Translate this page into:
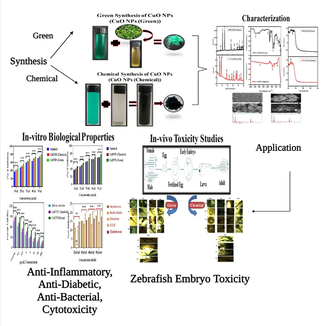
Green and chemical synthesis of CuO nanoparticles: A comparative study for several in vitro bioactivities and in vivo toxicity in zebrafish embryos
⁎Corresponding authors. gannadurai@msuniv.ac.in (Annadurai G), mahamed@ksu.edu.sa (Maqusood Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
CuO NPs is being applied for various applications including catalyst, electronics, cosmetics, and biomedicines due to its exceptional physicochemical properties. Physical and chemical routes for CuO NPs synthesis raised the concern of environmental hazard due to use of high energy, high temperature, and toxic chemicals. Conversely, green procedure is considered as an inexpensive and eco-friendly approach. To compare in vitro bioactivities and in vivo toxicity, CuO NPs was synthesized by two different methods; green and chemical routes. Leaf extract of a medicinal plant (Salacia reticulate) was used as a reducing and capping agent for green synthesis of CuO NPs. Sodium hydroxide was applied for chemical route of CuO NPs preparation. The in vivo toxicity of both CuO NPs (green and chemical) was examined in zebrafish (Dania rerio) embryos. Several in vitro biological activities e.g. antibacterial, cytotoxicity (in human cells), antidiabetic, and anti-inflammatory activities of both CuO NPs (green and chemical) was also assessed. Prepared CuO NPs (green and chemical) was characterized by SEM-EDX, FTIR, XRD, and UV–Vis spectroscopy. Several biomarkers such as hatching rate, malformation, and mortality rate of zebrafish embryos showed that green prepared CuO NPs exerted much lesser toxicity as compared to chemically synthesized CuO NPs. Antibacterial activity of green CuO NPs against Gram-negative (Escherichia coli, Staphylococcus aureus, and Enterobacter) and Gram-positive (Bacillus subtilis and Pseudomonas aeruginosa) bacteria was higher than chemical CuO NPs. Cytotoxicity results showed that human breast cancer cells (MCF-7) were more sensitive to green CuO NPs than chemical CuO NPs. Furthermore, antidiabetic and anti-inflammatory activities of green CuO NPs was greater than those of chemically prepared CuO NPs. This work revealed that in vitro bioactivities of green synthesized CuO NPs was better than chemically synthesized CuO NPs. Green CuO NPs also displayed higher biocompatibility towards zebrafish than chemical CuO NPs. This work highlights the importance eco-friendly green synthesis of CuO NPs for biomedical applications.
Keywords
CuO NPs
Green vs. chemical methods
Zebrafish
Cytotoxicity
Antibacterial activity
Antidiabetic activity
Anti-inflammatory activity
1 Introduction
The creation of nanoscale materials is expanding consistently due to their diverse applications from aerospace, agriculture, to biomedical and bioengineering (Ahamed et al., 2021). It is likewise realized that the particles and molecules of metals or non-metals in nanoscale range display extraordinary characteristics in comparison to their parent bulky materials (Ahamed et al., 2015). There are several physical, chemical, and green routes for the synthesis of nanoscale materials (Noorjahan et al., 2015). Physical and chemical methods of nanoparticles (NPs) synthesis are not advisable for their application in biomedical fields due to the use of high energy, high temperature, and toxic chemicals (Ahamed et al., 2022a). Green synthesis of NPs is an inexpensive and eco-friendly process that does not require toxic chemicals (Ahamed et al., 2022b). In green route, biological compounds such as sugar, polyphenols, alkaloids, and phenolic acids are utilized as a reducing and capping agent for reduction of metal ions into NPs in a very controlled way (Kuppusamy et al., 2016). Utilization of harmless biological compounds in green procedure is now providing a better option for synthesis of various types NPs for biomedical and clinical applications.
The Salacia reticulata has been employed in traditional medicine for treatment and prevention various disorders including asthma, amenorrhea, and dysmenorrhea (Morikawa et al., 2021). Besides, water extracts of leaves of Salacia reticulata has also shown potential for the prevention of diabetes and obesity as its multiple effects such as the ability to increase the plasma insulin level and lower the lipid peroxide level of the kidney (Yoshino et al., 2009). Salacia reticulate leaves contain several phytochemicals that can be utilized in green synthesis of various NPs. However, information on application Salacia reticulate in the synthesis of bio-medically relevant NPs is largely lacking.
CuO NPs has been shown potential for diverse application such as catalyst, battery, gas-sensor, heat transfer fluid, electronics, and cosmetics because of its superior physicochemical properties (Shah and Lu, 2018). CuO NPs is also found to be utilized in various biomedical applications including antimicrobial, biosensor, and cancer therapy (Ahamed et al., 2014; Siddiqui et al., 2013). In terms of healing, activation of pro- and anti-inflammatory pathways are important for the enlargement of protective strategies to shield the regenerative tissue from damage caused by imbalances in cytokines, oxidants, and antioxidants (Kenneth et al., 2009). Role of NPs in antidiabetic activity is one of the thrust areas of nanotechnology as it was estimated that by the year 2030, diabetes mellitus affected population will rise up to 366 million worldwide (Dhiraj et al., 2019).
There is limited information on comparative bioactivity/toxicity of green and chemically prepared CuO NPs. This study was designed to examined the comparative biological activity (in vitro) and toxicity (in vivo) of CuO NPs prepared by two different routes; green and chemical methods. Leaves extract of Salacia reticulata was applied as a reducing and capping agent for green synthesis of CuO NPs, while sodium hydroxide was used for chemical synthesis of CuO NPs. Prepared samples were characterized by UV–vis spectrophotometer, FTIR, XRD, and SEM-EDX techniques. The in vitro bioactivities such as antidiabetic, anti-inflammatory, cytotoxicity, and antibacterial activity of both CuO NPs (green and chemical) was assessed. Comparative toxicity of both types CuO NPs was also explored in zebrafish (Dania rerio) embryos. The zebrafish model represents a feasible alternate to the mammalian prototypes presently used in toxicity testing and other biological studies. Zebrafish are easier and less expensive to house and care for as compared to rodent models (Garcia, et al., 2016; Kumari et al., 2017).
2 Experimental plan
2.1 Preparation of leaf extract
Copper nitrate (CuN2O6·3H2O), polyvinylpyrrolidone (PVP), and sodium hydroxide (NaOH) was purchased from Organic Trading (Pvt) Ltd (Colombo, Sri Lanka). The Salacia reticulata leaves were obtained from department of agriculture, eastern university, Sri Lanka (EUSL). The Salacia reticulata leaves were washed thoroughly with distilled water and cut into small pieces. The 10 g of Salacia reticulata leaves pieces were transferred to 80 mL of distilled water and boiled for 20 min. Then, solution was filtered to obtain extract. The leaf extract was stored at 4 °C until further use.
2.2 Green synthesis of CuO NPs
Fig. 1 shows green route CuO NPs preparation. In brief, 3 mL of leaf extract was added to 40 mL of aqueous copper nitrate solution (1 mM) in a 250 mL flask. This solution was incubated for 24 h at 4 0C. A change in color from blue to green suggested the formation of colloidal suspension of CuO NPs. Colloidal suspension was further dried at 120 °C for 2 h to get the CuO NPs powder.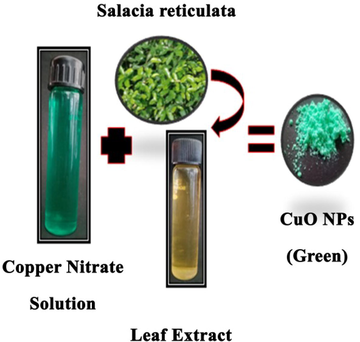
Green synthesis of CuO NPs.
2.3 Chemical synthesis of CuO NPs
Fig. 2 represents chemical route of CuO NPs synthesis. The initial precursors were copper nitrate, NaOH, and PVP. Briefly, PVP and copper nitrate (0.1 M) was dissolved in 50 mL distilled water. Then, aqueous solution of NaOH (0.5 M) added dropwise to above solution and stirred for 30 min to complete the precipitation process. Precipitate was washed several times and dried at 120 °C for 2 h. Finally, precipitate was calcined at 500 °C for 4 h to get powder of CuO NPs.
Chemical synthesis of CuO NPs.
2.4 Characterization of prepared samples
Optical absorption was recorded at UV–Vis spectrophotometer. FTIR was performed in the range of 500–4000 cm−1. Crystallinity and phase purity was examined by X-Ray diffraction. Morphology was assessed scanning electron microscopy (SEM).
2.5 Zebrafish maintenance and their breeding to embryos
Zebrafish (Dania rerio) were maintained in wealth section loaded up with the adapted water (75 g NaHCO3, 18 g ocean salt, 8.4 g CaSO4, per 1000 L). We housed wild-type adult zebrafish (Dania rerio) (Aquatic Environments) in an independent framework (Aquatic Habitats), maintained and bred zebrafish as reported earlier (Browning et al., 2009). In brief, two pairs of mature zebrafish placed into a clean incubator and applied a light (14 h)-dark (10 h) cycle to trigger fertilization and breeding of embryos. Eggs were gathered into a petri dish and washed multiple times with E3 medium.
2.6 Embryos toxicity study
The test is predominantly founded on the incipient organism test system created by Schulte and Nagel (1994). Briefly, 100 eggs were placed into a 96-well multi-plates with 10 eggs for every well. Then, each was loaded with E3 medium containing CuO NPs (green and chemical). Well without NPs (only E3 medium) serves as a control. The 96-well plate was covered with the plastic film and set in an enlightenment hatchery at 28 ± 1 °C with a light (14 h)-dark (10 h) cycle. The immediate perception was acted in the well under a light magnifying instrument associated with a camera gadget at indicated time focuses (24, 48, 72, and 96 hpf). The toxicological endpoints included mortality, gastrula improvement, tail separation, eyes, somite arrangement, circulatory framework, heartbeat, pigmentation, otic container, bring forth rate, distortions, and length of hatchlings were recorded. After 96 hpf exposure, the chorion was thoroughly taken out from the unhatched eggs with little youngsters. The hatchlings were situated on the parallel side and shot. The length of hatchlings was estimated utilizing advanced picture investigation (Scion Picture, ver. 4.0.3.2.). The half bring hatching rate (HT50) was determined utilizing Sigmoid Fit by Beginning 8.0 (Beginning Lab, US).
2.7 Antidiabetic assay
The α-glucosidase inhibition assay was performed as reported earlier (Butala et al., 2018). For this assay, 10–50 µl/mL of the test samples (CuO NPs) were aliquoted into 96-well plates and diluted with 0.02 M sodium phosphate buffer (pH 6.9). Then 50 µl of α- glucosidase was added to above mixture. Further, 50 µl of p-nitrophenyl- glucopyranoside (pNPG) (3 mM) was mixed as a substrate and reaction solutions further incubated for 20 min at 37 °C. Next, 50 µl of Na2CO3 (0.1 M) was added to reach the reaction at stationary phase. Absorbance was documented at 405 nm wavelength through a microplate reader.
2.8 Anti-inflammatory activity
The diclofenac sodium was used as a standard reagent for denaturation of bovine serum albumin (BSA). Both CuO NPs (green and chemical) were used for inhibitions of BSA denaturation process to test the scavenging activity as described earlier (Gunathilake et al., 2018). CuO NPs was dissolved in dimethylformamide (DMF) and diluted with 0.2 M of phosphate buffer (pH 7.4). The obtained DMF concentration in all the solutions was maintained by<2.5%. The 4 mL containing the NPs with the different concentrations (10–50 µl/ml) was mixed in 1 mL of BSA (1 mM) incubated for 15 min at 37 °C and then heated to 51 °C for 20 min. After cooling samples to room temperature the turbidity was estimated at 660 nm by UV–vis spectrophotometer.
2.9 Cytotoxicity assay
MTT assay was used to evaluate the cytotoxicity of CuO NPs (green and chemical) in human keratinocytes (HaCaT) and human breast cancer cells (MCF-7) (Ahamed et al., 2019). Both cell lines were obtained from National Centre for Cell Sciences (NCCS, Pune, India). Cells (1x105 cells/mL) were cultured in 96-well plates with Dulbecco's modified Eagle medium (DMEM) containing 10% FBS and 1% penicillin/streptomycin. Cells were allowed to attach the surface for 24 h at 37 °C with 5% CO2 supply. Cells were then treated for 24 h with various concentrations of NPs (0.1, 0.5, 1, 5, 10, 50, and 100 µg/mL). After exposure, 0.5 mg/mL of MTT added to each well and incubated for 4 h at 37 °C. Metabolically active cells formed formazan crystals, which were dissolved in SDS. The absorbance of solution was recorded 570 nm using an ELISA reader.
2.10 Antibacterial activity
Both gram-negative (Escherichia coli, Staphylococcus aureus, and Enterobacter) and gram-positive (Bacillus subtilis and Pseudomonas aeruginosa) bacterial strains were applied to test the antibacterial potential of prepared samples. These bacterial strains were received from Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology, Chandigarh, India. Both types of bacterial strain were maintained in the nutrient agar medium at 37 °C. Antibacterial activity of Salacia reticulate leaf extract and CuO NPs (green and chemical) were analysed utilizing a standard agar well dispersion strategy (Mulvaney, 1996). The Muller Hinton agar containing the microbial inoculum was spread everywhere on the Petri plate. Permit it to cool for quite a while, and afterward a well of distance across 8–10 mm was punched aseptically with a sterile cock borer or a tip. Different concentrations of NPs and extract was added into the well, and agar plates were incubated under sterile conditions. The NPs diffused into the agar media and restrain the development of organisms. At that point, the antibacterial movement of the nanoparticles was distinguished by the presence of the restraint zone around the agar well.
3 Results and discussion
3.1 X-ray diffraction study
XRD spectra of CuO NPs (green and chemical) is presented in Fig. 3. The estimated particle size for CuONPs (chemical) was around 84 nm, while CuONPs (green) size was around 42.2 nm. Sharp peaks indicating the crystalline nature of both prepared samples. The XRD patterns of NPs produced chemically and biologically match the reference patterns for CuO NPs in the available literature (Premana and Yulizar, 2017; Nagabhushana et al., 2016). XRD spectra illustrates that the strength and quality of XRD peaks vary based on the methods of preparation (green vs. chemical).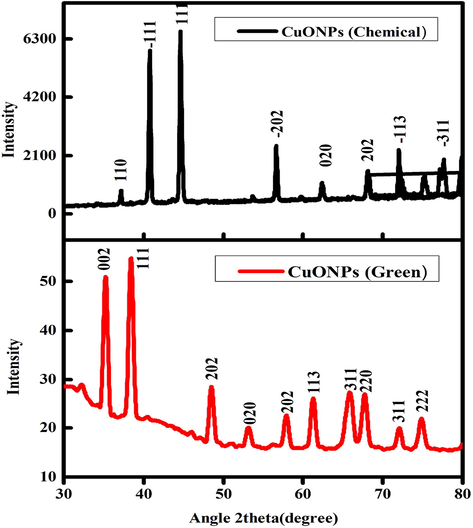
XRD spectra of CuO NPs (Chemical and Green).
3.2 FTIR study
FTIR analysis was done to perceive the possible biomolecules that are accountable for the creation of CuO NPs. Fig. 4 represents the FTIR spectra of CuO NPs (green and chemical) was recorded in the range of 500–4000 cm−1. FTIR spectra of both samples show a smooth comparable curvature with slight difference. The broad peak observed at 3533 cm−1 corresponds to the O–H and N–H bond stretching vibrations. The vibrations might be appropriate to phenolic compounds in attendance in the solution (Mamun et al., 2017). The minute peak at 2024 cm−1 correspond to the stretching vibrations of the compounds containing C≡N bonds. The sharp peak at 1630 cm−1 corresponds to the presence of C = N or C = O stretching. Likely, peak at 1412 cm−1 indicate sp3 C–H bending or acyl C-O (or phenol C-O) stretching and 1324 cm−1 denotes C–H stretching. Additionally, peak at 1184 cm−1 denotes alkoxy C-O. The unsaturated C–H bending appears under 771 cm−1 that might be due to the presence of bioactive phytochemicals. These compounds might impart capping which helps in maintaining the stability of CuO NPs. Moreover, a sharp peak found in the infrared spectrum at low frequencies at 673–414 cm−1 corresponds to CuO vibrations which were in accordance with earlier reports (Kumari et al., 2017). CuO NPs (green) bands around 1420 cm−1 and 1163 cm−1 are due to the characteristic frequency of inorganic ions. CuO NPs (chemical) displayed prominent peaks at 3011 cm−1, 1420 cm−1, 1163 cm−1, 869 cm−1, 452 cm−1. A strong intensity band at 1,058 cm−1 may be due to S-O-C stretching. The stretching vibrations assigned to the C-S linkage occur in the region of 800–600 cm−1 and the weak S-S stretching vibration falls between 600 and 400 cm−1 (Upadhyay et al., 2020). A comparative strong peak was observed CuO NPs (green) synthesized broad peak at 1184 cm−1 and CuO NPs (chemical) synthesized broad peak 1163 cm−1 is due to C- O stretching of the capping (Nasrullah et al., 2020).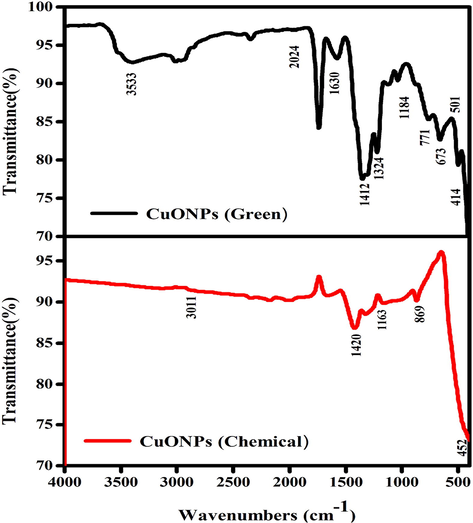
The FTIR spectar CuO NPs (Chemical and Green).
3.3 UV–vis spectroscopy
Fig. 5 represents the UV–visible absorption spectra of both CuO NPs (green and chemical) with slight shifting of absorption peak. In green synthesis, a change in color from blue to green of colloidal suspension suggests the formation of CuO NPs. Hence, the absorption peak for the green synthesized CuO NPs was recorded at 240 nm. In chemical route color of solution changed from colourless to black. As a consequences of UV–visible absorption peak for the chemically prepared CuO NPs was observed at 230 nm.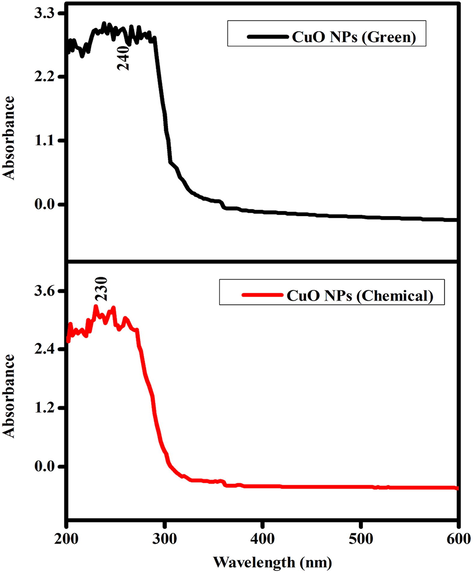
UV–visible absorbance CuO NPs (Green and Chemical).
3.4 SEM-EDX study
Morphology of prepared CuO NPs (chemical and green) was investigated by SEM-EDX (Figs. 6a and b). These images suggested that CuO NPs prepared by green method was more dispersed than chemically prepared CuO NPs. This could be due to adsorption phytochemical on the surface of NPs during green synthesis. Size calculated from SEM images for both types of NPS (green and chemical) were in agreement with size recorded from XRD data. As seen from the SEM images, green CuO NPs has much lower particle size than chemical CuO NPs. The change in size was due to presence natural capping agent during green preparation (Upadhyay et al., 2020). EDX spectra showed that Cu and O were main elements in prepared CuO NPs (green and chemical). Tables 1a and b represent elemental composition of CuO NPs (green and chemical). These results were in agreement with previous studies (Sukumar et al., 2020).Fig. 7a.Fig. 7b.Fig. 8a.Fig. 8b..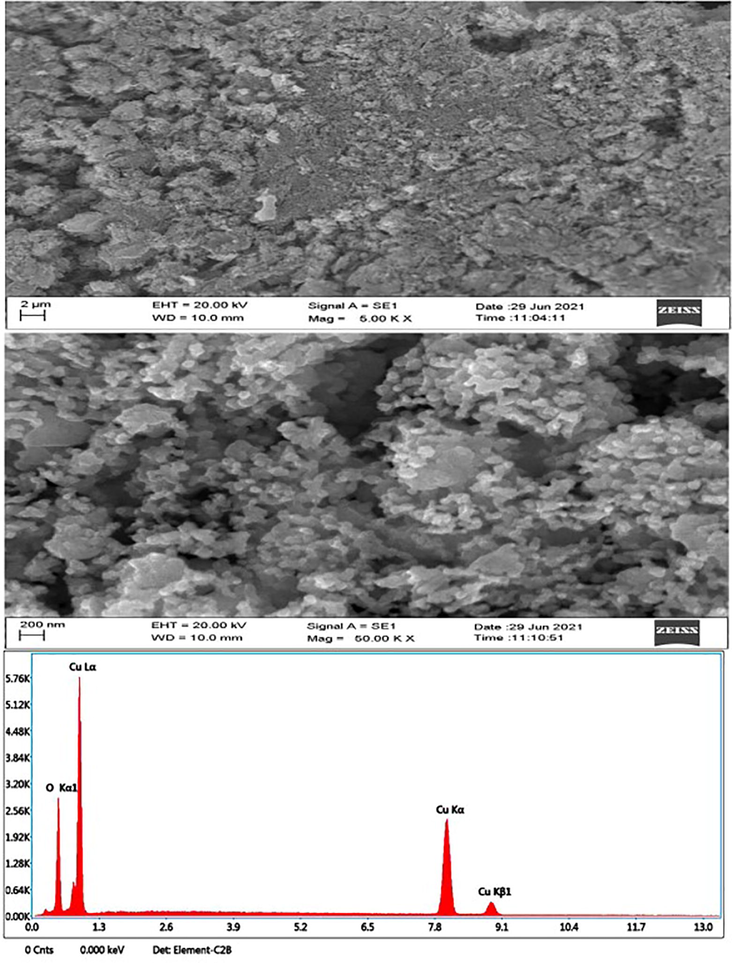
SEM-EDX study of CuO NPs (Green).
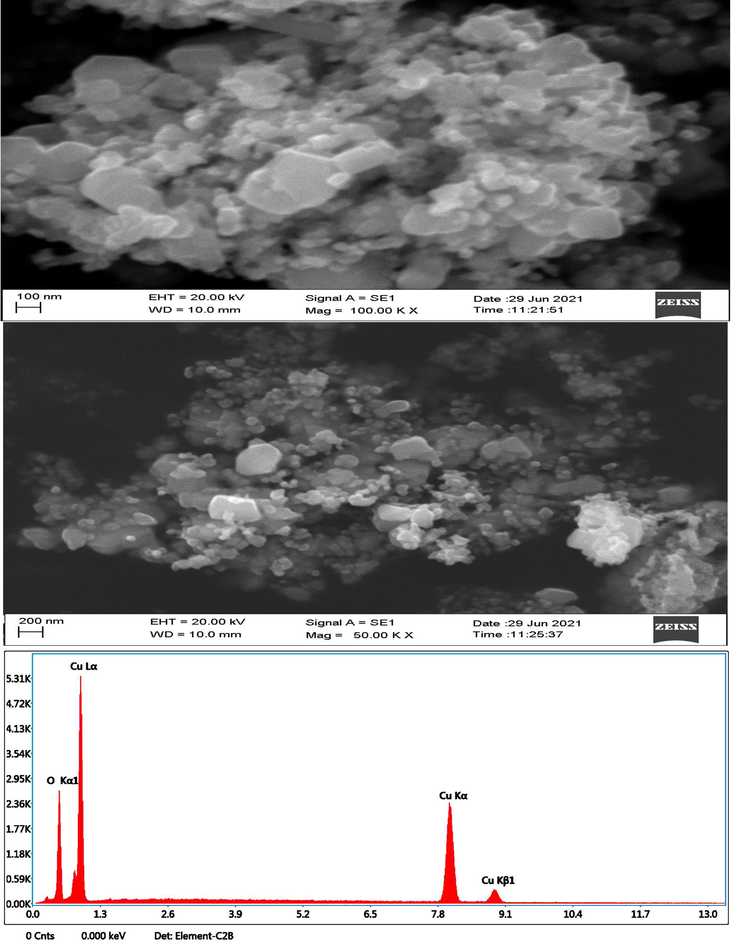
SEM-EDX study CuO NPs (Chemical).
S.No
Element
Weight %
Atomic %
1
O K
17.8
46.2
2
CuK
82.2
53.8
S.No
Element
Weight %
Atomic %
1
O K
17.2
45.2
2
CuK
82.8
54.8
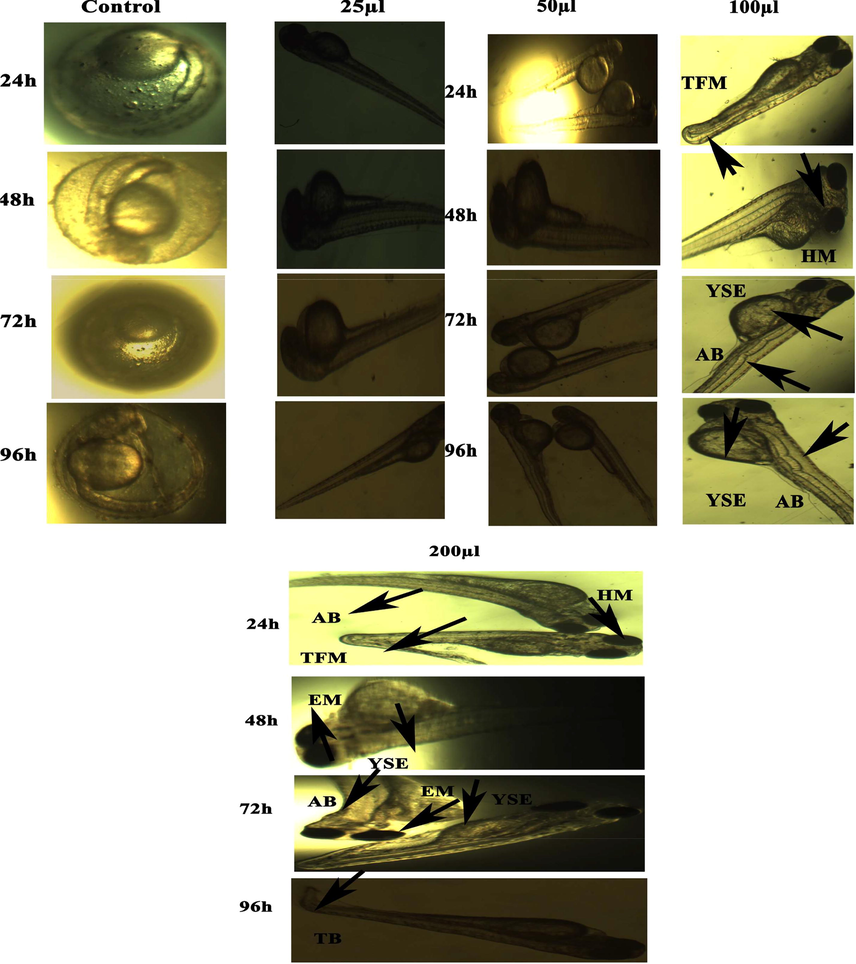
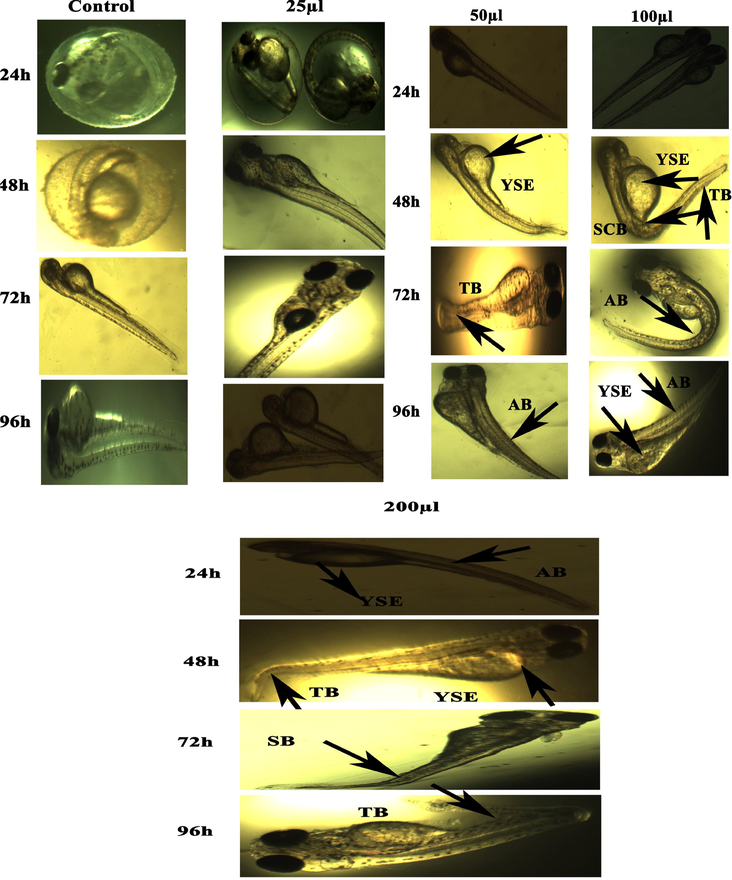
Representative images of zebrafish embryos and larvae exposed to CuO NPs (Green). The control group shows the normal appearance at 24 hpf, 48 hpf, 72 hpf, and 96 hpf. Tail bent (TB), yolk sac edema (YSE), head malformation (HM), axis bent (AB) and & tail fold malformation (TFM) denoted the malformation after exposure to 100–200 µl of CuO NPs (Green) for 48–96 hpf.
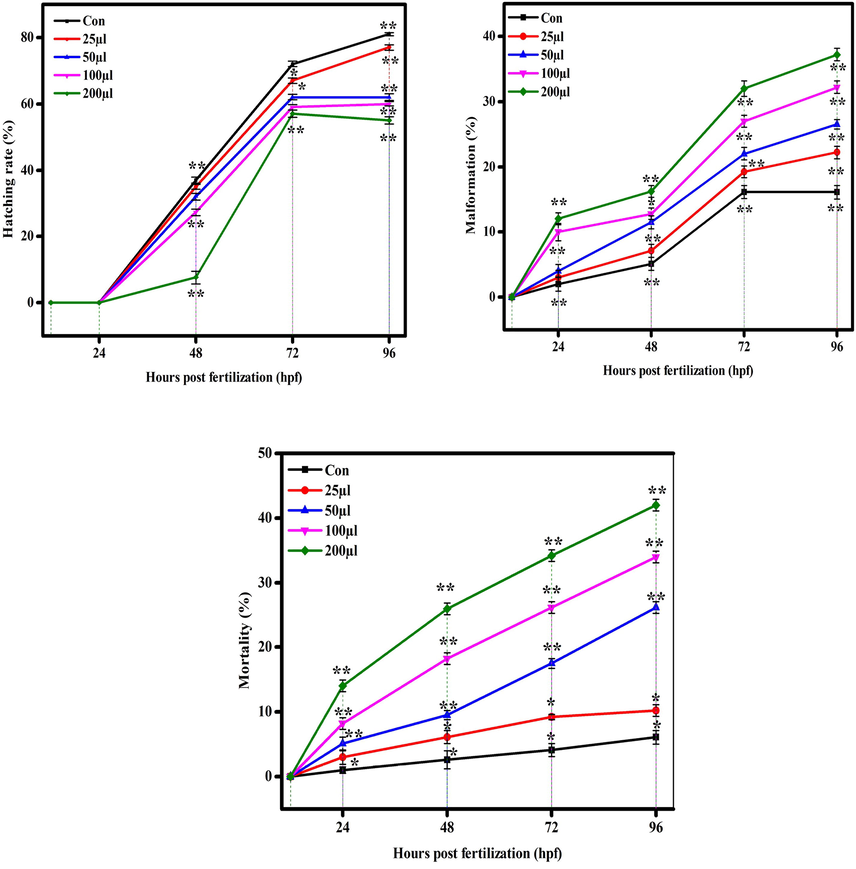
Hatching, malformation, and mortality rate in zebrafish against CuO NPs (Green). The data are presented as mean ± SD of three experiments. The data were analysed statistically by one way analysis of variance (ANOVA) followed by Dunnett’s Multiple range test (Tukey’s post-hoc test) using GraphPad Prism software. Significance “*” and “**” represent p < 0.05 and p < 0.01, respectively.
Representative images of zebrafish embryos and larvae exposed to CuO NPs (Chemical). The control group shows the normal appearance at 24 hpf, 48 hpf, 72 hpf, and 96 hpf. Tail bent (TB), yolk sac edema (YSE), head malformation (HM), axis bent (AB) and & tail fold malformation (TFM) denoted the malformation after exposure to 100–200 µl of CuO NPs (Chemical) for 48–96 hpf.
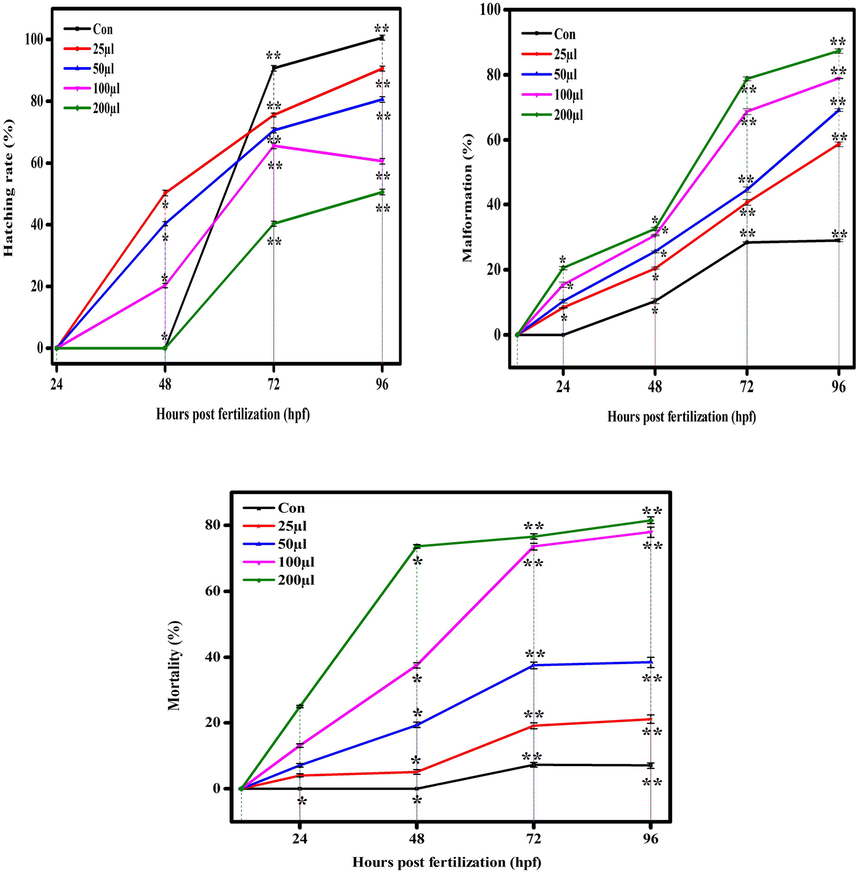
Hatching, malformation, and mortality rate in zebrafish against CuO NPs (Chemical). The data are presented as mean ± SD deviation of three experiments. The data were analysed statistically by one way analysis of variance (ANOVA) followed by Dunnett’s Multiple range test (Tukey’s post-hoc test) using GraphPad Prism software. Significance “*” and “**” represent p < 0.05 and p < 0.01, respectively.
3.5 Toxicity study in zebrafish embryos and larvae
There are several reports demonstrated the toxicity of metal and metal oxide NPs in zebrafish embryos (Asharani et al., 2008; Zhu et al., 2008). Physicochemical characteristics e.g. shape, size, and surface area are responsible factors for toxicity of metal oxide NPs in zebrafish embryos. Figures 7 (a and b) and 8 (a and b) show the comparative toxicity of CuO NPs (green vs. chemical). Large amount of CuO NPs (green and chemical) deposited on egg surfaces. The mortality of zebrafish eggs and larvae exposed to various concentrations (25, 50, 100, 200 µl/ml) of CuO NPs (green and chemical) was evaluated for certain time periods. At 200 µl/ml, CuO NPs exhibited significant anomalies. As shown in Figures 7 and 8, exposure to CuO NPs (green and chemical) disturbed the development embryos characterized by several malformations e.g. spinal card bent (SB), yolk sac edoema (YSE), tail bent (TB), head malformation (HM), axis bent (AB), eye malformation (EM), tail fold malformation (TFM), and delayed hatching rate at higher concentrations (100 and 200 µl/ml) for 24–96 hpf. CuO NPs also displayed significant killing of zebrafish embryos. Table 2 also shows the comparison of touch responses of embryos after exposure to CuO NPs (green vs. chemical). These results demonstrated that green CuO NPs produce much less toxicity to zebrafish than chemical CuO NPs. Earlier studies also reported the such malformations in zebrafish embryos after exposure to CuO NPs, ZnO NPs, ZrO NPs, and SiO2 NPs (Bai et al., 2010, Duan et al., 2013, Ganesan et al., 2016).
Concentration µl/ml
Touch and Swim Response
Legend
CuO NPs (Green)
CuO NPs (Chemical)
Control
++++
++++
++++ Fast responce
25
++++
+++
+++ Medium responce
50
++++
++
++ Slow responce
100
++++
+
+ Very Slow responce
200
+++
–
- No responce
3.6 Antidiabetic activity
To reduce hyperglycaemia therapeutically, carbohydrate-digesting enzymes such as α-glucosidase must be inhibited to prevent the breakdown of carbohydrates into monosaccharides that are major contributor in high sugar level in the blood (Etxeberria et al., 2012). In this study, antidiabetic activity (α-glucosidase inhibition assay) of CuO NPs (green and chemical) is presented in Fig. 9. Results showed that both types of CuO NPs (green and chemical) induce dose-dependent inhibition of α-glucosidase enzyme activity in the concentration range of 10–50 μl/mL. The IC50 values of antidiabetic activity for green and chemical CuO NPs was 22 μl/mL and 26 μl/mL, respectively (Table 3). Moreover, antidiabetic activity of green CuO NPs was slightly higher than chemically produced CuO NPs. Hence, green prepared CuO NPs has potential to be further studied for the treatment of diabetes.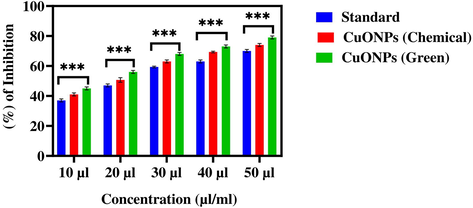
The α-glucosidase inhibition activity of CuO NPs (Green and Chemical). The data are presented as mean ± SD of three replications. The data were analysed statistically by Two-way analysis of variance (ANOVA) followed by Dunnett’s multiple range test (Tukey’s post-hoc test) using GraphPad Prism Software. Bars labeled with “***” represent statistically significant results (p < 0.001).
Assays
Parameters/cell lines
IC50 value (µl/mL)- Standard
IC50 value (µl/mL) CuO NPs (Chemical)
IC50 value (µl/mL) CuO NPs (Green)
Antidiabetic Activity
α-glucosidase assay
29
26
22
Anti-inflammatory Activity
BSA denaturation
267
274
109
Cytotoxicity
HaCaT cells
0.4
0.3
0.6
MCF-7 cells
0.3
0.36
0.42
3.7 Anti-inflammatory activity
Anti-inflammatory activity of CuO NPs (green and chemical) was measured using bovine serum albumin (BSA) denaturation assay. Different concentrations (10–50 µg/mL) of both CuO NPs was applied for anti-inflammatory assay. Results showed that both CuO NPs exhibit anti-inflammatory activity in a dose-dependent manner (Fig. 10). Similarly, a recent study observed that the anti-inflammatory activity of CuO NPs using the BSA denaturation approach (Gnanasundaram and Balakrishnan, 2017). Another study also reported the anti-inflammatory activity of Ag NPs (Prabakaran and Mani, 2019). The IC50 values of anti-inflammatory activity for chemical and green CuO NPs was 274 μl/mL and 109 μl/mL, respectively (Table 3). This data suggested that green prepared CuO NPs is a better agent in creation of active medicines for inflammatory diseases.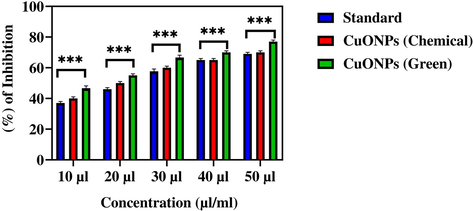
Percentage of bovine serum albumin (BSA) inhibition activity of CuO NPs (Green and Chemical). The data are presented as mean ± SD of three replications. The data were analysed statistically by Two-way analysis of variance (ANOVA) followed by Dunnett’s multiple range test (Tukey’s post-hoc test) using GraphPad Prism Software. Bars labeled with “***” represent statistically significant results (p < 0.001).
3.8 Cytotoxicity study
MTT assay is a widely accepted method to study the cytotoxicity of metal oxide NPs (Ahamed et al., 2021). In the present work, the cytotoxicity of leaf extract (Salacia reticulata) and CuO NPs (Chemical and Green) was assessed in human keratinocyte (HaCaT) and human breast cancer (MCF-7) cells (Figs. 11a and b). Cells were exposed to different concentrations of leaf extract and CuO NPs (0.1–100 µg/mL) for 24 h. Results showed that leaf extract both types of CuO NPs decreased the viability of two cells in a dose-dependent manner. The IC50 value of both types of CuO NPs (green and chemical) for HaCaT and MCF-7 cells is given presented in Table 3. These results suggested that green synthesized CuO NPs exert higher cytotoxicity towards MCF-7 cancer cells than those of CuO NPs prepared by chemical method.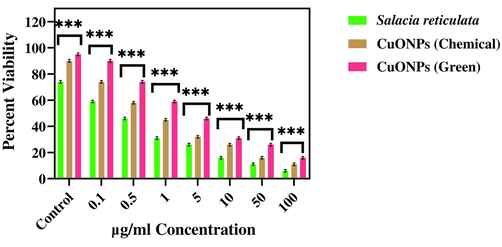
Cytotoxicity assay in normal human keratinocyte (HaCaT) cells after the treatment with several concentrations of CuO NPs (Green and Chemical) and leaf extract of Salacia reticulata. Data represent mean ± SD of three replications. The data were analysed statistically by two-way analysis of (ANOVA) followed by Dunnett’s multiple range test (Tukey’s post-hoc test) using GraphPad Prism Software. Bars labeled with “***” represent statistically significant results (p < 0.001).
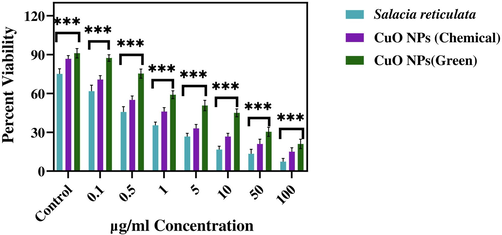
Cytotoxicity assay in human breast cancer (MCF-7) cells after the treatment with several concentrations of CuO NPs (Green and Chemical) and leaf extract of Salacia reticulata. Data represent mean ± SD of three replications. The data were analysed statistically by two-way analysis of (ANOVA) followed by Dunnett’s multiple range test (Tukey’s post-hoc test) using GraphPad Prism Software. Bars labeled with “***” represent statistically significant results (p < 0.001).
Results of the present study are in agreement with several previous reports. Our ealier study reported that CuO NPs induced cytotoxicity in human liver cancer (HepG2) cells in a concentration dependent manner. (Siddiqui et al., 2013). Different metal NPs, metal oxide NPs and metal oxide-based nanocomposites prepared by chemical and green methods also demonstrated anticancer activity against several typs of human cancer cells (Ahamed et al., 2021a, 2021b, 2022a, 2022b). In this study, we did not explore the potential mechanisms of anticancer activity of prepared samples. Role of oxidative stress in anticancer activity of NPs is actively being investigated. However, exact mechanisms of anticancer potential of nanoscale materials still remains a daunting task.
3.9 Antibacterial efficacy
Percentage of inhibition of bacterial growth due to leaf extract and CuO NPs (green and chemical) is presented in Figs. 12a-c. These figures suggested that CuO NPs display excellent antibacterial activity against both gram-positive and gram-negative bacteria. Zone of inhibition images provided in Fig. 13 that also indicates the potential inhibition against bacterial pathogens by tested materials. These results suggested the dose-dependent antibacterial activity of leaf extract and CuO NPs (green and chemical) (Table 4).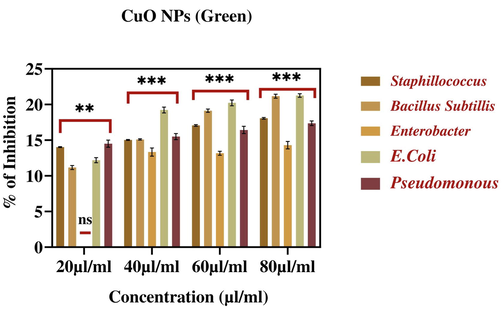
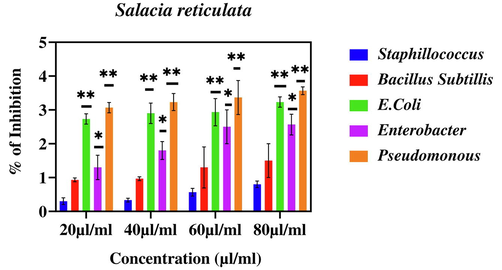
Percentage inhibition of bacterial growth against CuO NPs (Green). The data are presented as mean ± SD of three replications. The data were analysed statistically by Two-way analysis of variance (ANOVA) followed by Dunnett’s multiple range test (Tukey’s post-hoc test) using GraphPad Prism software. Significance “**” represent p < 0.01, “ns” represent No Significance. Bars labelled with “***” represent statistically significant results p < 0.001.
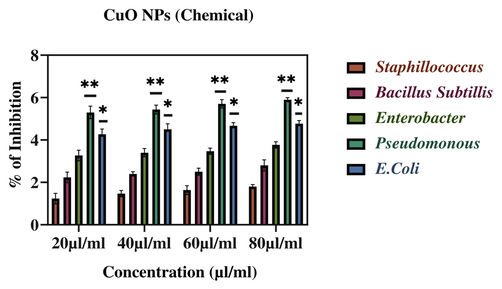
Percentage inhibition of bacterial growth against CuO NPs (Chemical). The data are presented as mean ± SD of three replications. The data were analysed statistically by Two-way analysis of variance (ANOVA) followed by Dunnett’s multiple range test (Tukey’s post-hoc test) using GraphPad Prism software. Significance “*” and “**” represent p < 0.05 and p < 0.01.
Percentage inhibition of bacterial growth against leaf extract of Salacia reticulata. The data are presented as mean ± SD of three replications. The data were analysed statistically by Two-way analysis of variance (ANOVA) followed by Dunnett’s multiple range test (Tukey’s post-hoc test) using GraphPad Prism software. Statistically Significance “*” and “**” represent p < 0.05 and p < 0.01.
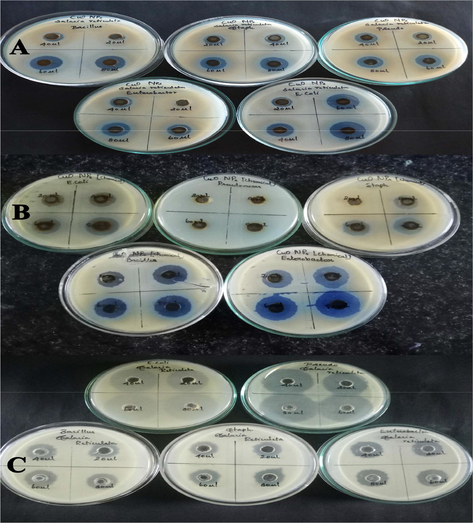
Zone of inhibition assay. (A) CuO NPs-Green. (B) CuO NPs-Chemical). (C) Leaf extract of Salacia reticulate.
Sample
Bacteria
20 µl/ml
40 µl/ml
60 µl/ml
80 µl/ml
CuO NPs (Green)
Staphyllococcus
14.03 ± 0.05
15.03 ± 0.05
17.03 ± 0.1
18.06 ± 0.1
Bacillus
11.1 ± 0.2
15.1 ± 0.1
19.1 ± 0.2
21.1 ± 0.2
Enterobacter
0
13.3 ± 0.5
13.1 ± 0.2
14.3 ± 0.5
E.Coli
12.2 ± 0.3
19.2 ± 0.4
20.2 ± 0.4
21.2 ± 0.2
Pseudomonous
14.5 ± 0.5
15.5 ± 0.43
16.4 ± 0.5
17.3 ± 0.3
CuO NPs (Chemical)
Staphyllococcus
1.2 ± 0.2
1.4 ± 0.1
1.6 ± 0.2
1.8 ± 0.1
Bacillus
2.2 ± 0.2
2.4 ± 0.1
2.5 ± 0.17
2.8 ± 0.26
Enterobacter
3.26 ± 0.25
3.4 ± 0.2
3.46 ± 0.15
3.7 ± 0.15
E.Coli
4.26 ± 0.25
4.5 ± 0.2
4.6 ± 0.15
4.7 ± 0.15
Pseudomonous
5.3 ± 0.3
5.4 ± 0.2
5.7 ± 0.2
5.9 ± 0.1
Salacia reticulata
Staphyllococcus
0.3 ± 0.1
0.33 ± 0.05
0.5 ± 0.1
0.8 ± 0.1
Bacillus
0.93 ± 0.05
0.96 ± 0.05
1.3 ± 0.6
1.5 ± 0.5
Enterobacter
1.3 ± 0.3
1.8 ± 0.26
2.5 ± 0.5
2.56 ± 0.3
E.Coli
2.7 ± 0.1
2.9 ± 0.3
2.93 ± 0.40
3.2 ± 0.1
Pseudomonous
3.0 ± 0.1
3.2 ± 0.2
3.3 ± 0.5
3.5 ± 0.1
Moreover, green prepared CuO NPs shown better antibacterial activity against Bacillus and E. Coli when compared to other three pathogenic bacteria (Fig. 13). Importantly, green produced CuO NPs demonstrated substantial antibacterial activity against both gram-positive and gram-negative bacterial pathogens. Our results are supported by previous findings of antibacterial activity of different types of NPs (Ahamed et al., 2014; Jain and Mehata, 2017; Rautela et al., 2019; Rokade et al., 2016).
4 Conclusions
In summary, CuO NPs was produced by two different methods; chemical and green routes. Several in vitro biological activities and in vivo toxicity in zebrafish was examined against both types of CuO NPs (green and chemical). Results showed that green prepared CuO NPs exhibits higher antidiabetic and anti-inflammatory activity than those of chemically synthesized CuO NPs. Antibacterial activity of green CuO NPs against gram-positive and gram-negative bacterial pathogens was higher in comparison to CuO NPs produced by chemical method. Human breast cancer cells (MCF-7) displayed greater vulnerability against green CuO NPs than those of chemical CuO NPs. Interestingly, green CuO NPs exhibited less toxicity to zebrafish embryos than chemically synthesized CuO NPs. This study offers an inexpensive and eco-friendly approach for green synthesis CuO NPs with the potential of its application in pharmaceutical industries.
5 Ethics approval
All the animal experiments were carried out in compliance with the standard ethical guidelines and under the control of the Sri Paramakalyani Centre for Excellence in Environmental Science-Manonmaniam Sundaranar University of Animal Ethics Committee.
Acknowledgement
The authors extend their sincere appreciation to the Researchers Supporting Project (RSP-2021/129) at the King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere. 2019;216:823-831.
- [CrossRef] [Google Scholar]
- Enhanced anticancer performance of eco-friendly-prepared Mo-ZnO/RGO nanocomposites: Role of oxidative stress and apoptosis. ACS Omega. 2022;7(8):7103-7115.
- [CrossRef] [Google Scholar]
- SnO2-doped ZnO/reduced graphene oxide nanocomposites: synthesis, characterization, and improved anticancer activity via oxidative stress pathway. Int. J. Nanomedicine. 2021;16:89-104.
- [CrossRef] [Google Scholar]
- Assessment of the lung toxicity of copper oxide nanoparticles: Current status. Nanomedicine (Lond.). 2015;10(15):2365-2377.
- [CrossRef] [Google Scholar]
- Ahamed, M., Alhadlaq, H.A., Khan M.A.M., Karuppiah, P., Al-Dhabi, N.A., 2014. Synthesis, characterization and antimicrobial activity of copper oxide nanoparticles J. Nanomater. 2014, 637858. https://doi.org/10.1155/2014/637858
- Facile green synthesis of ZnO-RGO nanocomposites with enhanced anticancer efficacy. Methods. 2022;199:28-36.
- [CrossRef] [Google Scholar]
- A novel green preparation of Ag/RGO nanocomposites with highly effective anticancer performance. Polymers. 2021;13(19):3350.
- [CrossRef] [Google Scholar]
- Toxicity of silver nanoparticlesinzebrafishmodels. Nanotechnology.. 2008;19(25)
- [CrossRef] [Google Scholar]
- Toxicity of zinc oxide nanoparticles to zebrafish embryo: a physicochemical study of toxicity mechanism. J. Nanopart. Res.. 2010;12(5):1645-1654.
- [Google Scholar]
- Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale.. 2009;1:138.
- [CrossRef] [Google Scholar]
- An ayurvedic anti-diabetic formulation made from curcuma longa L. and Emblica officinalis L. Inhibits α-amylase, α-glucosidase, and starch digestion, in vitro. Starch. 2018;70:1700182.
- [CrossRef] [Google Scholar]
- Gnidia glauca- and plumbago zeylanica-ediated synthesis of novel copper nanoparticles as promising antidiabetic agents. Adv. Pharmacol. Sci.. 2019;2019:9080279.
- [CrossRef] [Google Scholar]
- Toxic effects of silica nanoparticles on Zebrafish embryos and larvae. PLoS ONE. 2013;8(9)
- [CrossRef] [Google Scholar]
- Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin Ther Targets.. 2012;16:269-297.
- [CrossRef] [Google Scholar]
- Acute and sub-lethal exposure to copper oxide nanoparticles causes oxidative stress and teratogenicity in zebrafish embryos. J. Appl. Toxicol.. 2016;36(4):554-567.
- [Google Scholar]
- Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther.. 2016;161:11-21.
- [CrossRef] [Google Scholar]
- Gnanasundaram, I., Balakrishnan, K., 2017. Synthesis and evaluation of anti-inflammatory activity of silver nanoparticles from Cissus vitiginea leaf extract. J. Nanosci. Tech. 3, 266–269.
- Influence of boiling, steaming and frying of selected leafy vegetables on the in vitro anti-inflammation associated biological activities. Plants. 2018;7:22.
- [CrossRef] [Google Scholar]
- Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep.. 2017;7:15867.
- [CrossRef] [Google Scholar]
- Mechanistic insight to ROS and apoptosis regulated cytotoxicity inferred by green synthesized CuO nanoparticles from Calotropis gigantean to embryonic zebrafish. Sci Rep.. 2017;7:16284.
- [CrossRef] [Google Scholar]
- Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications-an updated report. Saudi Pharm. J.. 2016;24:473-484.
- [CrossRef] [Google Scholar]
- Characterization of phytoconstituents and evaluation of antimicrobial activity of silver-extract nanoparticles synthesized from Momordica charantia fruit extract. BMC Complement Altern. Med.. 2017;17:1-7.
- [CrossRef] [Google Scholar]
- A review of antidiabetic active thiosugar sulfoniums, salacinol and neokotalanol, from plants of the genus Salacia. J. Nat. Med.. 2021;75:449-466.
- [CrossRef] [Google Scholar]
- Surface plasmon spectroscopy of nano sized metal particles. Langmuir. 1996;12:788-800.
- [CrossRef] [Google Scholar]
- Facile, EGCG., assisted green synthesis of raspberry shaped CdO nanoparticles. J. Alloys Compd.. 2016;669:232-239.
- [Google Scholar]
- Green and chemical syntheses of CdO NPs: A comparative study for yield attributes, biological characteristics, and toxicity concerns. ACS Omega. 2020;5(11):5739-5747.
- [CrossRef] [Google Scholar]
- Green Synthesis and Characterization of Zinc Oxide Nanoparticles from Neem (Azadirachta indicia) IJSETR. 2015:5751-5753.
- [Google Scholar]
- Potency of Parkia speciosa Hassk seed extract for green synthesis of CdO nanoparticles its characterization. IOP Conf. Ser. Mater. Sci. Eng.. 2017;188:012018
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of silver nanoparticles synthesized from Eichhornia crassipes: An in vitro study. J. Pharmacogn. Phytochem. 2019;8:2556-2558.
- [Google Scholar]
- Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol.. 2019;10:5.
- [CrossRef] [Google Scholar]
- Pure green chemical approach for synthesis of Ag2O nanoparticles. Green Chem. Lett. Rev.. 2016;9:216-222.
- [CrossRef] [Google Scholar]
- Testing acute toxicity in the embryo of zebrafish, Brachydanio rerio, as an alternative to the acute fish test: Preliminary results. ALTA.. 1994;22:12-19.
- [CrossRef] [Google Scholar]
- Morphology, large scale synthesis and building applications of copper nanomaterials. Constr. Build. Mater.. 2018;180:544-578.
- [CrossRef] [Google Scholar]
- Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocellular carcinoma cells. PLoS ONE. 2013;8(8):e69534.
- [CrossRef] [Google Scholar]
- Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia bonducella Seed Extract and Their Applications. ACS Omega.. 2020;5:1040-1051.
- [CrossRef] [Google Scholar]
- Green and chemically synthesized ZnO nanoparticles: A comparative study. IOP Conference Series: Mat. Sci. Eng.. 2020;798(1)
- [CrossRef] [Google Scholar]
- Anti-diabetic activity of a leaf extract prepared from Salacia reticulata in mice. Biosci. Biotechnol. Biochem.. 2009;73:1096-1104.
- [CrossRef] [Google Scholar]
- Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J Environ Sci Health A Tox Haz Subst Environ (Eng). 2008;43:278-284.
- [CrossRef] [Google Scholar]







