Translate this page into:
Gold nanoparticles stabilized by starch polymer and their use as catalyst in homocoupling of phenylboronic acid
⁎Corresponding author at: Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Ubon Ratchathani University, Warin Chamrap, Ubon Ratchathani 34190, Thailand. sanoe.c@ubu.ac.th (Sanoe Chairam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
In this study, gold nanoparticles (Au NPs) stabilized by a starch polymer have been successfully prepared and characterized via a number of techniques including X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), UV-visible spectroscopy (UV-vis), transmission electron microscopy (TEM), and dynamic light scattering (DLS) measurements. The catalytic activity of starch-stabilized Au NPs was also examined toward the homocoupling of phenylboronic acid in water using oxygen in air as oxidant at an ambient temperature (25 ± 1 °C). Several parameters including the catalyst loading, base equivalent (eq.), base type, and reaction time were studied. This study offers a simple, inexpensive and environmentally friendly procedure for the stabilization of colloidal gold catalysts using a hydroxyl-rich structure of starch polymer with a great promise through potential applications in related fields.
Keywords
Gold nanoparticles
Starch support
Homocoupling
Phenylboronic acid
1 Introduction
Nanoparticles constitute one of the best examples of nanoscience, that is, unique and novel properties (Astruc et al., 2005). Gold nanoparticles (Au NPs) occupy an important role in catalysis (Hashmi and Hutchings, 2006), not only for aerobic oxidation CO to CO2 (Haruta et al., 1989), but also for many chemical reactions such as selective chemisorption (Fukushima et al., 1979) and hydrogenation (Jia et al., 2000), and carbon-carbon coupling reactions (Carrettin et al., 2005; Hopkinson et al., 2011; Wegner and Auzias, 2011). In fact, it was observed that the catalytic activity of Au decreases considerably as the average particle size of Au NPs increases, and very small Au clusters between 3 and 10 Au atoms are the most active gold species for various organic reactions (Oliver-Meseguer et al., 2012). However, the problem is that these clusters tend to grow and are impossible to stabilize under the reaction conditions, increasing their size gradually. In the most common strategy for fabricating nanosized gold catalysts, a large number of supports such as metal oxides, polysaccharides, proteins and organic polymers are frequently used to stabilize Au NPs against their aggregation for efficient catalysis. As supports, a number of organic polymers can provide the stabilization of nanoparticles not only because of steric, but also by a weak binding to the surface of particles, playing the role of ligands in the catalytic cycle of Au catalysts (Alexandridis, 2011). A conventional polymer, poly(N-vinyl-2-pyrrolidone) (PVP) is the most commonly used polymer for the stabilization of Au NPs, because it fulfils both steric and ligand requirements. Tsunoyama et al. (2004) first reported that small and monodisperse Au NPs stabilized by PVP are highly active for the homocoupling reaction of phenylboronic acid in water; however, no effort was made in this study to maximize the yield of biphenyl as a major product.
Water as a reaction solvent has received much attention in coupling reactions catalyzed by supported Au NPs because of its environmental friendliness. Besides the use of synthetic polymers as supports, a natural biopolymer is considered as a suitable water-compatible support for the stabilization of Au catalysts because it is highly abundant in nature, it can easily be chemically modified, and it has a high sorption capacity for metal ions. In this context, Primo and Quignard (2010) recently reported that a biopolymer can also stabilize Au NPs by interaction of the N and O atoms of chitosan with Au NPs. Au NPs supported on a large surface area, porous chitosan have been found to be an active catalyst for the carbon-carbon bond formation.
Starch is a natural polyhydroxylated macromolecule mainly consisting of a large number of glucose units connected by glycosidic bonds to form amylose and amylopectin chains. This polysaccharide presents interesting dynamic supramolecular associations facilitated by inter- and intramolecular hydrogen bonding resulting a helical structure, which can act as a template for the nanoparticle growth. The extensive number of hydroxyl groups possibly facilitated metal ions by electrostatic binding in the helical structure of starch polymer (Raveendran et al., 2003; Pienpinijtham et al., 2012). Though starch as a natural polymer is cheap and widely used in various applications, less attention has been paid to the stabilization of nanoparticles in catalysis. Due to its high amylose content (Hoover et al., 1997), mung bean starch is easy to make a starch gel or paste, in this study it is selected as a model for the stabilization of Au NPs. In order to investigate their potential application, starch-stabilized Au NPs were successfully applied as catalyst for the carbon–carbon homocoupling of phenylboronic acid in water.
2 Experimental
All reagents were at least analytical grade. Hydrogen teterachloroaurate(III) trihydrate (HAuCl4·3H2O, ≥99.99%), trisodium citrate (Na3C6H5O7, 99%), and phenylboronic acid (96%), dodecane (C12H26, 99.99%) were purchased from Sigma-Aldrich. Sodium bicarbonate (NaHCO3), sodium carbonate (Na2CO3), potassium carbonate (K2CO3) and potassium hydroxide (KOH) were obtained from Acros-Organic. A starch polymer (100% mung bean starch produced by Sit Thi Nan Co., Ltd. Thailand) was purchased from supermarkets worldwide. All the glassware used were cleaned with 10%HNO3, and then washed with de-ionized (DI) water (R ≥ 18.2 MΩ·cm, purified by a Nanopore Ultrapure Water System).
The typical procedure for the preparation of Au NPs synthesized by the reduction of chloroaurate anions [AuCl4]− solution with trisodium citrate in an aqueous starch solution is given as follows. A 0.9 g mung bean starch (5 mmol with respected to glucose unit) was placed in a three-neck round-bottom flask containing 50 mL of boiling water under vigorously magnetic stirring. After the complete dissolution of starch, a 50 µL of 1% HAuCl4 was added and then allowed to form a Au3+:starch complex. After 30 min, 5 mL of 1 mM trisodium citrate was rapidly added. A controlled experiment was carried out without the addition of trisodium citrate. The reaction color immediately turned from pale yellow to wine-red, indicating the formation of Au NPs. The reaction flask was then further magnetically stirred for 30 min. After cooling to room temperature while stirring continuously, the solution was diluted to 100 mL and then stored in a dark bottle at 4 °C when not in use.
Gold nanoparticles stabilized by a starch polymer (Au NPs: Starch) was characterized by the following techniques. The XPS measurements were carried out using Vacuum Generators AXIS Ultra DLD electron spectrometer. The pressure in the analyzer was ca. 2 × 10−8 Torr. X-rays from the Mg Kα line at 1253.6 eV (15 kV, 20 mA) were used for an excitation. Photoelectrons were corrected in a constant analyzer energy mode with pass energy of 80 eV. The crystal structure was characterized by using Philip X’pert-MDP powder X-ray diffractometer (XRD) operating the Cu Kα radiation at the wavelength of 1.54056 Å, 30 mA and 40 kV. The diffraction patterns were recorded from 20° to 80° 2θ at ambient temperature of about 25 °C with the time per step of 1 min/step and the scan step size of 0.02 2θ/s. The absorption spectra were recorded using PerkinElmer Lambda25 UV-vis absorption spectrometer with the path length of standard rectangular quartz cells (Hellma Analytics, 1 cm) over the wavelength ranging from 300 nm to 800 nm. The nanostructure was examined using transmission electron microscopy (TEM). The TEM images were recorded employing a JEOL JSM-2010 transmission electron microscope by accelerating at a voltage of 200 kV. A drop of colloidal nanogold solution was dispersed on a Formvar-coated Cu grid for TEM measurements. The average hydrodynamic diameter of the stabilized Au NPs was determined by a dynamic light scattering (DLS) instrument (MAL 500261 Particle Size Analyzer). The DLS experiment was carried out employing a 90° angle detector with 35 mW solid state laser detector operating at a wavelength of 658 nm.
The catalytic activity of Au NPs:Starch was examined toward the homocoupling reaction of phenylboronic acid in water using oxygen in air as oxidant. Briefly, in a 5 mL round-bottom flask, phenylboronic acid (0.45 mmol), a base (3 eq.), Au NPs-St catalyst (5 mL, 0.625 mol%Au determined by Pin AACle 900 T Perkin Elmer atomic absorption spectrometer, AAS) and water (5 mL) were placed. The reaction mixture was vigorously stirred under air at an ambient temperature (approximately 25 ± 1 °C) for a given time. Subsequently, the reaction was quenched with 1 M HCl, and the products were then extracted with AcOEt (5 mL × 3) using dodecane as internal standard. The combined organic phase was dried over anhydrous Na2SO4. The resulting mixture was characterized by 1H NMR Bruker Avance 300 MHz, while the percent yield was examined by Shimadzu GC-17 A gas chromatography (GC).
Biphenyl: 1H NMR (300 MHz, CDCl3, 298 K) (δ, ppm): 7.61 (t, 4 H, ArH), 7.53 (t, 4 H, ArH), 7.42 (d, 2 H, ArH)
3 Results and discussion
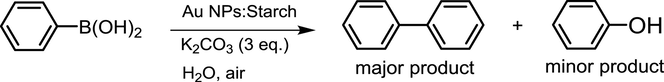
The Au oxidation state was analyzed by XPS measurements. A survey spectrum and core-level spectra of Au 4f (82–90 eV), C 1s (282–290 eV) and O 1s (528–536 eV) regions were systematically recorded (Fig. 1a). From core-level spectra of Au 4f, two peaks were observed at 83.7 and 87.4 eV, which correspond to Au(0) 4f7/2 and Au(0) 4f5/2, respectively, The Au(III) peaks at 86.9 and 90.6 eV were not observed in the XPS spectrum, which is in accordance with the literature values reported for Au(0) stabilized by starch polymer (Tajammul Hussain et al., 2008). The results indicate the complete reduction of Au(III) to Au(0) by trisodium citrate using starch as a stabilizing agent, and a hydroxyl-rich structure of starch polymer could possibly stabilize Au NPs by electrostatic binding via the O atoms. A reaction mechanism to obtain small Au NPs by citrate reduction is reported in the existing literature (Ojea-Jiménez et al., 2010). The crystallinity was further characterized by XRD (Fig. 1b). The major diffraction peaks appeared at around 37.3°, 44.5, 64.7, and 77.3 were obviously observed, which are assigned to the diffraction of (111), (200), (220), and (311) lattice planes of metallic gold with face-centered cubic (fcc) structure (Joint Committee on Powder Diffraction Standards, JCPDS, file No. 04-0784), which is in agreement with the literature report (Sun and Xia, 2002). The XRD results indicated that Au NPs were successfully synthesized by the citrate reduction. The formation of colloidal Au NPs was also investigated by UV-vis spectroscopy (Fig. 1c). The starch solution did not appear the absorption band, while a wine-red Au NPs:Starch solution exhibited the absorption band at approximately 520 nm, which corresponds to a characteristic surface plasmon resonance (SPR) band of colloidal gold particles (Eustis and El-Sayed, 2006).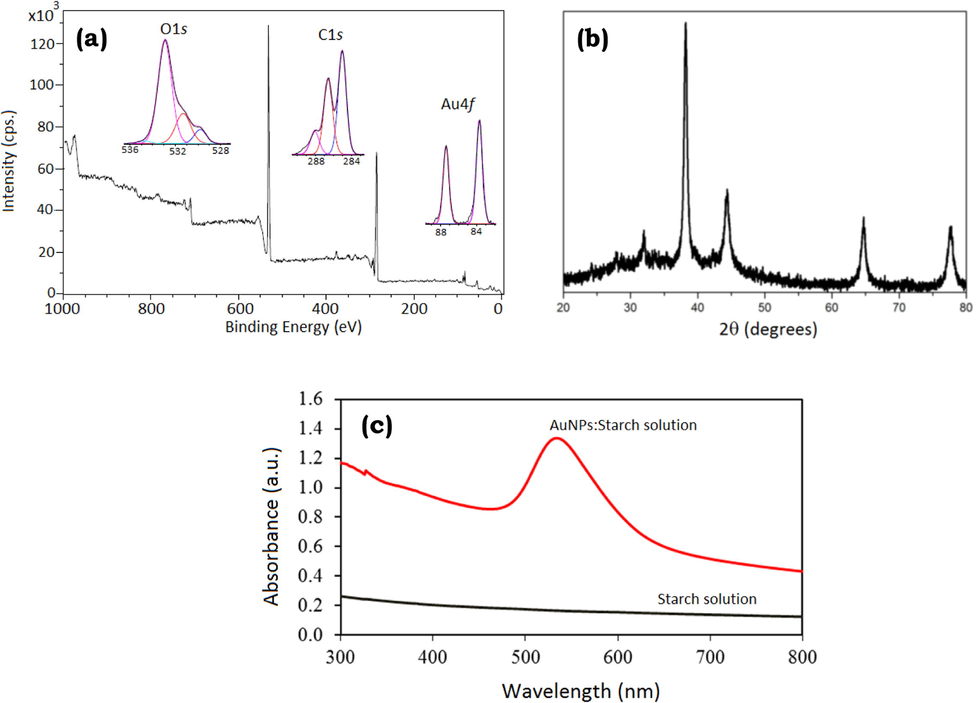
(a) XPS, (b) XRD, and (c) UV-vis spectra of Au NPs:Starch (d).
TEM analysis has become a routine and very important technique for the catalyst characterization. In this study, the particle size and morphology of the prepared gold colloid were also examined. The TEM images show that the starch-stabilized Au NPs are spherical in shape with an average particle size about 10 ± 2.5 nm (Fig. 2a–c). This value is also in agreement with the previous reports (Raveendran et al., 2003, Chairam et al., 2009), using starch as a protecting agent for the stabilization of metal nanoparticles. Electron diffraction is widely used to determine the crystallinity as well as the phases in catalysts. Comparing to XRD, it is more sensitive to nanometer-sized particles and local structures. The selected area electron diffraction (SAED) pattern was also recorded from TEM images to obtain the crystallographic structure. By a ring pattern, the diffraction spots suggested that each nanoparticle of Au was a polycrystalline product (Fig. 2d). In order to confirm the particle size, the as-prepared nanoparticles were characterized by a DLS measurement. The results exhibited a narrow particle size distribution with an average diameter of about 10.83 nm (Fig. 2e). This finding is also in agreement with those obtained from the TEM analysis.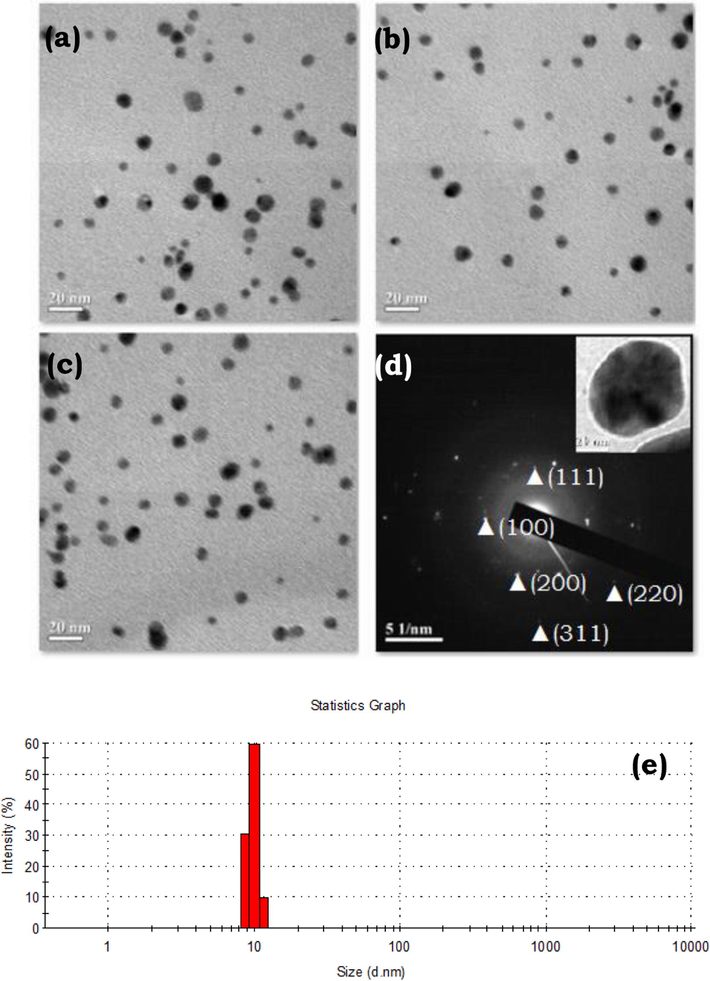
(a–c) TEM images, (d) SAED pattern, and (e) DLS measurement of gold colloid.
The carbon-carbon forming reaction catalyzed by colloidal gold particles is a powerful and convenient synthetic method in organic chemistry. In this study, the catalytic activity of Au NPs stabilized by starch was examined toward homocoupling of phenylboronic acid in water. The experimental conditions including the catalyst loading, base equivalent (eq.), base type, and reaction time were studied to maximize the yield of biphenyl. As well known, the amount of catalyst has a great influence on the catalytic activity, so the effect of catalyst loading was first studied by varying Au NPs:Starch while keeping various parameters constant (Fig. 3a). It has been experimentally observed that the coupling reaction did not proceed at all in the absence of Au catalyst. With a 0.625 mol% Au, biphenyl was detected as a major product with a good yield, while phenol was a minor product. The results obtained here is also in agreement with the previous reports (Tsunoyama et al. (2004)). Both products almost did not change significantly when increasing to 1.25 mol% of catalyst. So, 0.625 mol% Au NPs was selected as the best catalyst loading for further investigations of other parameters in our work.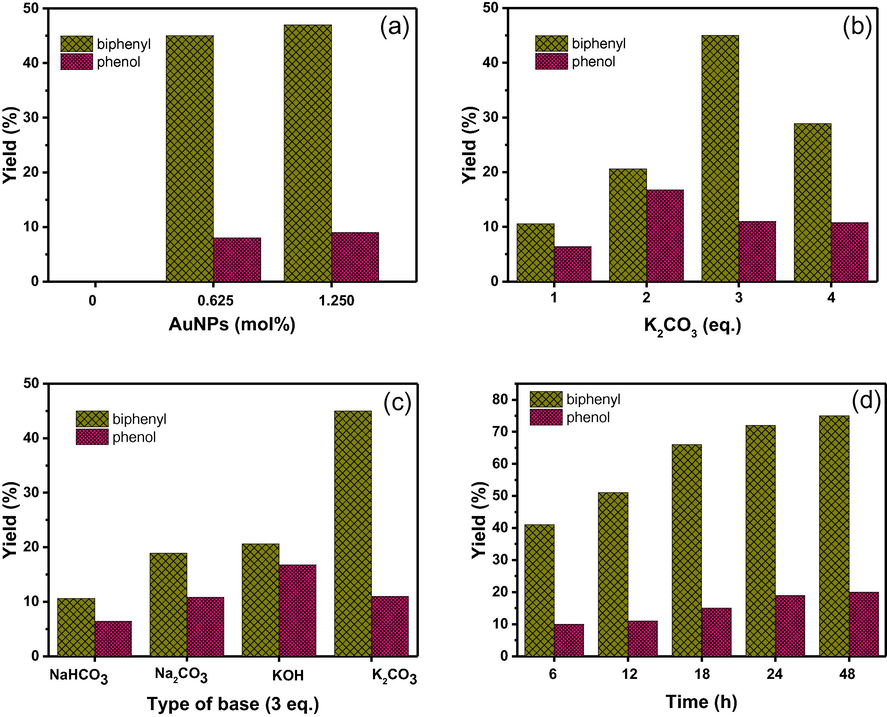
Effects of (a) catalyst loading, (b) equivalent of base (using K2CO3), (c) type of base, and (d) reaction time on the homocoupling of phenylboronic acid.
As well known, the formation of biphenyl product do not proceed without the use of base. Initially, K2CO3 was used as a model base to promote the homocoupling of phenylboronic acid. It was found that the percent yield of biphenyl gradually increased from 1 to 3 eq., and then decreased when increasing to 4 eq. (Fig. 3b). Thus, base at 3 eq. was selected for the homocoupling reaction. In order to study the effect of base, inorganic bases, such as NaHCO3, Na2CO3, KOH, and K2CO3 at 3 eq. were taken into consideration for the coupling reaction (Fig. 3c). Obviously, the results showed that K2CO3 when compared with others afforded the products with the highest percent yield, so K2CO3 was chosen as a suitable base for the homocoupling reaction. Finally, the reaction time was studied by quenching the reaction at different time intervals. It was found that at the early stage of the reaction the percent yields of products increased with increasing the reaction time. After 24 h, the percent yields for both products almost did not change significantly (Fig. 3d). Thus, the suitable reaction time for the homocoupling reaction should be carried out at 24 h.
The recycling of Au NPs:Starch catalyst was investigated under optimal conditions for homocoupling of phenylboronic acid. The reaction was carried out by using phenylboronic acid (0.45 mmol), K2CO3 (3 eq.), Au NPs:Starch catalyst (0.625 mol%Au in water (5 mL) under vigorously magnetic stirring at an ambient temperature (25 ± 1 °C) for 24 h. The as-prepared gold catalyst still exhibited a good catalytic activity with a satisfactory yield of biphenyl after being recycled for five successive runs. This is mainly attributed to colloidal gold particles with a large surface area, and a hydroxyl-rich structure of starch polymer also plays an important role for stabilizing highly dispersed Au NPs. This simple, inexpensive and environmentally friendly procedure ought to be interesting for the stabilization of gold colloids using a hydroxyl-rich structure of biopolymer from starches as stabilizer with a great promise through other potential applications in related fields.
Based on our evidence and the suggested mechanism from the existing literature (Tsunoyama et al., 2004, Wang et al., 2013), the possible mechanism for homocoupling of phenylboronic acid over Au NPs stabilized by a starch polymer is descried as follows (Fig. 4). Initially, a superoxo species is generated by the oxidation process from O2 adsorption on the surface of nanosized gold particles stabilized by starch polymer. Subsequently, the phenylboronic acid reacts with hydroxide ion to yield a Ph-B(OH)3− species acting as a nucleophile. Then, the transmetalltion process is occurred from a positively charged Au site attacked by a Ph-B(OH)3− species, and leads to the phenyl and boron peroxyl fragments adsorbed on the surface of nanosized gold particles. The products of both biphenyl and phenol resulted from a direct transformation of phenylboronic acid. If the transmetalltion process occurs again, two phenyl fragments would be adsorbed on the surface of Au catalyst; however, a by-product of phenol can be formed in this step. Then, the biphenyl is produced from the reductive elimination step in which two phenyl fragments react each other. The catalytic reaction can be recyclable when the active Au(0) species is obtained.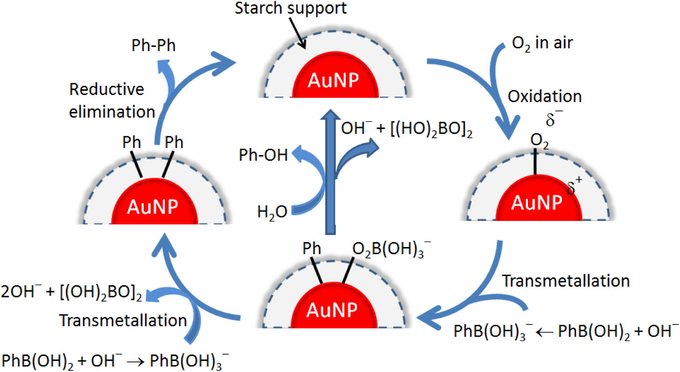
The possible mechanism for the homocoupling of phenylboronic acid over starch-stabilized Au NPs to form biphenyl and phenol.
4 Conclusion
In conclusion, gold nanoparticles (Au NPs) stabilized by a starch polymer have been successfully prepared, and characterized by a number of techniques including XPS, XRD, UV-vis, TEM, SAED, and DLS measurements. The homocoupling reaction of phenylboronic acid was carried out to investigate the catalytic activity of stabilized Au NPs. The starch-stabilized Au NPs still exhibited a good catalytic activity with a satisfactory yield of biphenyl after being recycled for five successive runs. Based on the experimental data, a possible mechanism for homocoupling of phenylboronic acid catalyzed by colloidal gold particles is suggested. The Au(0) species is thought to be the active sites for the reaction, and a base is required to activate phenylboronic acid through the participation of surface hydroxyl groups of starch polymer. This simple, inexpensive and environmentally friendly catalytic route ought to be interesting for carbon-carbon forming reaction catalyzed by starch-stabilized gold catalysts through potential applications in related fields.
Acknowledgements
This work was supported by the Thailand Research Fund (TRF, MRG5480037), the Center of Excellent for Innovation in Chemistry (PERCH−CIC), Office of the Higher Education Commission, Ministry of Education, and Faculty of Science, Ubon Ratchathani University (UBU).
References
- Gold nanoparticle synthesis, morphology control, and stabilization facilitated by functional polymers. Chem. Eng. Technol.. 2011;34:15-28.
- [Google Scholar]
- Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed.. 2005;44:7852-7872.
- [Google Scholar]
- Supported gold catalyzes the homocoupling of phenylboronic acid with high conversion and selectivity. Angew. Chem. Int. Ed.. 2005;44:2242-2245.
- [Google Scholar]
- Starch vermicelli template-assisted synthesis of size/shape-controlled nanoparticles. Carbohydr. Polym.. 2009;75:694-704.
- [Google Scholar]
- Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev.. 2006;35:209-217.
- [Google Scholar]
- Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal.. 1989;115:301-309.
- [Google Scholar]
- Physicochemical characterization of mung bean starch. Food Hydrocolloids. 1997;11:401-408.
- [Google Scholar]
- AuI/AuIII catalysis: an alternative approach for C-C oxidative coupling. Chem. Eur. J.. 2011;17:8248-8262.
- [Google Scholar]
- Selective hydrogenation of acetylene over Au/Al2O3 catalyst. J. Phys. Chem. B. 2000;104:11153-11156.
- [Google Scholar]
- Small gold nanoparticles synthesized with sodium citrate and heavy water: Insights into the reaction mechanism. J. Phys. Chem. C. 2010;114:1800-1804.
- [Google Scholar]
- Small gold clusters formed in solution give reaction turnover numbers of 107 at room temperature. Science. 2012;338:1452-1455.
- [Google Scholar]
- Green synthesis of size controllable and uniform gold nanospheres using alkaline degradation intermediates of soluble starch as reducing agent and stabilizer. Macromol. Res.. 2012;20:1281-1288.
- [Google Scholar]
- Chitosan as efficient porous support for dispersion of highly active gold nanoparticles: design of hybrid catalyst for carbon-carbon bond formation. Chem. Commun.. 2010;46:5593-5595.
- [Google Scholar]
- Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc.. 2003;125:13940-13941.
- [Google Scholar]
- Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298:2176-2179.
- [Google Scholar]
- Size control synthesis of starch capped-gold nanoparticles. J. Nanopart. Res.. 2008;11:1383.
- [Google Scholar]
- Colloidal gold nanoparticles as catalyst for carbon−carbon bond formation: Application to aerobic homocoupling of phenylboronic acid in water. Langmuir. 2004;20:11293-11296.
- [Google Scholar]
- Aerobic homocoupling of phenylboronic acid on Mg–Al mixed-oxides-supported Au nanoparticles. J. Catal.. 2013;298:186-197.
- [Google Scholar]
- Gold for C-C coupling reactions: a swiss-army-knife catalyst? Angew. Chem. Int. Ed.. 2011;50:8236-8247.
- [Google Scholar]







