60, 100 mg/ml) which may be due to the increase of H2O2 concentration from the surface of ZnO nanoparticales The zone of inhibition was found to be highest in case of Streptococcus aureus (29 mm) when compared to other bacteria like Streptococcus mutans (28 mm), Clostridium absonum (27 mm), Escherichia coli (24 mm) as shown in Fig. 5 and Table 2. Senthilkumar et al demonstrated the antibacterial activity of biosynthesized ZnO nanoparticles against the bacterial strain Streptococcus aureus with zone of inhibition 28 mm (Senthilkumar and Sivakumar, 2014). Similarly Chinnammal Janaki et al. reported the antibacterial activity of ZnO nanoparticles against Staphylococcus aureus (10 mm), C. albicans (10 mm), Penicillium notatum (12 mm) (Chinnammal Janaki et al., 2015).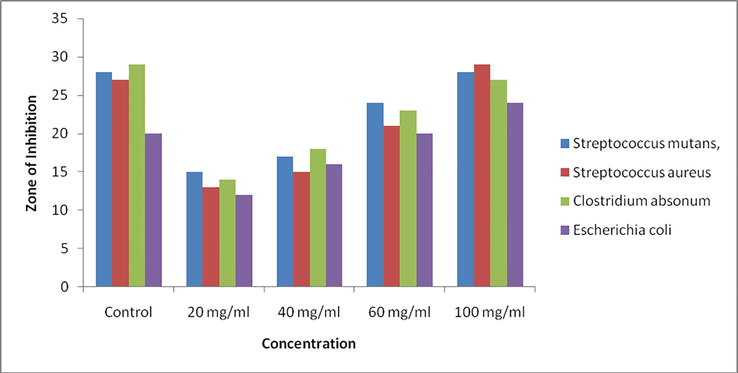
Antibacterial activity of synthesized ZnO Nano particles.
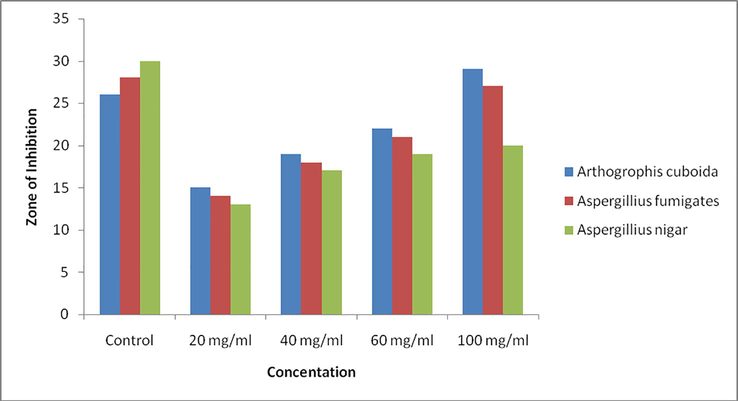
Antifungal activity of synthesized ZnO Nano particles.
Generally the synthesized nanoparticles of metal oxide carry positive charge and micro organism carry negative charge thus this interaction creates an “electromagnetic” attraction between the microorganism and treated surface due to this excitation the concentration of excited singlet oxygen increases. Singlet oxygen’s are mostly reactive and subsequently enhance the antimicrobial activity.
(b) Anti fungal Activity
The antifungal activity of synthesized ZnO nanoparticles was also investigated against selected fungal apathogen such as Arthogrophis cuboida, Aspergillius fumigates and Aspergillius nigar. The diameter of zone of inhibition was measured in mm. In the disc diffusion method synthesized ZnO nanoparticles show significant antifungal activity against all the selected fungal strains. Result is shown in Table 3 and Fig. 6.
Name of Micro organism
Zone of inhibition (diameter in mm) at various concentrations
Control
20 mg/ml
40 mg/ml
60 mg/ml
100 mg/ml
Arthogrophis cuboida
26
15
19
22
29
Aspergillius fumigates
28
14
18
21
27
Aspergillius nigar
30
13
17
19
20
As shown in table the antifungal activity of synthesized nanoparticles is increases as the concentration increases (20, 40, 60, 100 mg/ml). It is found that on comparing the obtained antifungal result that the synthesized nanoparticles show best result in case of Arthogrophis cuboida (zone of inhibition 29 mm). Minimum zone of inhibition was reported in case of Aspergillius nigar (zone of inhibition 20 mm). Jamdagni et al reported the antifungal activity of synthesized ZnO nano particle againes the following antifungal strains A. alternata (64 mm), A.niger (16 mm), B. cinerea (1 2 8), F. oxysporum (64 mm) (Jamdagni et al., 2018).
4 Conclusion:
The synthesis of medicinally useful nanoparticles using chemical synthesis techniques produces lots of toxicity to the environment. So in the present paper the production ZnO nanoparticles was carried out by green synthesis. The Euphorbia hirta (asthmatic plant) leaves extract was used effectively for the above synthesis. The biological synthesis of zinc nanoparticles using leaf extract of Euphorbia hirta provide an environmental friendly, simple and efficient route for synthesis of nanoparticles. The use of plant extract avoids usage of harmful and toxic reducing and stabilizing agents. The characterization of ZnO nanoparticles were carried out using different techniques like XRD, FTIR, SEM and UV–Vis etc. To analyze the antimicrobial activity of the sample, the samples are subjected disc diffusion method. The samples were experimented against some bacteria like Staphylococcus aureus and many more followed by some fungi like Aspergillus niger, Cuboida etc. As the production of ZnO NPs is cost effective, rapid and environmentally benign this trouble-free, single step procedure of E. hirta extracts mediated synthesis appears to be appropriate for large scale production. Hence it is concluded that the ZnO NPs synthesized in the present research can be a potential candidate for different medicinal and biological related applications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical Screening and antimicrobial study of Euphorbia hirta extracts. J. Med. Plants Stud.. 2017;5:185-188.
- [Google Scholar]
- Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem.. 2013;4:29.
- [Google Scholar]
- Antibacterial, magnetic, optical and humidity sensor studies of β-CoMoO4-Co3O4 nanocomposites and its green synthesis and characterization. J. Photochem. Photobiol., B. 2018;183:233-241.
- [Google Scholar]
- Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem.. 2009;11:926-930.
- [Google Scholar]
- Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci.. 2016;23:517-523.
- [Google Scholar]
- Synthesis, Characteristics and Antimicrobial activity of ZnO nanoparticles. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015
- [CrossRef] [Google Scholar]
- Green synthesis of ZnO nanoparticles by Aspalathus linearis: structural & optical properties. J. Alloy. Compd.. 2015;646:425-430.
- [Google Scholar]
- Synthesis of zinc oxide nanoparticle from Hibiscus rosa-sinensis leaf extract and investigation of its antimicrobial activity. Res. J. Pharm. Biol. Chem.. 2013;4(2):1137-1142.
- [Google Scholar]
- Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int.. 2012;22(6):693-700.
- [Google Scholar]
- Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustainable Chem. Eng.. 2013;1(7):703-712.
- [Google Scholar]
- Nanoparticles based DNA conjugates for detection of pathogenic microorganisms. Int. Nano Lett.. 2016;6:139-146.
- [Google Scholar]
- Green palladium and palladium oxide nanoparticles synthesized via Aspalathus linearis natural extract. J. Alloy. Compd.. 2017;695:3632-3638.
- [Google Scholar]
- Synthesis and characterization of ZnO nanoparticles for efficient gas sensors. Arch. Appl. Sci. Res.. 2013;5(6):20-28.
- [Google Scholar]
- Preparation of ZnO nanostructures by chemical precipitation method. Synth. React. Inorg., Met.-Org., Nano-Met. Chem.. 2011;41(7):814-819.
- [Google Scholar]
- Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett.. 2013;3:30.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. Journal of King Saud University – Science. 2018;30:168-175.
- [Google Scholar]
- Bioinspired synthesis of pure massicot phase lead oxide nanoparticles and assessment of their biocompatibility, cytotoxicity and in-vitro biological propertie. Nanomedicine. 2017;12(15):1767-1789.
- [Google Scholar]
- Beet juice-induced green fabrication of plasmonic AgCl/Ag nanoparticles. ChemSusChem. 2012;5(12):2435-2441.
- [Google Scholar]
- Synthesis, characterization and evaluation of antimicrobial activity of zinc oxide nanoparticles. J. Biochem. Technol.. 2012;3(5):S151-S154.
- [Google Scholar]
- Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate and turmeric extracts. ACS Sustainable Chem. Eng.. 2014;2:1717-1723.
- [Google Scholar]
- Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem.. 2008;10(8):859-862.
- [Google Scholar]
- Production and characterization of protein encapsulated silver nanoparticles by marine isolate Streptomyces parvulus SSNP11. Indian J. Microbiol.. 2014;54(3):329-336.
- [Google Scholar]
- Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater. Chem. Phys.. 2011;129:558-563.
- [Google Scholar]
- Rambutan peels promoted biomimetic synthesis of bioinspired zinc oxide nanochains for biomedical applications. Spectrochim Acta Part A Mol. Biomol. Spectrosc.. 2013;112:384-387.
- [Google Scholar]
- Physical properties of fish gelatin-based bio-nanocomposite films incorporated with ZnO nanorods. Nanoscale Res. Lett.. 2013;8:364-369.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: structure and optical properties. Mater. Res. Bull.. 2011;46:2560-2566.
- [Google Scholar]
- Sol–gel synthesis of zinc oxide nanoparticles using Citrus aurantifolia extracts. Ceram. Int.. 2013;39:S545-S548.
- [Google Scholar]
- Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZNO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci.. 2014;6:461-465.
- [Google Scholar]
- Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett.. 2015;7:219-242.
- [Google Scholar]
- Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol.. 2016;34(7):588-599.
- [Google Scholar]
- A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol.. 2018;16:1-28.
- [Google Scholar]
- Advances in nanodiagnostic techniques for microbial agents. Biosens. Bioelectron.. 2014;51:391-400.
- [Google Scholar]
- Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. ISRN Nanotechnol.. 2012;2012:372505
- [Google Scholar]
- Physical properties of CdO nanoparticles synthesized by green chemistry via Hibiscus Sabdariffa flower extract. J. Alloy. Compd.. 2016;655:314-320.
- [Google Scholar]
- Rambutan peels promoted biomimetic synthesis of bioinspired zinc oxide nanochains for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2015;137:250-258.
- [Google Scholar]
- Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids) J. Nanoparticle Res.. 2007;9:479-489.
- [Google Scholar]
- Size controlled synthesis of ZnO nanoparticles and their photoluminescence properties. J. Lumin.. 2007;122–123(1–2):195-197.
- [Google Scholar]







