Translate this page into:
Gossypetin mitigates doxorubicin-induced nephrotoxicity: A histopathological and biochemical evaluation
⁎Corresponding authors. umar.ijaz@uaf.edu.pk (Muhammad Umar Ijaz), smarazi@ksu.edu.sa (Suhail Razak)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
Doxorubicin (DOX) is an anticancer agent that is generally used to treat wide range of malignancies. Gossypetin (GTIN) is a well-known bioflavonoid that shows multiple therapeutical potentials. The experiment aimed to elucidate the nephroprotective impact of GTIN against renal damage caused by DOX. 24 Sprague-Dawley (SD) rats were separated into 4 groups: Control, DOX (3 mg/kg i.p.), DOX + GTIN (3 mg/kg i.p. + 30 mg/kg by oral gavage), and GTIN (30 mg/kg. orally) for the evaluation of renal markers, apoptotic markers, antioxidant and inflammatory profile. The outcomes of the experiment apprised that DOX exposure induced a pronounced decrease in enzymatic activities of antioxidants including glutathione peroxidase (GPx), superoxide dismutase (SOD), glutathione reductase (GSR), catalase (CAT), glutathione-S-transferase (GST) as well as glutathione (GSH) accompanied by an increment in reactive oxygen species (ROS) as well as malondialdehyde (MDA) levels. Likewise, DOX intoxication promoted escalation in the urea, kidney injury molecule-1 (KIM-1), creatinine, as well as neutrophil gelatinase-associated lipocalin (NGAL) level, on the other hand instigating a remarkable decline in creatinine clearance level. DOX induction brought a considerable escalation in interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), nuclear factor kappa-B (NF-κB) as well as cyclooxygenase-2 (COX-2). In addition, DOX administration depicted a considerable raised in Caspase-9, Bax as well as Caspase-3 levels, while lowered Bcl-2 level. DOX treatment also prompted histomorphological impairment. GTIN + DOX co-treatment ameliorated all the above-stated damages in the kidneys. In conclusion, GTIN can effectively mitigate the renal impairments induced by DOX-prompted nephrotoxicity.
Keywords
Doxorubicin
Gossypetin
Nephrotoxicity
Renal markers
Inflammatory markers
1 Introduction
DOX, an efficient chemotherapeutic agent, belongs to the family of anthracycline antibiotics (Ijaz et al., 2023). It is widely used to cure multiple types of malignancies such as lung, ovary, blood and breast cancer (Khames et al., 2019). Despite its major advantages in clinical oncology, it also depicts adverse side effects on healthy cells due to its ability to cause non-targeted toxicity (Moutabian et al., 2022). DOX depicts cellular toxicity by targeting certain molecules in the cell. The anti-cancer property of DOX is due to its intercalation into DNA molecules, leading to inhibition of the synthesis of DNA, RNA and protein (Carvalho et al., 2009). DOX exposure triggers the inhibition of topoisomerase II activity, autophagy dysregulation and redox cycling blockage owing to ROS production (Zilinyi et al., 2018). Moreover, it also instigates pulmonary, testicular, hematological and cardiac damage that restricts its clinical application in the field of oncology (Ayla et al., 2011).
Kidneys are an important organ that regulates homeostasis, acid-base balance, blood pressure, and toxic metabolites (Hertzberg et al., 2017). DOX prompts irreversible damage to the kidney tissues owing to its limited regeneration and healing ability. Although the exact process to trigger nephrotoxicity remains unclear but various studies believed that oxidative (OS) stress mediated by radical formation is the primary reason for kidney damage (Owumi et al., 2021). DOX disrupts cellular activities of the antioxidant profile as well as enhances the MDA level (Wu et al., 2021). Furthermore, escalation in creatinine and urea levels is evidence of progressive kidney damage (Afsar et al., 2020).
Flavonoids, a polyhydroxy phenolic compound, are abundantly found in flowers, wine, grains, fruits and vegetables (Ishtiaq et al., 2022). GTIN is a well-known bioflavonoid that contains 6 OH groups. It can be isolated from Hibiscus sabdariffa and many other flowers. It is evinced to exhibit multiple pharmacological properties such as anti-inflammatory (Lin et al., 2021), antibacterial, antioxidant and anti-cancer (Xie et al., 2019). Hence, the aforementioned therapeutical properties of GTIN open a new avenue for research and impelled us to determine its remedial effects against DOX-instigated renal toxicity.
2 Materials and methods
2.1 Chemicals
DOX and GTIN (CAS no. 489–35-0) were bought from Sigma-Aldrich (Germany).
2.2 Experimental animals
SD rats (weight; 180–200 g, n = 24, age; 6–7 weeks old) were placed in well-ventilated cages at the standard condition (humidity: 42 ± 7, photoperiod: 12 h light/dark cycle, and normal range of temperature: 20–26 °C) in animal research laboratory of University of Agriculture, Faisalabad (UAF). They were served with a commercial diet (Oxbow Essentials adult rat food, Oxbow Animal Health, Murdock, USA) and offered access to tap water. Rats were handled by following the protocol of animal protection and handling committee of UAF.
2.3 Experimental layout
SD rats were categorized into 4 groups (six in each). Administration of GTIN and DOX were given to the following: Control group, DOX intoxicated group (3 mg/kg. i.p.), GTIN + DOX co-administrated group (30 mg/kg of GTIN by oral gavage + 3 mg/kg of DOX i.p.) as well as GTIN group (30 mg/kg. orally). Doses of DOX and GTIN was taken in accordance to the previous investigation of Mustafa et al. (2022) and Khan et al. (2015) respectively. The trial continued for thirty days. On 31st day of the trial, rats were euthanized via cervical dislocation and cardiac blood was obtained to analyze the profile of the serum sample. Both kidneys were taken out from rats and left kidney was kept in zipper storage bags as well as preserved at −80 °C to analyze the biochemical assay, whereas right kidney was preserved in formalin (10 %) to observe histopathology of renal tissues. Before analysis, the left kidney (0.5 mg) was also homogenized in phosphate buffer saline (3 mL) followed by centrifugation at 12000 rpm for 15 min.
2.4 Biochemical assay
CAT activity was measured by following the methodology of Aebi (1984). GPx and SOD activities were accessed in compliance with the process explained by Lawrence and Burk (1976) and Sun et al. (1988). GSR and GST activities were investigated according to the protocol of Factor et al. (1998) and Couri and Abdel-Rahman (1979). Moreover, GSH level was ascertained by following the technique of Sedlak and Lindsay’s (1968). MDA and ROS level was measured as per the methodology of Placer et al. (1966) and Hayashi et al. (2007). For the estimation of total protein, Merck's Total Protein Kit was (Cat. No. TP0100-1KT) purchased.
2.5 Renal biomarkers evaluation
The kits provided by abcam were used to evaluate the urea (Cat No. ab83362), creatinine (Cat No. ab65340) as well as creatinine clearance levels (Cat No. ab65340) via an ELISA plate reader. Urinary KIM-1 as well as serum NGAL levels were measured following the constructor's guidelines via KIM-1 Quantikine ELISA & NGAL Quantikine ELISA-Kits, respectively. (Changning, China).
2.6 Inflammatory mediator evaluation
Inflammatory mediators such as IL-6 (CSB-E04640r), NF-κB (CSB-E13148r), IL-1β (CSB- E08055r), TNF-α (CSB-E07379r) along with COX-2 (CSB-E13399r) were determined via an ELISA kit in accordance with manufacturer’s guidelines, BioTek, Winooski, VT, USA.
2.7 Pro/anti-apoptotic markers assessment
Caspase-9 (CSB-E08863r), Bax (CSB-EL002573RA), Caspase-3 (CSB-E08857r) along with Bcl-2 (CSB-E08854r), levels were checked using ELISA (Cusabio Technology Llc, Houston, USA) kits in accordance with the constructor's instructions.
2.8 Histopathology
Renal tissues from the right kidney were fixed in a 10% formalin for 2 days. Then, dehydration was performed in progressively ascending grades of (80, 90 and 100 percent respectively) alcohol. Renal tissues were fixed in paraffin wax followed by cutting (4 µm slices) of the paraffin-embedded specimen via microtome. Hematoxylin-eosin (H & E) stain was used for staining renal tissues. Finally, tissues were observed under the microscope for histopathological analysis (Nikon, 187842, Japan). Photographs were taken with a MoticTM 5.0-megapixel camera.
2.9 Statistical evaluation
Results were displayed as Mean ± SE. The variation among the control and other experimental groups were investigated using one-way ANOVA, followed by Tukey's test using Minitab software.
3 Results
3.1 Effect of GTIN and DOX on biochemical assays
DOX induction brought a pronounced (P < 0.05) decline in enzymatic activities of antioxidants (GPx, GSR, SOD, GST, CAT as well as GSH) accompanied by an increment in ROS along with MDA level in the DOX-administrated rats than in control rats. Nonetheless, the treatment of rats with GTIN and DOX substantially restored the (P < 0.05) antioxidants enzyme activity, whereas reduced ROS and MDA levels in GTIN + DOX group as compared with DOX exposed group. Nevertheless, an insignificant difference among the average score of the GTIN-supplemented and control group was observed (Table 1). Values that do not hold a similar superscript depicts significant difference.
Parameters
Groups
Control
DOX
GTIN + DOX
GTIN
CAT (U/mg protein)
8.76 ± 0.12a
4.46 ± 0.14c
7.20 ± 0.18b
8.87 ± 0.16a
GPx (U/mg protein)
17.88 ± 0.40a
7.37 ± 0.09c
13.70 ± 0.76b
17.94 ± 0.41a
SOD (U/mg protein)
6.72 ± 0.09a
2.98 ± 0.18c
4.98 ± 0.10b
6.78 ± 0.09a
GSR (nM NADPH oxidized/min/mg tissue)
5.48 ± 0.09a
1.73 ± 0.08c
3.23 ± 0.12b
5.51 ± 0.11a
GST (nM/min/mg protein)
27.40 ± 1.29a
9.10 ± 0.27c
20.02 ± 0.74b
28.39 ± 1.40a
GSH (µM/g tissue)
17.66 ± 0.44a
8.42 ± 0.28c
13.59 ± 0.48b
17.98 ± 0.48a
MDA (nmol/g)
0.77 ± 0.03c
2.79 ± 0.08a
1.32 ± 0.02b
0.75 ± 0.02c
ROS (µmol/g)
1.41 ± 0.04c
9.58 ± 0.12a
3.05 ± 0.08b
1.39 ± 0.05c
3.2 Effects of GTIN and DOX on renal biomarkers
DOX exposure substantially (P < 0.05) augmented the urea, creatinine, KIM-1, as well as NGAL level accompanied by a remarkable decrease in creatinine clearance in DOX-intoxicated rats than in control group. Contrarily, the GTIN + DOX group revealed that urea, creatinine, KIM-1, as well as NGAL levels were brought down (P < 0.05), while creatinine clearance was brought up followed by GTIN + DOX co-treatment compared to DOX-treated group. Additionally, insignificant difference was spotted among the control and GTIN-treated group (Table 2). Values that do not hold a similar superscript depicts significant difference.
Parameters
Groups
Control
DOX
GTIN + DOX
GTIN
Urea (mg/dL)
14.96 ± 0.94c
38.72 ± 1.81a
25.32 ± 1.28b
14.93 ± 0.95c
Creatinine (mg/dL)
1.34 ± 0.04c
3.81 ± 0.08a
2.43 ± 0.06b
1.32 ± 0.02c
Creatinine clearance (mL/min)
1.72 ± 0.02a
0.31 ± 0.03c
1.18 ± 0.03b
1.71 ± 0.07a
KIM-1 (mg/mL)
0.30 ± 0.04c
1.81 ± 0.03a
0.97 ± 0.07b
0.29 ± 0.02c
NGAL (ng/mL)
0.64 ± 0.03c
2.03 ± 0.05a
1.10 ± 0.05b
0.63 ± 0.03c
3.3 Effects of GTIN and DOX on inflammatory mediators
DOX induction brought a remarkable augmentation (P < 0.05) in inflammatory mediators’ levels (IL-6, NF-κB, IL-1β, TNF-α along with COX-2) in the DOX-administrated rats with match to the control. Moreover, the administration of rats with GTIN + DOX considerably reduced the above-mentioned inflammatory markers levels in the (GTIN + DOX) co-treated rats in contrast with the DOX-treated rats. Nevertheless, an insignificant difference between the mean values of control and GTIN group were observed (Table 3). Values that do not hold a similar superscript depicts significant difference.
Parameters
Groups
Control
DOX
GTIN + DOX
GTIN
NF-kB (ng/g tissue)
16.57 ± 1.05c
69.95 ± 2.51a
31.13 ± 2.03b
16.48 ± 1.36c
TNF-α (ng/g tissue)
7.49 ± 0.25c
20.60 ± 1.17a
11.74 ± 0.88b
7.44 ± 0.24c
1L-1β (ng/g tissue)
25.97 ± 1.60c
84.69 ± 2.54a
37.18 ± 1.64b
25.72 ± 1.19c
IL-6 (ng/g tissue)
6.24 ± 0.32c
27.53 ± 1.36a
12.44 ± 0.66b
6.15 ± 0.30c
COX-2 (ng/g tissue)
23.42 ± 1.22c
74.40 ± 2.64a
33.44 ± 1.22b
23.33 ± 1.23c
3.4 Effects of GTIN and DOX on pro-or-anti-apoptotic markers
DOX exposure to rats elicited a remarkable elevation (P < 0.05) in Caspase-9, Bax as well as Caspase-3 levels, whereas lessened the level of Bcl-2 in DOX-intoxicated rats than in control. GTIN + DOX co-treatment reversed the (P < 0.05) above-stated changes in pro/anti-apoptotic markers in GTIN + DOX group with match to DOX group. However, GTIN-exposed rats depicted a standard level of these markers in the GTIN (only) supplemented group, which was comparable with control group (Table 4). Values that do not hold a similar superscript depicts significant difference.
Parameters
Groups
Control
DOX
GTIN + DOX
GTIN
Bax (pg/ mL)
2.32 ± 0.07c
9.78 ± 0.55a
4.03 ± 0.16b
1.96 ± 0.32c
Bcl-2 (ng/ mL)
17.20 ± 0.87a
5.77 ± 0.67c
11.88 ± 0.78b
17.35 ± 0.96a
Caspase-9 (pg/ mL)
3.64 ± 0.06c
20.76 ± 1.51a
7.52 ± 0.49b
3.59 ± 0.05c
Caspase-3 (pg/ mL)
1.20 ± 0.06c
11.84 ± 0.64a
3.27 ± 0.22b
1.16 ± 0.06c
3.5 Effects of GTIN and DOX on histology
DOX exposure instigated a considerable deterioration as well as detachment of epithelial cells, bowman capsule dilation, disruption of renal tubules and glomerular contraction. Whereas, GTIN + DOX exposure mitigated the above-stated adverse changes in renal morphology in the GTIN + DOX co-administrated group than in DOX group, reflecting the renoprotective role of GTIN. GTIN and control group demonstrated almost the same renal morphology (Fig. 1).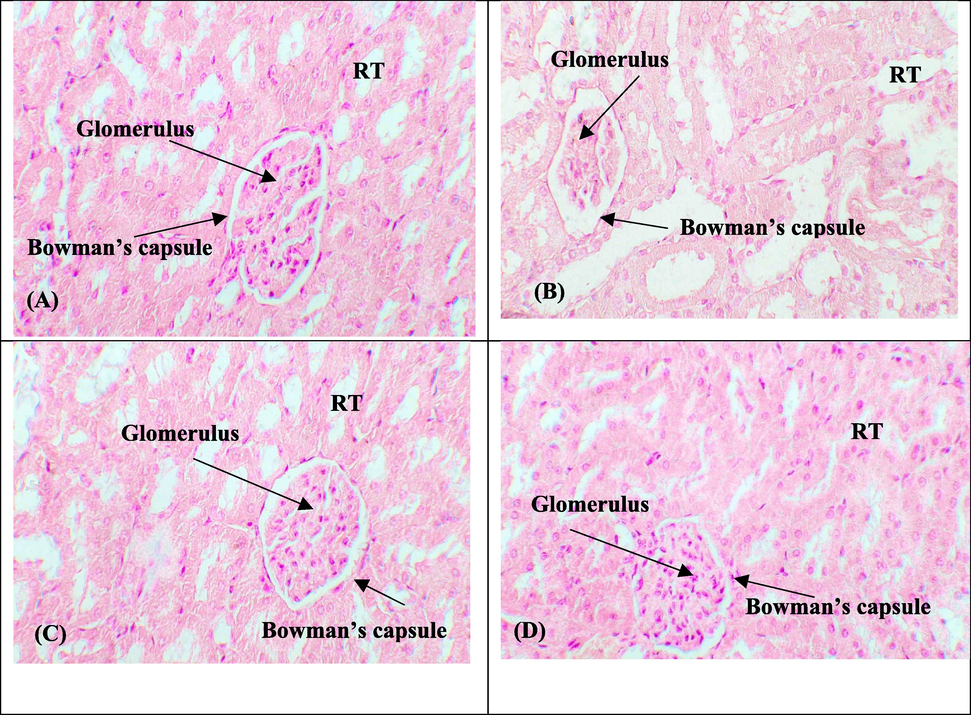
Remedial potential of GTIN on DOX instigated renal histological damages. (A) Control group (B) DOX intoxicated group (3 mg/kg) (C) GTIN + DOX co-treated group (30 mg/kg + 3 mg/kg) (D) GTIN supplemented group (30 mg/kg). RT: Renal tubules.
4 Discussion
According to our results, the biochemical analysis illustrated a profound decline in the profile of GPx, GSR, SOD, CAT, GST, and GSH accompanied by an increment in ROS and MDA levels after DOX administration. The instability in ROS level and antioxidant profile prompts OS that ultimately triggers oxidative impairment (Aziz et al., 2021; Mustafa et al., 2022). Moreover, MDA escalation depicts lipid peroxidation. SOD binds with two oxygen radicals to form hydrogen peroxide, while CAT converts H2O2 to oxygen and H2O by inhibiting the generation of cytotoxic ions, (OH–) (Ighodaro and Akinloye, 2018). GPx also mitigates OS by reducing hydrogen peroxide and lipid peroxide levels (Safhi, 2018). Hence, it is imperative to alleviate ROS level by regulating the normal antioxidant profile that subsequently declines renal OS. GTIN supplementation significantly escalated the antioxidant enzymes activities accompanied by an alleviation in ROS and MDA levels. The outcomes of our investigation were in compliance with Samant and Gupta (2022), who highlighted that the antioxidant ability and free radical scavenging property of GTIN in neurons that contribute to regaining the enzymatic profile and ROS and MDA level.
According to the current study, DOX intoxication augmented the urea, creatinine, KIM-1, as well as NGAL level, while a phenomenal reduction was detected in creatinine clearance level. Urea and creatinine, are used to monitor renal functions (Sahu et al., 2020). Creatinine is formed by the breakdown of phosphocreatine catalyzed by creatine kinase and it is removed from the kidney through glomerular filtration (Sepulveda, 2019). Any oxidative damage to renal tissues escalated the urea and creatinine level accompanied by lower creatinine clearance. Moreover, KIM-1, a transmembrane protein, does not normally present in urine but its presence implies injury in proximal tubules (Dobrek et al., 2016). NGAL is also seen in urine, kidney tubules, and blood following renal damage (Mori et al., 2005). However, co-administration of GTIN + DOX lowered the urea, creatinine, KIM-1, as well as NGAL levels that is probably associated with an improved glomerular filtration rate reflected by higher creatinine clearance.
DOX administration augmented inflammatory responses that are associated with the increased inflammatory mediator levels (IL-6, NF-κB, IL-1β, TNF-α along with COX-2). NF-κB, a transcription factor, is activated by various stresses (oxidative agents) in the cell leads to the regulation of a variety of proinflammatory mediators i.e., COX-2 and TNF-α. A previous study about DOX exposure on renal tissues depicted a remarkable increase in NF-κB expression, which ultimately boosted the release of other inflammatory markers (Hussain et al., 2021). According to our findings, GTIN treatment suppressed the level of inflammatory markers. Our results are further endorsed by earlier study, which predicted that structural features of flavonoids might be responsible to suppress inflammation (Nile et al., 2018).
DOX intoxication elicited a considerable augmentation in Bax, Caspase-9 along with Caspase-3 levels, while lessened the Bcl-2 level. Bax, Caspase-9 and Caspase-3, (pro-apoptotic markers), are the key indicator of apoptosis compared with Bcl-2, which is an anti-apoptotic marker. Cellular OS stimulates the caspase-3 level in the tissues, that promotes the apoptotic process and results in apoptotic damage (Liu et al., 2012). Moreover, disturbance in Bax as well as Bcl-2 level encourage cytochrome c eviction from mitochondrial membrane into the cytoplasm. Cytochrome c eviction promotes the stimulation of Caspase-9 and Caspase-3, which ultimately prompts apoptosis (Katiyar et al., 2005). GTIN + DOX co-administration mitigated all the above said irregularities by upregulating Bcl-2 and lowering Caspase-9, Bax and Caspase-3 levels owing to its anti-apoptotic potential.
Histomorphological observation of DOX-administrated rats showed deterioration and detachment of epithelial cells, glomerular contraction, degeneration of tubules and dilation of the bowman capsule. These alternations promoted renal impairment associated with the production of free radicals-induced lipid oxidation in renal tissues. Our findings were analogous to Mohamed et al. (2022), who illustrated glomerular contraction, necrosis, vacuolization, epithelial cell detoriations and bowman capsule dilation followed by DOX exposure. However, GTIN supplementation effectively mitigated nephrotoxicity and morphological damages prompted by DOX in renal tissues. The renoprotective properties of GTIN might be directly linked with its free radical scavenging potential and anti-inflammatory potential.
5 Conclusion
In conclusion, GTIN treatment elicited excellent attenuative effects against DOX nephrotoxicity. GTIN holds some promising therapeutic strategies to prevent any changes in the level of renal biomarkers, oxidative markers, apoptotic markers, inflammatory markers, antioxidants activity as well as histological architecture of renal tissues. Collectively, it can be concluded that GTIN may have clinical prospects in future to treat renal dysfunction in humans and other animals.
Funding
The authors extend their appreciation to the Deputyship of Research and Innovation, ‘Ministry of Education’ in Saudi Arabia for funding this research (IFKSUOR3-279-1).
Author contribution
MUI, KA and HAK designed the study, conceived the study, and analyzed the results. KA and MUI conceived an initial part of the study, performed the experiment, histology and helped in compiling the results. SR, AA, MI, HA, and TA helped in writing the results. MUI, SR, KZ, MI, TA, HA, and AA made a substantial contribution in the interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript.
Acknowledgement
The authors extend their appreciation to the Deputyship of Research and Innovation, ‘Ministry of Education’ in Saudi Arabia for funding this research (IFKSUOR3-279-1).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; Improvement by Acacia hydaspica tannin-rich ethyl acetate fraction. Saudi J. Biol. Sci.. 2020;27:2251-2260.
- [CrossRef] [Google Scholar]
- Doxorubicin-induced nephrotoxicity: protective effect of nicotinamide. Int. J. Cell Biol.. 2011;2011:1-9.
- [CrossRef] [Google Scholar]
- Effect of Engineered Nickel Oxide Nanoparticles on Antioxidant Enzymes in Freshwater Fish, Labeo rohita. Pak. Vet. J.. 2021;41:424-428.
- [CrossRef] [Google Scholar]
- Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem.. 2009;16:3267-3285.
- [CrossRef] [Google Scholar]
- Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol.. 1979;3:451-460.
- [Google Scholar]
- Urinary kidney injury molecule-1 (KIM-1) excretion in rats with experimental cystitis induced by oxazaphosphorines. Prz. Lek.. 2016;73:805-812.
- [Google Scholar]
- Disruption of redox homeostasis in the transforming growth factor-α/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J. Biol. Chem.. 1998;273:15846-15853.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;631:55-61.
- [CrossRef] [Google Scholar]
- Acute kidney injury-an overview of diagnostic methods and clinical management. Clin. Kidney J.. 2017;10:323-331.
- [CrossRef] [Google Scholar]
- Antioxidant and anti-inflammatory effects of crocin ameliorate doxorubicin-induced nephrotoxicity in rats. Oxid. Med. Cell. Longev.. 2021;2021
- [CrossRef] [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex. J. Med.. 2018;54:287-293.
- [CrossRef] [Google Scholar]
- Therapeutic efficacy of chrysoeriol on doxorubicin-induced liver damage by improving biochemical and histological profile in rats. J. Food Nutr. Res.. 2023;11:57-62.
- [CrossRef] [Google Scholar]
- Therapeutic Effect of Oroxylin A Against Bisphenol A-induced Kidney Damage in Rats: a Histological and Biochemical Study. Pak. Vet. J.. 2022;42:511-516.
- [CrossRef] [Google Scholar]
- Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol. Cancer Ter.. 2005;4:207-216.
- [CrossRef] [Google Scholar]
- Nicorandil combats doxorubicin-induced nephrotoxicity via amendment of TLR4/P38 MAPK/NFκ-B signaling pathway. Chem. Biol. Interact.. 2019;311:108777
- [CrossRef] [Google Scholar]
- Gossypetin ameliorates ionizing radiation-induced oxidative stress in mice liver—a molecular approach. Free Radic. Res.. 2015;49:1173-11111.
- [Google Scholar]
- Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun.. 1976;71:952-958.
- [CrossRef] [Google Scholar]
- Anti-atherosclerotic effect of gossypetin on abnormal vascular smooth muscle cell proliferation and migration. Antioxidants. 2021;10:1357.
- [CrossRef] [Google Scholar]
- Liu, N.S., Du, X., Lu, J., He, B.P., 2012. Diva reduces cell death in response to oxidative stress and cytotoxicity. 7, pp. 1-13. https://doi.org/10.1371/journal.pone.0043180.
- Anti-inflammatory, anti-apoptotic, and antioxidant roles of honey, royal jelly, and propolis in suppressing nephrotoxicity induced by doxorubicin in male albino rats. Antioxidants. 2022;11:1029.
- [CrossRef] [Google Scholar]
- Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig.. 2005;115:610-621.
- [Google Scholar]
- The cardioprotective effects of nano-curcumin against doxorubicin-induced cardiotoxicity: a systematic review. Biofactors. 2022;48:597-610.
- [CrossRef] [Google Scholar]
- Isorhamnetin: a flavonoid, attenuated doxorubicin-induced testicular injury via regulation of steroidogenic enzymes and apoptotic signaling gene expression in male rats. Toxicol. Res.. 2022;11:475-485.
- [CrossRef] [Google Scholar]
- Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J. Biochem. Mol. Toxicol.. 2018;32:22002.
- [CrossRef] [Google Scholar]
- Luteolin attenuates doxorubicin-induced derangements of liver and kidney by reducing oxidative and inflammatory stress to suppress apoptosis. Hum. Exp. Toxicol.. 2021;40:1656-1672.
- [CrossRef] [Google Scholar]
- Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem.. 1966;16:359-364.
- [CrossRef] [Google Scholar]
- Nephroprotective effect of Zingerone against CCl4-induced renal toxicity in Swiss albino mice: molecular mechanism. Oxid. Med. Cell. Longev.. 2018;2018:1-7.
- [CrossRef] [Google Scholar]
- Naringenin mitigates antituberculosis drugs induced hepatic and renal injury in rats. J. Tradit. Complement. Med.. 2020;10:26-35.
- [CrossRef] [Google Scholar]
- Gossypetin-based therapeutics for cognitive dysfunction in chronic unpredictable stress-exposed mice. Metab. Brain Dis.. 2022;37:1527-1539.
- [CrossRef] [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- Sepulveda, J.L., 2019. Challenges in routine clinical chemistry testing analysis of small molecules. In: Accurate results in the clinical laboratory. Elsevier, pp 101-140. https://doi.org/10.1016/B978-0-12-813776-5.00009-1.
- A simple method for clinical assay of superoxide dismutase. Clin. Chem.. 1988;34:497-500.
- [CrossRef] [Google Scholar]
- Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother.. 2021;137:111308
- [CrossRef] [Google Scholar]
- Gossypetin is a novel MKK3 and MKK6 inhibitor that suppresses esophageal cancer growth in vitro and in vivo. Cancer Lett.. 2019;442:126-136.
- [CrossRef] [Google Scholar]
- The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: the role of autophagy. Molecules. 2018;23:1184.
- [CrossRef] [Google Scholar]
Further Reading
- Nephroprotective Effects of Delphinidin against Bisphenol A Induced Kidney Damage in Rats. Pak. Vet. J.. 2022;43:189-193.
- [CrossRef] [Google Scholar]
- Pomegranate Peel Extract and Quercetin Possess Antioxidant and Hepatoprotective Activity against Concanavalin A-induced Liver Injury in Mice. Pak. Vet. J.. 2021;41:197-202.
- [CrossRef] [Google Scholar]







