Translate this page into:
Green synthesis and evaluation of anti-microbial, antioxidant, anti-inflammatory, and anti-diabetic activities of silver nanoparticles from Argyreia nervosa leaf extract: An invitro study
⁎Corresponding author at: Centre of Molecular Medicine and Diagnostics (COMManD) Department of Biochemistry, Saveetha Dental College & Hospitals Saveetha Institute of Medical and Technical Sciences Saveetha University, Chennai 600077, India. selvarajj.sdc@saveetha.com (Selvaraj Jayaraman),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The aim of the study is to develop an environmentally friendly development of isolating silver nanoparticles using Argyreia nervosa plant leaves and examine their potential pharmacological properties, including antibacterial, anti-inflammatory, antioxidant, and anti-diabetic activities. Various analytical techniques were employed to examine the isolated silver nanoparticles, encompassing UV spectroscopy, XRD, SEM, EDX, FTIR, zeta potential measurement, and particle size determination. Furthermore, Argyreia nervosa-mediated silver nanoparticles exhibited strong antioxidant, anti-inflammatory activity, and good anti-diabetic activity. Argyreia nervosa-mediated nanoparticles showed a significant antibacterial activity against selected pathogens. The findings indicate that Argyreia nervosa demonstrates encouraging pharmacological properties. However, additional in vivo research focusing on molecular-level investigations is necessary to substantiate these findings and explore the potential of Argyreia nervosa-mediated silver nanoparticles as a viable drug for antioxidant, antibacterial, and anti-diabetic purposes.
Keywords
Nanotechnology
Silver nanoparticle
Argyria nervosa
Traditional medicine
Antidiabetic activity
1 Introduction
Promising and multi-disciplinary area of biotechnology combined with nanotechnology known as nanobiotechnology. Nanotechnology is an emerging and rapidly expanding field that is revolutionizing science and technology by focusing on the development of novel nanomaterials (Albrecht et al., 2006). In recent years, biosynthetic techniques based on nanoparticle (NP) preparation have used biological microbes like fungi, bacteria, and plant extracts. Metal-based nanoparticles can be produced using numerous chemical, biological, physical, and hybrid processes (Tiwari et al., 2008). Nanotechnology has applications in many fields including medicine, biotechnology, chemistry, physics, and material science. In parallel, metal-based nanoparticles such as gold (Au), selenium (Se), silver (Ag), zinc sulfide (ZnS) and zinc oxide (ZnO) gained more attention due to their specialty and specificity (Malarkodi et al., 2014; Vanaja et al., 2014). Among these NPs, silver nanoparticles (AgNPs) are easy to synthesize and user-friendly metals. Moreover, AgNPs are effective, chemically stable, and has been shown to have antibacterial, antiviral, antifungal, and easily synthesized (Balavijayalakshmi and Ramalakshmi, 2017; Palanisamy et al., 2023). Silver-impregnated topical dressings are now often used to treat wounds, burn-related infections, and chronic ulcers. Since the chemical methods-based synthesis of nanoparticles have harmful effects as they are environmentally hazardous, in the current study, we used physical method-based nanoparticle preparation as these methods are user-friendly, easy to synthesize, and harmless methods (Raveendran et al., 2003).
Medicinal herbs and plant extracts are now widely recognized as effective medications worthy of respect and value, and they play an important part in modern medicine. According to the WHO, 80% of the total population uses herbs for primary healthcare. The Indian subcontinent is home to a wide variety of herbal plants used in the Indian system of medicine (Mukherjee et al., 2012; Saminathan et al., 2022). Plant-based treatments have traditionally been recognized as the very first line of defense in sustaining health and treating disease (Ballabh and Chaurasia, 2007). Argyreia nervosa (A. nervosa) is one of the most medically valuable plants, it has pharmacologic activities like hypoglycemic, anti-inflammatory, antiulcer, antimicrobial, immunomodulatory, anticonvulsant, etc. A. nervosa is commonly known as morning glory, Hawaiian Baby Wood Rose, and elephant Creeper. The plant has the potential to attain a height exceeding 10 m. It is a woody shrub, mostly found edges of the lake and semi-deciduous forests of the Western Ghats of India (Thombre et al., 2014). Traditionally used for gonorrhea and chronic ulcer management. This is also used for an ornamental plant because of its attractive leaves and flower colors (Paulke et al., 2015). Leaf has potential anti-cancer and anti-bacterial activity (Subramanyam et al., 2021). Bloom is funnel-shaped violet or lavender in color. while leaves contain, β-sitosterol, 1-tricontanol, flavonoids, lipids, and quercetin (Singhal et al., 2011). Roots are used for obesity management, diabetes, tuberculosis, ulcer, and wound management (Milimita et al., 2013). The seeds in the dried-out flower pods are black in color. Each flowering pod contains three to five seeds (Meher and Padhan, 2011). Based on these realities, current research focused on AgNPs isolated from A. nervosa leaf extracts for their characterization, and analysis of in-vitro biological activities.
2 Methodology
2.1 Chemicals
In this study, silver nitrate extra pure AR, 99.9 %, DPPH, ABTS, potassium persulfate, ascorbic acid, α-amylase. α-glucosidase, and p-NPG were purchased from Sigma. Standard drugs ciprofloxacin and acarbose were procured from the pharmacy. The microbial pathogens such as Escherichia coli (E. coli), and Pseudomonas aeruginosa (P. aeruginosa) were sourced from Pharmacology Department, Saveetha dental college, Chennai.
2.2 Extract preparation
The fresh A. nervosa leaves were collected from Velliangiri Hills, India. The plant sample was authenticated by the National Institute of Siddha Ministry of Ayush, Chennai (certificate no: NISMB5592023, supplementary Fig. S1). The leaves were cleaned with normal water to prepare the plant extract, followed by a rinse with distilled water. After the air-drying process, the leaves samples were in the shade for 5–7 days, they were finely powdered using a mixer grinder. 5 g of the powdered leaves were mixed with sterilized water (100 ml) and heated to 60 °C for 30 min and then allowed to cool. After filtration through Whatman filter paper, the resultant clear extract was collected and kept at 4 °C for the following process.
2.3 AgNPs synthesis from the A. nervosa extract
5 ml of prepared A. nervosa aqueous extract was added to 20 mM pure aqueous silver nitrate (AgNO3) solution and kept for 60 min to reduce silver nitrate to silver nanoparticles (AgNPs). The color of the solution changed from yellow to dark brown after the reduction of Ag+ by Ag0 nanoparticles were confirmed that the synthesis of AgNPs (Balavijayalakshmi and Ramalakshmi, 2017).
2.4 Nanoparticle characterization
The confirmation of AgNP synthesis was done using UV–Vis absorption spectra. To investigate the structure and composition of the synthesized AgNPs, XRD analysis was conducted using a Bruker instrument, Germany. FTIR spectroscopy was used to study the possible functional groups involved in the synthesis and stabilization of AgNPs. The shape and size of the AgNPs were characterized using SEM, and the presence of elemental Ag was confirmed using EDX. Zeta potential was measured using a Microtra instrument, which is an important parameter for characterizing stability in aqueous nanosuspensions. The particle size investigation method determined the average particle size of the synthesized AgNPs.
2.5 Inoculum preparation and anti-bacterial activity of A. nervosa AgNPs
A disc diffusion process was employed to estimate the bacterial efficiency of AgNPs derived from A. nervosa. In this study, ciprofloxacin was used as an antibiotic agent. The concentration range of AgNPs tested was 10–30 μg/ml. The agar plates were incubated at 37 °C for a duration of 12 h. On a subsequent day, the inhibition zone for each sample was measured and recorded in millimetres (mm) (Shanmugam et al., 2021).
2.6 DPPH activity
A range of concentrations for A. nervosa AgNPs (100–500 μg/ml) and plant extracts were obtained by performing serial dilution with a 1 mg/ml stock solution. Subsequently, the mixture was incubated for half an hour and read at 517 nm using a spectrophotometer (Hatano et al., 1990). The inhibition (%) for scavenging activity was calculated using the formula:
2.7 ABTS antioxidant assay
Test sample concentrations range of 100–500 μg/ml for both AgNPs and plant extract were used for antioxidant assay. Briefly, 50 μl of the sample and 950 μl of the solution ABTS made up of the 1 ml reaction mixture of the standard and extracts. For 10 s, the reaction solution was vortexed and read at 734 nm. In this analysis, ascorbic acid served as the standard, and the percentage (%) was calculated using Eq. (1).
2.8 Hydrogen peroxide (H2O2) activity
Concentrations ranging from 100 to 500 μg/ml of leaf extract and AgNPs were employed in the experiment with ascorbic acid as the standard. Briefly, a mixture containing 50 μl of H2O2 (5 mM) solution was prepared and incubated at room temperature (26 ± 2 °C) for a duration of 20 min and read at 610 nm. Eq. (1) was utilized to calculate the H2O2 radical scavenging activity.
2.9 Albumin denaturation inhibition
The albumin denaturation assay was carried out as described by Mizushima and Kobayashi (1968) Diclofenac sodium was used as a standard for this procedure. The reaction mixture consisted of sample concentrations levels ranging from 100 to 500 μg/ml, along with 1 % aqueous solution of albumin fraction. The absorbance of the mixture was measured within the range of 660 nm. The percentage (%) of inhibition was determined using Eq. (1) for calculation purposes.
2.10 α-Amylase inhibition
The antidiabetic activity of A. nervosa-mediated AgNPs was estimated in vitro by a standard protocol with acarbose as a reference substance and the absorbance was subsequently measured at 540 nm (Hansawasdi et al., 2000). Results were expressed as % of inhibition of α-amylase inhibitory activity.
2.11 α-Glucosidase activity
The α-glucosidase enzyme inhibitory activity was done by standard (Yarrappagaari, et al., 2020; Yin et al., 2008). The results were expressed in % of inhibition of α-glucosidase activity.
3 Statistical analysis
The data were expressed as the mean ± SEM of three replicates. One-way analysis of variance (ANOVA) was used, followed by Tukey's test. p < 0.05 had been considered statistically significant.
4 Results
4.1 Observation of color changes of AgNPs
The synthesized AgNPs are generally confirmed by their, range, shape, and surface area with dispersity. Apart from these characteristics, simple color development is also used to identify the presence of AgNPs. When silver solution was added to the extract, the plant aqueous extract color was altered from light brown to dark and deep brown color (Fig. 1). These color changes indicate the presence of AgNPs. In this linking, earlier studies showed comparable color changes to form dark brown color (Subramanyam et al., 2021). Due to the reduction of Ag+ ions converted to Ag0 atoms.
Plant extract + AgNo3 → AgNPs.
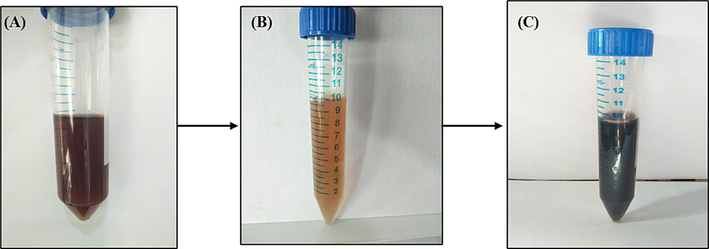
Represents AgNPs synthesized in A. nervosa leaf extract. (a) Leaf extract without the addition of silver nitrate (AgNo3), (b) After adding Ag solution, (c) A. nervosa Leaf extract with Ag solution.
4.2 UV–Vis spectral analysis of AgNPs
To find the exact concentration for the AgNPs preparation initially, we need to find the metal solution reactivity to our plant extract. At the preliminary stage of the synthesis, the reduction of Ag ions in the aqueous solution of the Ag complex was observed through UV–Vis spectroscopy, indicating the interaction with the components presents in the A. nervosa extract. For this UV spectroscopy analysis, 20 mM and 40 mM of Ag solution were used for the preparation of the A. nervosa mediated AgNPs preparation (Fig. 2a). The UV spectrum absorbance measurement for a 20 mM Ag solution yielded an absorbance value of 421 nm, while the 40 mM concentration exhibited an absorbance value of 424 nm. Based on these results, time-dependent analyses were conducted for the 20 mM concentration at different time intervals, including 0 h, 15 min, 30 min, 45 min, and 1 h (Fig. 2b).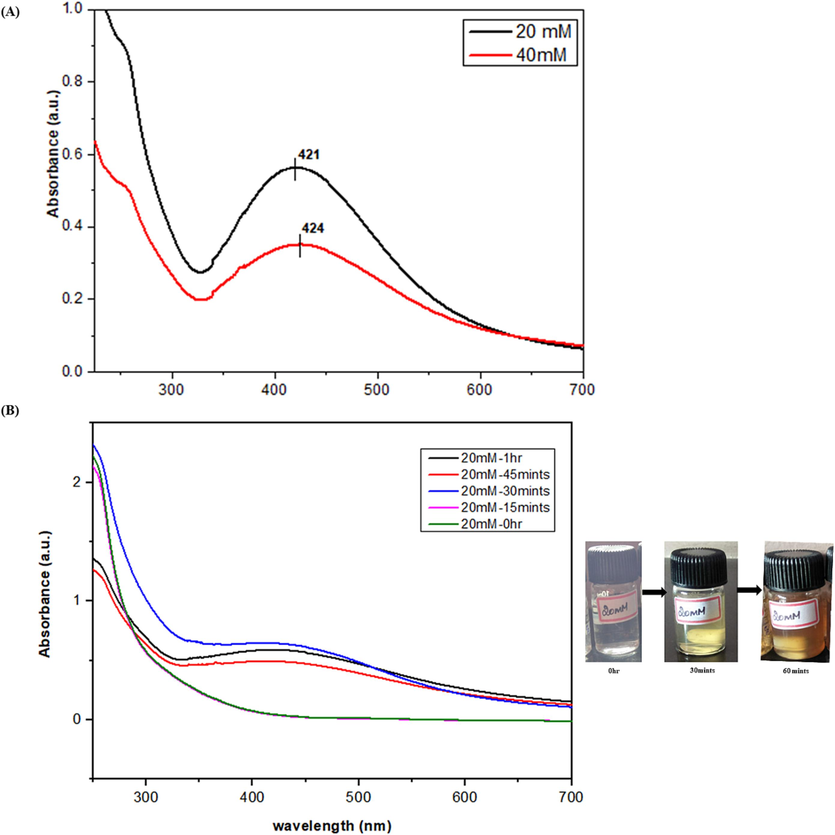
(a) UV- spectrum record of A. nervosa AgNPs in a concentration-dependent manner (20 mM and 40 mM). (b) Time-dependent manner analysis was performed on the duration of 0 hr, 15, 30, 45 min, and 1 hr.
4.3 XRD and FTIR investigation
The nature of crystalline AgNPs was verified by the XRD diffraction diagram (Fig. 3a). The calculation of the crystallite sizes of AgNPs synthesized using A. nervosa extract was performed using Debye-Scherrer's equation, as described by Gnanajobitha et al. (2013).
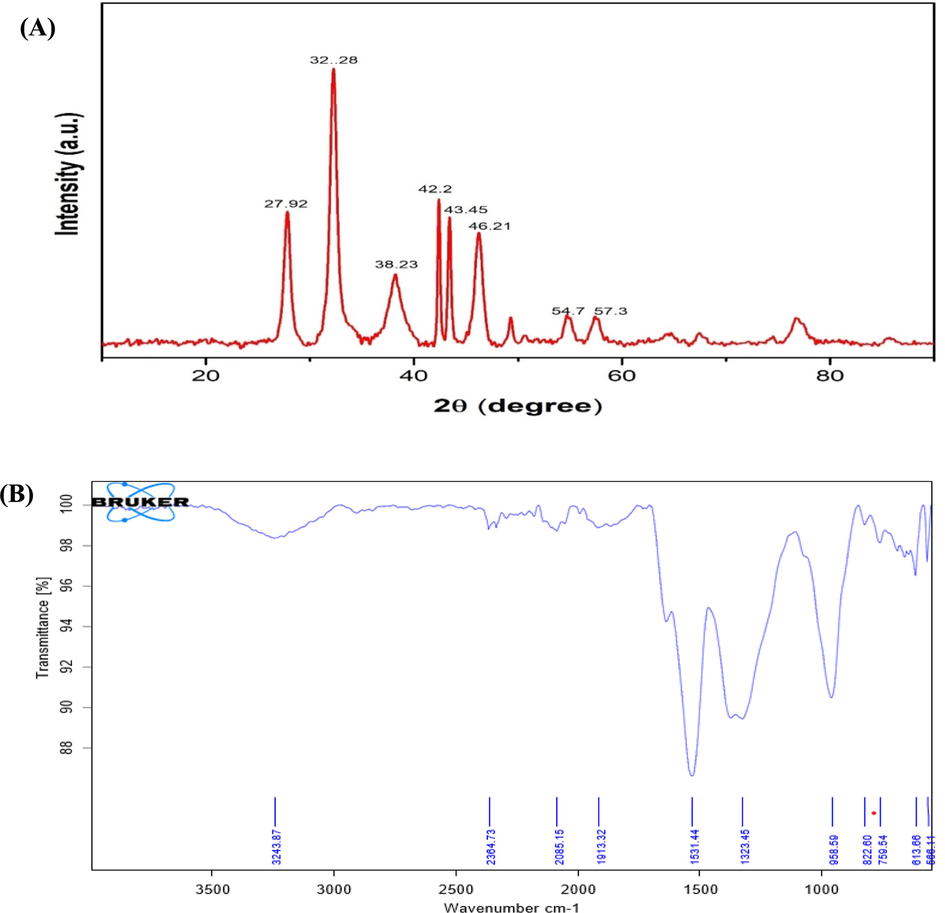
(a) The XRD pattern of A. nervosa mediated AgNPs, where the X-axis indicates the 2θ scale, and the Y-axis denotes the intensity of the AgNPs. (b) The FTIR spectrum of A. nervosa mediated AgNPs. Where the X-axis denotes the wavenumber in cm-1, and the Y-axis shows the transmittance of the AgNPs.
The inorganic biomolecules in the fucoidans responsible for AgNP formation were analyzed using the FT-IR analysis (Fig. 3b).
4.4 SEM and EDX analysis
Fig. 4a and b depicts the morphology of A. nervosa-mediated AgNPs. For SEM analysis, dried nanoparticles were utilized, and two magnification ranges were employed to visualize their presence namely 50 μm, and 100 nm. EDX results demonstrate the purity and the complete chemical composition of A. nervosa mediated AgNPs. The percentage of elements followed by Ag is 82.3 %, chlorine (Cl) 10 %, carbon (C) 5.2 %, and oxygen (O) 2.6 % respectively. The additional elements assisted as capping organic agents bound to the surface of the AgNPs (Fig. 4c).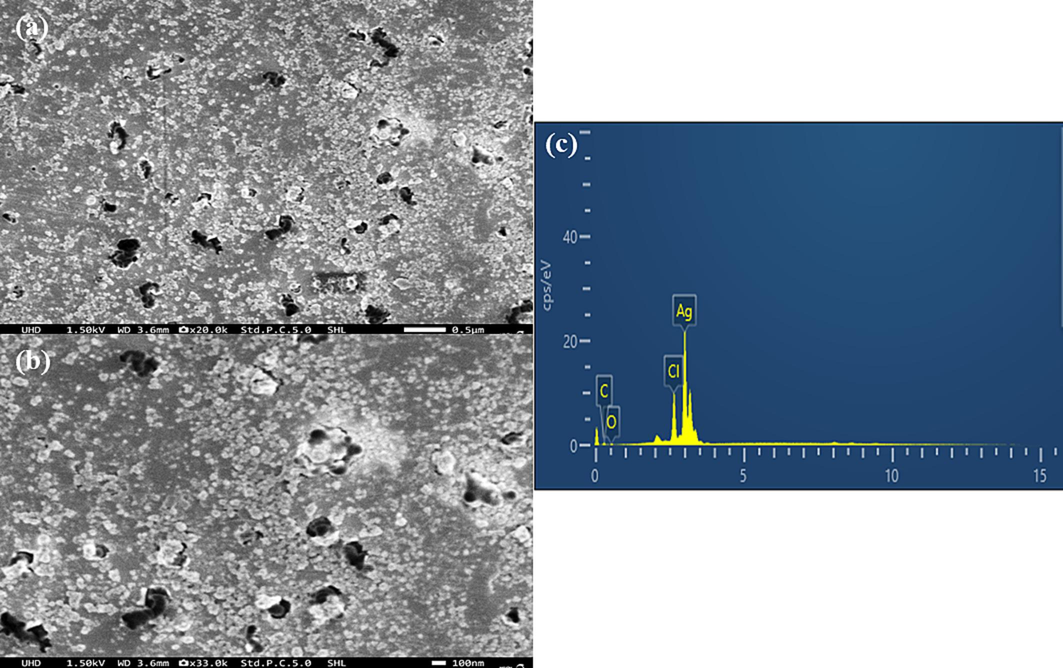
SEM Analysis of A. nervosa mediated AgNPs (a) 50 μm, (b) 100 nm. (c) EDX analysis of AgNPs prepared with A. nervosa leaf extract.
4.5 Zeta potential measurement and particle size analysis
The synthesized AgNPs were dispersed into the double distilled water to analyze the zeta potential. The zeta potential of the prepared AgNPs was −16.4 mV and the zeta deviation was 7.89 mV as shown in Fig. 5a. The particle size investigation results clearly demonstrated the distribution of AgNPs in the reaction mixture according to intensity, number, and volume of AgNPs respectively. Before analyzing particle size, NPs were suspended in 10 ml of water and sonicated for 30 s. The average particle size detected in 69.54 nm (Fig. 5b).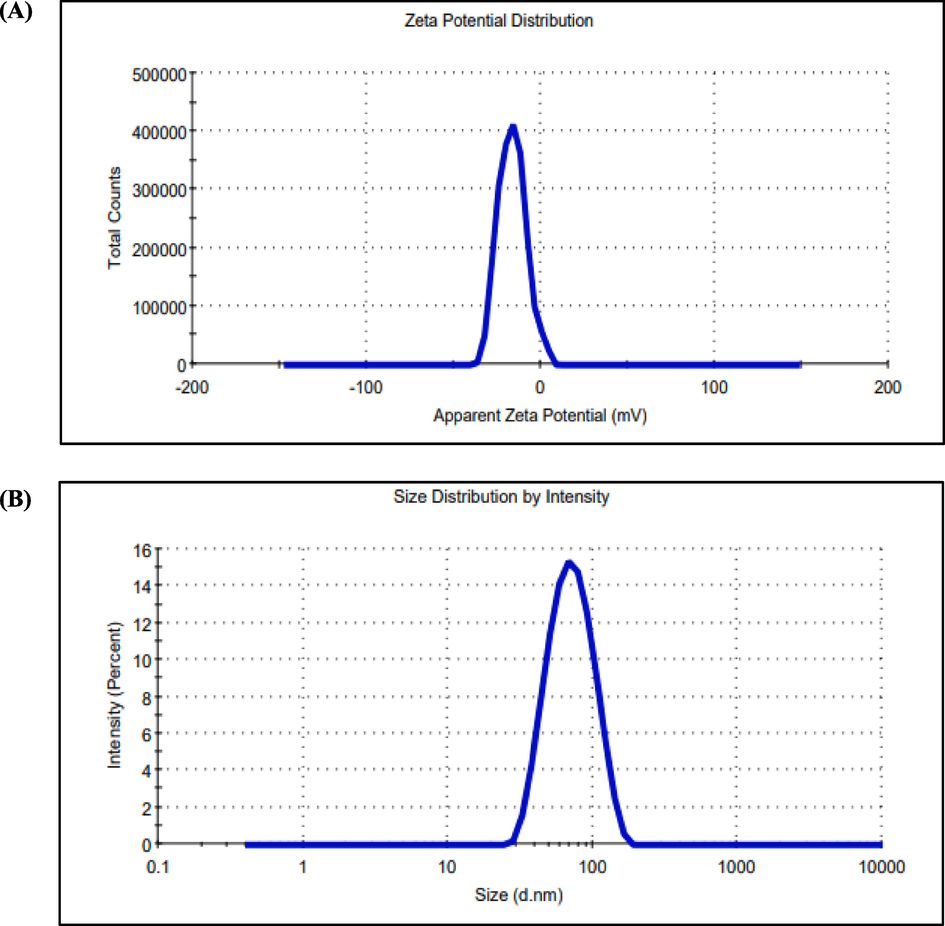
(a) Zeta potential measurement and (b) Particle size of AgNPs with respect to intensity, number, and volume of AgNPs.
4.6 Antimicrobial activity
The percent study investigated the antibacterial action of A. nervosa AgNPs against E.coli and P. aeruginosa. This study was carried out using the following samples: A. nervosa AgNPs at 10, 20, and 30ug concentration, ciprofloxacin (antibiotic control, 30ul concentration) (Fig. 6). Increasing concentration of A. nervosa AgNPs formed improved the ZOI for both tested species. A. nervosa-mediated AgNPs showed increased antimicrobial activity in terms of ZOI in both tested organisms (Table 1).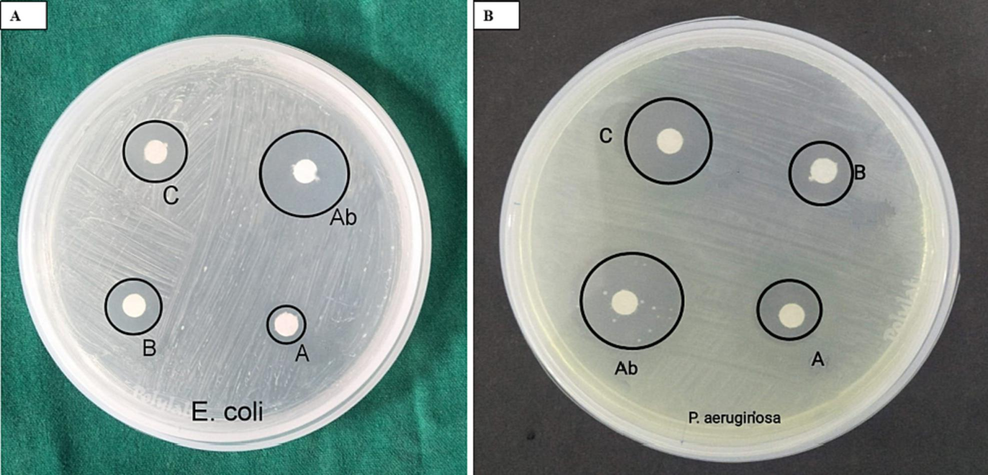
Photograph of petri dishes with loaded samples of A. nervosa mediated AgNPs against (a) E. coli, (b) p. aeruginosa. Ab-antibiotic drug (30 μl), a-10 μl, b-20 μl, c-30 μl concentrations.
Name of the pathogen
Standard (30 μl)
AgNPs ZOI (mm)
10 μl
20 μl
30 μl
E. coli
12.5
3.5
6
7.8
p. aeruginosa
15.5
5.5
7
10.5
4.7 DPPH antioxidant assay
The A. nervosa leaf extract and AgNPs antioxidant activity conformed via performing the DPPH free radical scavenging activity (Fig. 7a and Table 2a). This is a commonly used method for quantifying free radical scavenging action. A decrease in DPPH is indicated by a change in color from violet to yellow, demonstrating the capability of the extract to donate a proton to the DPPH radical (Kawra et al., 2020).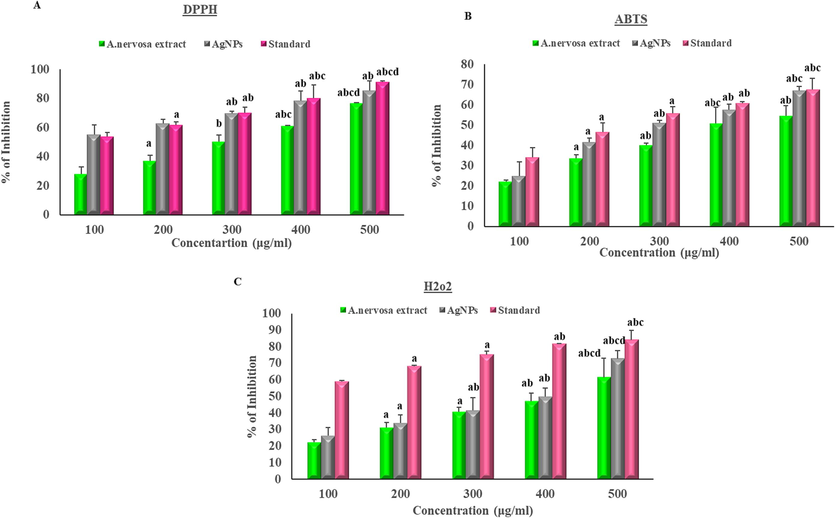
Effect of A. nervosa and AgNPs on (a) DPPH activity, (b) ABTS assay, (c) H2O2. The X-axis shows the different concentrations of AgNPs and extract and the Y-axis denotes the % of inhibition. (a-significance to 100 μg/ml, b-significance to 200 μg/ml, c-significance to 300 μg/ml, and d-significance to 400 μg/ml).
(A)
Concentration (μg/ ml)
DPPH % of scavenging activity
Extract
AgNPs
Standard
100
27.92 ± 5.1
55.33 ± 6.4
53.81 ± 2.7
200
37.15 ± 3.8a
62.81 ± 2.8
61.83 ± 2.1a
300
50.23 ± 4.8b
69.8 ± 1.3ab
70.13 ± 3.8ab
400
61.23 ± 0.2abc
78.33 ± 6.8 ab
80.1 ± 9.1abc
500
76.91 ± 0.1abcd
85.43 ± 6.6ab
91.3 ± 0.7abcd
(B)
Concentration (μg/ ml)
ABTS % of scavenging activity
Extract
AgNPs
Standard
100
22.04 ± 1
25 ± 7
34.19 ± 4.8
200
33.63 ± 1.6a
41.66 ± 2a
46.5 ± 4.7a
300
40.04 ± 1ab
51.13 ± 1.2ab
55.9 ± 3.2a
400
50.95 ± 8abc
57.63 ± 2.6ab
60.8 ± 0.9ab
500
54.56 ± 5.1ab
67.09 ± 2abc
67.5 ± 5.7abc
(C)
Concentration (μg/ ml)
H2O2 % of scavenging activity
Extract
AgNPs
Standard
100
22.48 ± 1.5
26.26 ± 5
58.98 ± 0.7
200
31.07 ± 3.1a
33.95 ± 5a
68.15 ± 0.7a
300
40.72 ± 2.7a
41.63 ± 7.5ab
75.15 ± 2a
400
46.94 ± 4.9ab
49.91 ± 5ab
81.58 ± 0.2ab
500
61.50 ± 11.6abcd
72.60 ± 5abcd
83.97 ± 6abc
4.8 ABTS assay
The current study examined the ABTS test on both AgNPs and extract of A. nervosa (Fig. 7b and Table 2b). ABTS is recognized as a protonated radical capable of accepting electrons from antioxidant compounds, resulting in a color change from blue to pink, as described by Gangwar et al. (2013).
4.9 H2O2 scavenging activity
H2O2 activity of A. nervosa leaves extract and AgNPs capped with plant extract showed H2O2 scavenging activity by transferring electrons to H2O2 and neutralizing it into water·H2O2 inhibition activity assay plays a dynamic part in the determination of antioxidant activity. The results obtained for the H2O2 scavenging activity closely resembled those obtained for the DPPH radical scavenging activity. The A. nervosa AgNPs displayed potential H2O2 radical action in a dose-dependent manner (Fig. 7c and Table 2c).
4.10 Albumin denaturation inhibition
In the present study, anti-inflammatory action was performed by albumin denaturation assay. The outcomes were compared to the standard drug diclofenac sodium. The assay confirmed the anti-inflammatory action of A. nervosa AgNPs whose efficacy was nearest to the standard drug as shown in Fig. 8a and Table 3a.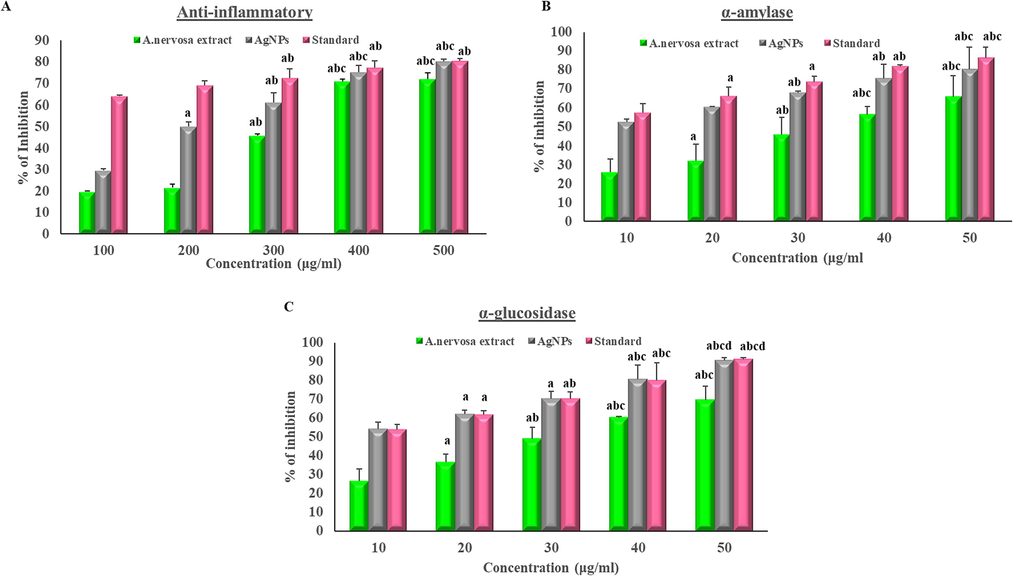
(a) Anti-inflammatory activity, (b) α-amylase assay, and (c) α-glucosidase assay. (The X-axis shows the different concentrations of AgNPs and extract and the Y-axis denotes the % of inhibition, (a-significance to 100 μg/ml, b-significance to 200 μg/ml, c-significance to 300 μg/ml, and d-significance to 400 μg/ml).
(A)
Concentration (μg/ ml)
Anti-inflammatory % of scavenging activity
Extract
AgNPs
Standard
100
19.82 ± 0.2
29.71 ± 0.7
63.89 ± 0.7
200
21.63 ± 1.6
50.08 ± 2.1a
68.99 ± 2.1
300
45.86 ± 0.9ab
61.30 ± 4.3ab
72.45 ± 4.3ab
400
70.97 ± 1abc
75.21 ± 3.2abc
77.31 ± 3.2ab
500
71.93 ± 2.9abc
80.19 ± 1.2abc
80.40 ± 1.2ab
(B)
Concentration (μg/ ml)
Alpha-amylase % of scavenging activity
Extract
AgNPs
Standard
10
26 ± 7
52.65 ± 1.7
57.62 ± 4.8
20
36.25 ± 8.8a
60.63 ± 0.3
66.08 ± 4.7a
30
46.15 ± 8.9ab
67.95 ± 0.9ab
73.56 ± 3.2a
40
56.7 ± 4.3abc
75.4 ± 7.6ab
81.75 ± 0.9ab
50
65.65 ± 11.4abc
80.4 ± ± 11.6abc
86.35 ± 5.7abc
(C)
Concentration (μg/ ml)
Alpha-glucosidase % of scavenging activity
Extract
AgNPs
Standard
10
26.6 ± 6.4
54.17 ± 3.7
53.81 ± 2.7
20
36.7 ± 4.3a
62.03 ± 2.1a
61.83 ± 2.1a
30
49.1 ± 5.9ab
70.4 ± 3.8a
70.13 ± 3.8ab
40
60.35 ± 0.6abc
80.5 ± 7.6abc
80.1 ± 9.1abc
50
69.65 ± 7.3abc
90.6 ± 1.4abcd
91.3 ± 0.7abcd
4.11 The activity of α-amylase inhibition
The study found that AgNPs can reduce the activity of enzymes that break down complex carbohydrates and increase glucose consumption, as previously described by Sengottaiyan et al. (2016) and Balan et al. (2016). In the current study, it was observed that an increase in the concentration of AgNPs resulted in a corresponding dose-dependent increase in the inhibition percentage of α-amylase (Fig. 8b and Table 3b).
4.12 α-Glucosidase inhibition
The α-glucosidase is an important enzyme in carbohydrate metabolism, according to research, inhibiting α-glucosidase can delay carbohydrate digestion, and absorption, and decrease blood sugar levels (Khan et al., 2014). AgNPs synthesized from A. nervosa showed a significant increase in α-glucosidase inhibitory action in a dose-dependent manner (Fig. 8c and Table 3c).
5 Discussion
Currently, there is a global focus among researchers to develop eco-friendly and cost-effective medicine. The current study purposed to investigate the synthesis and characterization of A. nervosa AgNPs, as well as their potential therapeutic effects in terms of antibacterial, antioxidant, anti-inflammatory, and anti-diabetic properties. By utilizing a green synthesis approach, the current study aimed to produce nanoparticles that are both effective and environmentally safe. The outcome of these findings could contribute to the development of new and more accessible treatments for patients in need.
The UV–visible spectroscopy also enables the quantitative analysis of AgNPs. By measuring the absorbance at a specific wavelength and comparing it to a calibration curve obtained from known concentrations of AgNPs, the concentration of the nanoparticles in a sample can be determined. This quantitative analysis is valuable for various applications, including nanoparticle synthesis, quality control, and assessing the stability of AgNPs in different conditions. In addition to size and concentration, UV–Vis spectral analysis can also provide an insight into the stability and surface chemistry of AgNPs. Changes in the absorption peak position, shape, or intensity over time or under different environmental conditions can indicate nanoparticle aggregation, surface modifications, or interactions with other molecules. UV–vis spec analysis was performed for two different concentrations, from those ranges, 20 mM time-dependent analyses were performed for various time durations such as 0 hr, 15 mints, 30 mints, 45 mints, and 1 hr absorbance peak was observed at 421 nm.
In XRD diffraction patterns showed four distinct peaks at 2θ = 27.784°, 32.192°, 38.095°, 42.429°, 43.400°, 46.165°, 49.079°, corresponding to (27.92), (32.28), (38.23), (42.2), (43.45), (46.21, (54.7), (57.3) planes respectively. The occurrence of functional groups in A. nervosa leaf extract leads to the efficient reduction of Ag ions to AgNPs. To get a good signal-to-noise ratio of AgNPs were taken in the range of 500–3400 cm. In the present study, the bio-synthesized A. nervosa AgNPs exposed some prominent peaks in FTIR examination such as 3243, 1531.44, and 1323.45 cm−1. The peaks observed at 3243 cm−1 can be attributed to the presence of O—H bonds, which are associated with both alcohols and phenols in the sample. These findings demonstrated that the leaf extract's various naturally occurring substances, such as tannins, polyphenols, and flavonoids, are actively involved in the reduction of Ag to AgNPs, as well as the capping and stability of the nanoparticles. These results correlated with previous research on green AgNPs production (Adoni et al., 2020; Palle et al., 2020).
The size of the particles ranged between 1 nm and 10 nm, indicating that they are nanoscale particles. The shape of AgNPs appeared to be nearly spherical, which is a desirable characteristic for nanoparticles. However, the SEM images indicated that the particles were agglomerated, which resulted in an uneven shape. Despite this, some particles had a circular shape, which suggests that the synthesis process could generate uniform particles. Overall, the SEM images offer important information about the physical properties of A. nervosa AgNPs. however additional characterization and analysis are required to understand their full behavior and properties. Analysis through EDX analysis confirmed the presence of elemental silver. The Zeta and particle size analysis results clearly demonstrated the distribution of AgNPs in the reaction mixture according to intensity, number, and volume of AgNPs respectively.
One of the major healthcare challenges is antibiotic resistance, which is caused by improper or excessive use of antibiotics. Hence, there is a demand on herbal remedies possessing substantial antibacterial potentials that hold enormous significance (Jansen et al., 2006). Nanotechnology offers desirable opportunities to improve the efficacy of herb-based drugs in antibiotic therapy. Nanoproducts can potentially increase the bioavailability, stability, and targeted administration of herbal constituents, enhancing their antibacterial activity.
The strong antimicrobial properties of Ag are well-established and have been extensively reported (Liu et al., 2010; Subramanyam et al., 2021). Earlier studies have provided an evidence of the antibacterial activity of AgNPs through mechanisms such as attachment to the bacterial cell wall or the generation of free radicals. Furthermore, the release of Ag ions from AgNPs is believed to be a significant contributor to their antibacterial effects, particularly through their interaction with thiol groups found in enzymes (Kim et al., 2007). The antibacterial activity of Ag can occur through several mechanisms which mechanism involves the attachment of AgNPs to the surface of bacterial cells, leading to structural damage and disruption of vital functions. This attachment can interfere with essential cell processes and ultimately result in bacterial cell death. Another mechanism involves the generation of reactive oxygen species (ROS) by AgNPs. These free radicals, such as can induce oxidative stress within bacterial cells. Further, oxidative stress can cause damage to cellular components, including, lipids, proteins, and DNA this leads to cell death or inhibition of microbial growth. Moreover, the release of Ag from AgNPs is a crucial aspect of their antimicrobial activity. The silver ions can interact with thiol groups present in enzymes that are essential for bacterial metabolism and cellular processes. This interaction disrupts the enzymatic activity, impeding crucial biochemical pathways and compromising bacterial survival (Yu et al., 2013). Overall, the antibacterial activity of Ag can be attributed to their attachment to bacterial cells, generation of free radicals, and their interaction with thiol groups of enzymes.
In this study A. nervosa leaf extracts exposure showed free radical scavenging action of 27 %, 37 %, 50 %, 61 %, and 76 % with the concentration of 100–500 μg/ml. Further, biologically synthesized A. nervosa AgNPs demonstrated free radical scavenging activity was 55 %, 62 %, 69 %, 78 %, and 85 % respectively on a concentration of 100–500 μg/ml. As the concentration level increased, there was a continuous increment in the inhibition level of DPPH radical formation. A similar finding was reported for A. nervosa leaf extract biosynthesized AgNPs, which demonstrated a significant ability to scavenge DPPH free radicals (Subramanyam et al., 2021).
The A. nervosa leaf extract revealed ABTS radical scavenging activity by 22 %, 33 %, 40 %, 50 %, and 54 % on a concentration of 100–500 μg/ml. Further, biologically synthesized A. nervosa AgNPs demonstrated free radical scavenging activity of 25 %, 41 %, 51 %, 57 %, and 67 % on a 100–500 μg/ml concentration. Standard drug inhibition (%) attained a range of 34 %, 46 %, 55 %, 60 %, and 67 % on a concentration of 100–500 μg/ml. Similar ABTS action of biologically produced AgNPs was found in earlier studies (Nallappan et al., 2021). A. nervosa root aqueous extract has previously proven that it has the potential ABTS radical scavenging activity against standard drugs (Shreedhara et al., 2009).
The A. nervosa leaf extract revealed H2O2 radical scavenging activity by 22 %, 31 %, 40 %, 46 %, and 61 % on a concentration of 100–500 μg/ml. Further, biologically synthesized A. nervosa AgNPs demonstrated free radical scavenging activity of 26 %, 33 %, 41 %, 49 %, and 72 % on a 100–500 μg/ml concentration. The accumulation of H2O2 in the biological system leads to the generation of hydroxy free radicals, which cause significant impairment of the cell membrane. The existing compounds capable of inhibiting these radicals have significant potential as therapeutic agents for alleviating the symptoms of oxidative stress (Kamalanathan et al., 2015). A. nervosa mediated AgNPs exhibited an improved scavenging effect than A. nervosa extract alone (Shanmugam et al., 2021).
The anti-inflammatory assay revealed extract maximum inhibition (%) obtained was 19 %, 21 %, 45 %, 70 %, and 71 % on a concentration level of 100–500 μg/ml. Maximum anti-inflammatory activity of A. nervosa AgNPs was detected as 29 %, 50 %, 61 %, 75 %, and 80 % respectively at a concentration of 100–500 μg/ml concentration levels. This finding is in accordance with the outcomes of other studies using AgNPs synthesized from diverse medicinal plants (Kedi et al., 2018). A. nervosa AgNPs were shown to be a good anti-inflammatory agent biosynthesized in an eco-friendly technique, without any kind of negative impacts as in chemically synthesized medications. In future A. nervosa AgNPs may be used as an alternative to chemically generated anti-inflammatory drugs used to treat inflammation-related disorders.
The α-amylase activity of A. nervosa extract was found to be 26 %, 36 %, 46 %, 56 %, and 65 % at concentrations of 10–50 μg/ml. The maximum α-amylase activity of A. nervosa AgNPs was observed as 52 %, 60 %, 67 %, 75 %, and 80 % at 10–50 μg/ml concentrations. The inhibition percentage of the standard drug ranged from 57 % to 86 % at concentrations of 10 to 50 μg/ml. Furthermore, the AgNPs synthesized from A. nervosa showed significantly higher levels of α-amylase inhibition activity compared to acarbose in all tested concentrations. This is consistent with the findings of other studies using AgNPs synthesized from different therapeutic plants (Jini and Sharmila, 2020). In the α-glucosidase assay A. nervosa leaves extract inhibition (%) obtained was 26 %, 36 %, 49 %, 60 %, and 68 % on a of 10–50 μg/ml concentrations. Maximum α-glucosidase activity of A. nervosa AgNPs was observed in the range of 54 %, 62 %, 70 %, 80 %, and 90 % at 10–50 μg/ml concentration. Green synthesized Nps from other medicinal plants showed similar outcomes (Jini and Sharmila, 2020). Additional research is essential to find out the possible activity of A. nervosa AgNPs role in diabetic management in animal or cell line studies.
6 Conclusions and recommendations
This study was focused on the production of NPs using plant materials, an emerging technique in nanotechnology. To develop a cost-effective and environmentally friendly method for synthesizing AgNPs using aqueous plant leaf extract from A. nervosa. We characterized the synthesized A. nervosa AgNPs using various techniques. Furthermore, our in vitro assay results showed that A. nervosa-mediated AgNPs have potential anti-bacterial, antioxidant, anti-inflammatory, and anti-diabetic activities. This green synthesis technique is a promising and more sustainable alternative to conventional methods. Further studies on animal and cell line models are warranted to explore the potential biological activities of A. nervosa-mediated AgNPs.
7 Limitation of the study
The study has some boundaries, over all the discoveries may be specific to Argyreia nervosa leaf extract-derived AgNPs and may not be applicable to AgNps synthesized from other plant extracts. The properties of Argyreia nervosa leaf extract may vary depending on factors like geographical location, environmental conditions, and the time of harvest. These variations may affect the reproducibility of the green synthesis process. Furthermore, the potential toxicity of the AgNPs to humans and the environment may not be comprehensively addressed. Additional research on their safety profiles, including cytotoxicity and environmental impact, is essential.
Acknowledgment
The authors thank the Research Supporting Project for funding this work through Research Supporting Project number (RSPD2023R708), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial, antioxidant, and dye degradation properties of biosynthesized silver nanoparticles from Artemisia annua L. Lett. Appl. Nanobiosci.. 2020;10:1981-1992.
- [Google Scholar]
- Green chemistry and the health implications of nanoparticles. Green Chem.. 2006;8:417-432.
- [Google Scholar]
- Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv.. 2016;6(44):40162-40168.
- [Google Scholar]
- Carica papaya peel-mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens. J. Appl. Res. Technol.. 2017;5:413-422.
- [Google Scholar]
- Traditional medicinal plants of cold desert Ladakh–used in the treatment of cold, cough, and fever. J. Ethnopharmacol.. 2007;2:341-349.
- [CrossRef] [Google Scholar]
- Antioxidant capacity and radical scavenging effect of polyphenol-rich Mallotus philippinensis fruit extract on human erythrocytes: An in vitro study. Sci. World J.. 2013;2013:279451
- [Google Scholar]
- Preparation and characterization of fruit-mediated silver nanoparticles using pomegranate extract and assessment of its antimicrobial activity. J. Environ. Nanotechnol.. 2013;1:04-10.
- [Google Scholar]
- Alpha-amylase inhibitors from roselle (Hibiscus sabdariffa Linn.) tea. Biosci. Biotech. Bioch.. 2000;5:1041-1043.
- [CrossRef] [Google Scholar]
- Effects of interaction of tannins with co-existing substances. VII. Inhibitory effects of tannins and related polyphenols on xanthine oxidase. Chem. Pharm. Bull.. 1990;5:1224-1229.
- [CrossRef] [Google Scholar]
- Bacterial resistance: a sensitive issue complexity of the challenge and containment strategy in Europe. Drug Resist. Updat.. 2006;9:123-133.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Mater. Today: Proc.. 2020;22:432-438.
- [Google Scholar]
- Antioxidant activities of leaf extracts of Euphorbia fusiformis Buch.-Ham. ex D. Don (Euphorbiaceae) Free Radic. Antioxid.. 2015;5(2):83-89.
- [Google Scholar]
- Preliminary phytochemical screening and antioxidant activity of five medicinal plants of Garhwal Himalaya: A comparative study. Vegetos. 2020;3:610-613.
- [Google Scholar]
- Eco-friendly synthesis, characterization, in vitro and in vivo anti-inflammatory activity of silver nanoparticle-mediated Selaginella myosurus aqueous extract. Int. J. Nanomed.. 2018;13:8537-8548.
- [Google Scholar]
- Synthesis and molecular docking studies of potent α-glucosidase inhibitors based on biscoumarin skeleton. Eur. J. Med. Chem.. 2014;81:245-252.
- [Google Scholar]
- Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;11:6903-6913.
- [Google Scholar]
- Biosynthesis and antimicrobial activity of semiconductor nanoparticles against oral pathogens. Bioinorg. Chem.. 2014;11:347167
- [CrossRef] [Google Scholar]
- A Literature Review on Argyreia Nervosa (Burm. F.) Bojer. Int. J. Res. Ayurveda Pharm.. 2011;5:1501-1504.
- [Google Scholar]
- Traditional uses and phytopharmacological aspects of Argyreia nervosa. J. Adv. Pharm. Res.. 2013;4:23-32.
- [Google Scholar]
- Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol.. 1968;3:169-173.
- [Google Scholar]
- Changing scenario for promotion and development of Ayurveda–way forward. J. Ethnopharmacol.. 2012;2:424-434.
- [CrossRef] [Google Scholar]
- Green biosynthesis, antioxidant, antibacterial, and anticancer activities of silver nanoparticles of Luffa acutangula leaf extract. Biomed Res. Int.. 2021;2021:5125681.
- [CrossRef] [Google Scholar]
- Synergistic antibacterial and mosquitocidal effect of Passiflora foetida-synthesized silver nanoparticles. Braz. J. Biol.. 2023;84:e263391.
- [Google Scholar]
- Green synthesis of silver nanoparticles by leaf extracts of Boerhavia erecta and spectral characterization and their antimicrobial, antioxidant ad cytotoxic studies on ovarian cancer cell lines. Lett. Appl. Nanobiosci.. 2020;9:1165-1176.
- [Google Scholar]
- Studies on the alkaloid composition of the Hawaiian Baby Woodrose Argyreia nervosa, a common legal high. Forensic Sci. Int.. 2015;249:281-293.
- [CrossRef] [Google Scholar]
- Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc.. 2003;125:13940-13941.
- [Google Scholar]
- Synthesis, characterization of silver nanoparticles from Punica granatum L. and its in vitro antidiabetic activity. Nanotechnol. Environ. Eng.. 2022;7:923-930.
- [Google Scholar]
- Synthesis and characterization of Solanum nigrum-mediated silver nanoparticles and its protective effect on alloxan-induced diabetic rats. J .Nanostruct. Chem.. 2016;6(1):41-48.
- [Google Scholar]
- Curcumin-chitosan nanocomposite formulation containing Pongamia pinnata-mediated silver nanoparticles, wound pathogen control, and anti-inflammatory potential. Biomed Res. Int.. 2021;23:3091587.
- [CrossRef] [Google Scholar]
- Free radical scavenging activity of aqueous root extract of Argyreia nervosa (Burm. f.) Boj. (convolvulaceae) J. Nat. Remedies. 2009;9(2):216-223.
- [Google Scholar]
- Wound healing activity of Argyreia nervosa leaves extract. Int. J. Appl. Basic Med. Res.. 2011;1:36-39.
- [Google Scholar]
- Argyreia nervosa (Samudra pala) leaf extract mediated silver nanoparticles and evaluation of their antioxidant, antibacterial activity, in vitro anticancer and apoptotic studies in KB oral cancer cell lines. Artif. Cells Nanomed. Biotechnol.. 2021;1:635-650.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using seed extract of Argyreia nervosa. Int. J. Pharm. Biol. Sci.. 2014;5:114-119.
- [Google Scholar]
- Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr. Sci.. 2008;95:647-655.
- [Google Scholar]
- Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinorg. Chem.. 2014;8:742346
- [Google Scholar]
- Eco-friendly synthesis of silver nanoparticles from the whole plant of Cleome viscosa and evaluation of their characterization, antibacterial, antioxidant and antidiabetic properties. Saudi J. Biol. Sci.. 2020;12:3601-3614.
- [CrossRef] [Google Scholar]
- Effect of galacto-mannan-oligosaccharides or chitosan supplementation on cytoimmunity and humoral immunity in early weaned piglets. Asian-Austr. J. Anim. Sci.. 2008;21:723-731.
- [Google Scholar]
- Silver nanoparticles in the environment. Environ. Sci. Process Impacts. 2013;15:78-92.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102955.
Appendix A
Supplementary material
The following are the Supplementary material to this article:Supplementary Fig. S1
Supplementary Fig. S1
Plant authentication certificate.







