Translate this page into:
Glycation of heme-protein, “myoglobin” by 3-deoxyglucosone: Implications in immunogenicity
⁎Corresponding authors. saeedmicrobiology@gmail.com (Mohd Saeed), s.ansari@uoh.edu.sa (Saheem Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The Reactive carbonyl species (RCS) like, 3-deoxyglucosone (3-DG) is a highly reactive intermediate of the glycation reaction which may result into the formation of protein advanced glycation end-products (AGEs), like myoglobin-AGEs (Mb-AGEs) which further lead to the structural perturbations. In the present work, we studied the glycation of myoglobin (Mb) by physico-chemical techniques and the resulting immunogenicity by ELISA techniques. The level of free lysine and arginine determined was found to be significantly decreased indicating the establishment of the glycation reaction. The glycated myoglobin (G-Mb) shows the increased absorbance at 280 nm and enhanced fluorescence emission at 350 nm excitation wavelengths. The levels of ketoamine and hydroxymethylfurfural (HMF) were recorded to be enhanced in G-Mb when compared to its native conformer. Furthermore, the New-Zealand white female rabbits immunized with native and its glycated analogue ‘G-Mb’ induced high titer antibodies as compared to the native one.
Keywords
Glycation
Myoglobin
Protein-AGEs
3-Deoxyglucosone
1 Introduction
The reaction between free amino groups of proteins and DNA with the reducing sugars leads to the structural and functional abnormalities in biomolecules; and this reaction is precisely called as glycation. This reaction may result into the development of other reactive intermediates called reactive oxygen species (in general free radicals). The free radicals are the key players in the development of metabolic derangements like diabetes, cancer and nephropathy.
Myoglobin (Mb) is a protein which is found in heart and skeletal muscle tissue which acts as an essential job in the storing and transporting of oxygen for the respiration of cellular machinery (Ghelani et al., 2018). In addition to O2 transport Mb acts as nitric oxide scavenger, to protect the respiration of mitochondria (Chung et al., 2006). Due to the increased glucose uptake via glucose transporter-1 (GLUT-1), there is high build-up of the blood glucose concentration. The increased build of glucose in the blood causes the terminal amino or free amino groups of myoglobin to undergo a non-enzymatic glycosylation reaction which results into the structural thus functional changes in the myoglobin macromolecule (Jürgens et al., 2000).
Like other proteins, non-enzymatic glycation of myoglobin (Mb) results in the generation of protein-advanced glycation end products (AGEs) (Miyazawa et al., 2012). The incubation of Mb with any reducing sugar like, glucose, produces fructosamine. Post fructosamine generation it leads to the formation of advanced glycation end-products (AGEs). 3-deoxyglucosone (3-DG) mediated glycation may induce modification of myoglobin by glucose by generating protein carbonyl compounds (Rosen et al., 2001). Therefore, the enhanced formation of AGEs linked with diabetes is usually the main cause in the progress of microvascular and macrovascular complications of diabetes mellitus (Singh et al., 2014).
Recent literature documents that AGEs, in the form of dicarbonyl, are also produced from the autoxidation of sugars and other metabolic pathways (Cantero et al., 2007; Thornalley, 2008; Lin et al., 2016). The buildup of dicarbonyl species, termed as carbonyl stress, is enhanced five to six times during diabetic complications (Jack and Wright, 2012). In diabetic patients, these are responsible for the pathogenesis and secondary consequences, leading to the structural and functional modifications of intracellular proteins (Singh et al., 2014). The dicarbonyl intermediates, such as 3-deoxyglucosone (3-DG) and methylglyoxal, produce AGEs that are exceedingly reactive than normal reducing sugars (Ashraf et al., 2015). The highly reactive dicarbonyl 3-DG is formed by the degradation of sugars that are found in a high-fructose corn syrup and many foods.
Myoglobin is a cytoplasmic heme-protein that is present exclusively in striated muscles (cardiovascular myocardium and skeletal muscle fibers) (Ordway and Garry, 2004). Its functions include buffering intracellular oxygen concentration, enhancing intracellular oxygen transport, inactivating nitric oxide (NO), and quenching the ROS (Kanatous et al., 2009). However, glycated Mb is unable to carry out these functions, which further advances carbonyl stress (Roy et al., 2004). In the current study, we examined the glycating capability of 3-DG with Mb. Physicochemical methods were utilized to describe the degree of alteration of Mb by 3-DG. In addition, the immunogenicity of native and 3-DG glycated Mb was also studied in New Zealand white female rabbits.
2 Materials and methods
2.1 Myoglobin modification
Commercially available Mb (50 µg/mL) was altered with 3-DG at different concentrations (5 to 20 mM) in 100 mM phosphate buffered saline (PBS), pH 7.4. After extensive dialysis against PBS to remove unbound constituents, the reactions were incubated at 37 °C from 1 to 12 days. The native myoglobin not treated with the 3-DG was served as a control.
2.2 Spectroscopic analysis of myoglobin after 3-DG modification
The UV–Vis spectroscopy of native and 3-DG glycated Mb were recorded between 200 and 400 nm of wavelength using Eppendorf biospectrophotometer according to a previously published literature (Dixit et al., 2014).
2.3 Fluorescence studies of myoglobin post 3-DG glycation
The fluorescence spectroscopy was performed on Cary-Eclipse Fluorescence Spectrophotometer. The native and 3-DG modified glycated samples were excited at 350 nm of wavelength and the emissions were recorded in the range between 350 and 500 nm of wavelength. The highest peak, i.e., increase in fluorescence intensity (FI) or emission was obtained at 450 nm which is a characteristic of the formation of advanced glycation end-products (AGEs) (Ahmad et al., 2011a). The shoot-up in the FI can be calculated from the following equation:
2.4 Quantification of carbonyl content
The carbonyl contents generated in native and modified (glycated) Mb samples was performed by using 2, 4-dinitrophenylhydrazine (DNPH) as per previously published literature (Ahmad et al., 2012). The final pellet (250 µL) was dissolved in 6 M guanidium hydrochloride solution and the absorbance was recorded at 360 nm for determination of carbonyl content concentration, an extinction coefficient of 22000 M−1cm−1 was used as described earlier (Ahmad et al., 2012).
2.5 Nitroblue tetrazolium assay
The amounts of ketoamine formed as a result of Mb glycation were measured by nitroblue tetrazolium (NBT) assay as explained elsewhere (Ansari et al., 2011). The absorbance of native and glycated Mb protein was taken at 525 nm, and content of ketoamine (nmol mL−1) was determined by dividing the absorbance by 12,640 M−1cm−1; which is an extinction coefficient.
2.6 Hydroxymethyl furfural (HMF) content
The quantitative estimation of HMF in native and glycated Mb samples were performed by thiobarbituric assay (TBA) (Ansari, et al. 2009). HMF is formed in the intermediate stage of the Maillard or glycation reaction. The quantification of HMF was completed by following steps-
One ml of native or 3-DG modified Mb samples was mixed with ‘one molar’ oxalic acid and thereafter heated for one hour.
40% of trichloroacetic acid (TCA) was mixed in the above samples at 24 h.
Thiobarbituric acid (TBA) was added after removal of precipitate through filtration and the mixture was further incubated for half an hour.
The quantity of HMF (molar extinction coefficient value 4 × 104 M−1cm−1) in nmol/ml was calculated using the molar extinction coefficient.
2.7 Free lysine estimation
The amount of free lysine in both the native and glycated Mb samples were determined by using 2,4,6 trinitrobenzene 1-sulphonic acid (TNBS) method as described in our previous literature (Khan et al., 2018). The absorbance of the reaction mixture was recorded at 346 nm against a blank on bio spectrum - kinetics spectrophotometer (Eppendorf) using quartz cuvette of 1 cm path length (Khan et al., 2018).
2.8 Free arginine estimation
Phenanthrequonine method was used for the estimation of free arginine content as described previously (Siddiqui et al., 2019). The fluorescence spectra were recorded at 395 nm emission wavelength using an excitation wavelength of 312 nm (Siqqidui et al., 2019).
2.9 Induction of antibodies against native and glycated Mb
The immunization experiment was approved by Institutional Animal Ethical Committee (IAEC) of the Integral University, India. The rabbits used in the experiment were handled meticulously with negligible suffering.
25 µg each of native Mb and its glycated analogue were put in 500 µL of PBS and were then emulsified in 500 µL of complete Freund’s adjuvant. The complex was infused intramuscularly at different destinations in the rear leg muscles. Subsequent infusions were administered with incomplete Freund's adjuvant every week with similar concentrations given as above for Mb and its glycated conformers. These doses were administered for six weeks such that all rabbit received 150 µg antigens in total. Post booster dose blood was isolated and serum was isolated from it. The isolated serum was decomplemented by warming at 56 °C for 30 min in the water bath. Pre-immune blood was collected before inoculation that served as a control (Shahab et al., 2012a).
2.10 Enzyme-Linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was performed on a polystyrene plate with minor adjustments as per the previous published literature (Shahab et al., 2012b; Ahmad et al., 2011b).
2.11 Competition ELISA
The antigen-binding specificity of isolated antibodies was estimated as per previous publications of our research group using competitive inhibition ELISA (Jairajpuri et al., 2007).
3 Results and discussion
3.1 Biophysical characterization of native and glycated Mb
Pilot tests were carried out to standardize the incubation time in addition to the concentration of 3-DG needed to glycate the commercially available Mb (Sigma-Aldrich, United States). Mb (50 µg/mL) was incubated with different concentrations of 3-DG (5, 10, and 20 mM) for various time interims (1 to 12 days) at 37 °C. The maximum hyperchromicity, at 280 nm, was observed at 12 days of incubation at 20 mM concentration of 3-DG. Additional incubation of Mb with the 3-DG did not bring about any further change in the hyperchromicity (Fig. 1a). Along these lines, Mb was incubated for 12 days with 20 mM of 3-DG in 100 mM PBS (pH 7.4). When compared with 280 nm peak of native Mb alone, the hyperchromicity at 4, 8, and 12 days were 56.2, 77.5, and 87.9%, respectively (Fig. 1). This increase in hyperchromicity might be due to the exposure of the chromophoric groups like aromatic amino acids which was caused because of the Mb protein glycation which lead to structural perturbations (Akhter et al., 2013).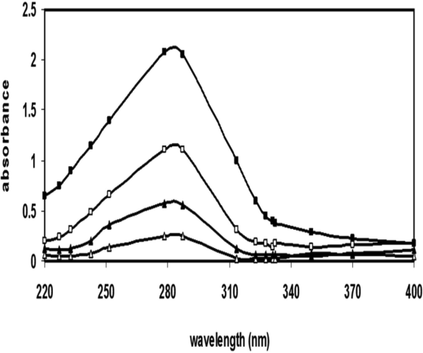
UV–Vis spectra of native Mb (-Δ-) and glycated Mb for 4 (▬▲▬), 8 (▬□▬) and 12 (▬■▬) days. All the experiment was repeated three times each.
The development of advanced glycation end-products in glycated Mb was affirmed by fluorescence spectroscopy as described in the methods section (Fig. 1b). Under identical conditions, native Mb did not demonstrate any fluorescence. An increase of 85.08% in fluorescence intenstity was seen in AGE-Mb when compared with the native Mb. This may be attributed to the presence of fluorescent AGEs that cause the glycation adducts to fluoresce.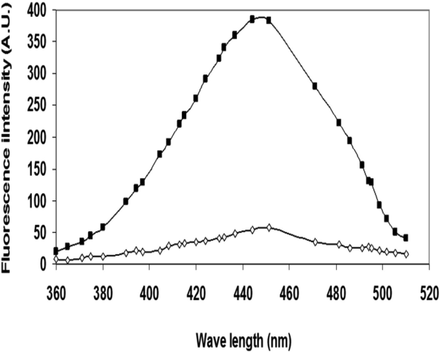
The fluorescence spectroscopy native Mb (▬□▬) and glycated Mb (▬■▬). The native and glycated conformer were excited at 350 nm. All the experiment was repeated three times each.
3.2 Biochemical parameters in native and modified Mb
Structural modifications of Mb upon glycation led to the increase in the carbonyl content. The carbonyl content was found to be enhanced from 3.65 ± 0.46 for native Mb to 31.534 ± 3.2 nmol mg−1 protein for glycated Mb (Table 1). Table 1 represents the increase/decrease in the biochemical/biophysical changes induced in the Myoglobin protein. The p value for the parameters are denoted as *p < 0.008; **p < 0.002; and ***p < 0.001. The changes in the Biochemical values were highly significant. The surge in the biochemical values was statistically significant for glycated Mb versus native Mb.
S.No.
Parameters
Native Mb
AGE-Mb
Percent Modification
1
Absorbance (at 280 nm)
0.135 ± 0.09
2.08 ± 0.12*
87.98% hyperchromicity
2
Fluorescence intensity (ex.340 nm)
57.51
382.31**
85.08% increase
3
Carbonyl content (nmol/mg protein)
3.65 ± 0.46
31.534 ± 3.2 **
88.43% increase
4
Ketamine content (nmol/mL protein)
2.78 ± 0.22
21.34 ± 0.95 **
86.97% increase
5
HMF content (nmol/mL protein)
3.31 ± 0.226
17.43 ± 0.72 ***
81.01% increase
6
Free Lysine
–
21% decrease
21% decrease
7
Free Arginine
–
36% decrease
36% decrease
For an early detection of glycation moieties, the ketoamine moieties in the glycation milieu were estimated by NBT. The analysis of native Mb alone indicated relatively insignificant ketoamine content; however, glycated Mb reported the highest ketoamine value at the seventh day of the incubation period. The normal ketoamine concentrations during the analyses of native and glycated Mb were 2.78 ± 0.22 nmol mL−1 and 21.34 ± 0.95 nmol mL−1 respectively (Table 1).
HMF was determined as thiobarbituric acid reactive substance (TBARS) after hydrolysis. The HMF content for glycated conformer of Mb was highest at the seventh day (17.43 ± 0.72 nmol mL−1 of protein), while native Mb had a relatively low level of HMF content (3.31 ± 0.226 nmol mL−1, Table 1). The higher yield of HMF reported by glycated Mb is in concurrence with the result of the NBT test. Ketoamines are converted to protein carbonyl compounds by means of a protein enediol creating superoxide radical. The protein carbonyl substance is a common biomarker of oxidation and glycation reaction of proteins.
Glycation also affects lysine residues which include ε-amino group along with arginine; therefore free lysine residue estimation is an essential parameter which should be included in the glycation reaction. Previous studies have shown that all the lysine residues are not involved in the glycation process; however, some specific lysine residues are known to be involved in it (Ghelani et al., 2018). Our results have shown 36% loss in free lysine residues in AGE-Mb as compared with the native Mb. (Table 1). The decreased percentage of free lysine residues in glycated Mb samples indicates the extent of glycation at lysine residues in the protein. Attachment of arginine residues to reducing sugar also results into AGEs formation. The reaction of arginine with phenanthrenequinone produces fluorescence which is recorded between 300 and 500 nm of wavelength. A significant reduction in free arginine residues of AGE-Mb samples was observed upon glycation with 3-DG. AGE-Mb showed a 21% reduction in arginine, comparatively with fluorescence intensity of the native Mb samples at 395 nm (Table 1).
3.3 In vivo immunogenicity study
We checked the antigenicity of 3-DG-modified Mb in New Zealand white (NZW) female rabbits. Not surprisingly, the native Mb was observed to be less immunogenic, while its glycated conformer was more immunogenic. The direct binding ELISA indicated enhanced antibody titer (>1:25,600) as compared to the native analogue (Fig. 2). The vigorous immune response in rabbits immunized from 3-DG-modified Mb suggests a structural alteration in Mb protein molecule. This would have been resulted into the generation of neo-epitopes on the protein Mb. These epitopes thus generated are acknowledged foreign by rabbit’s own immune system, which may have resulted into the formation of antibody molecules in order to fight against the glycated antigen, i.e., glycated Mb.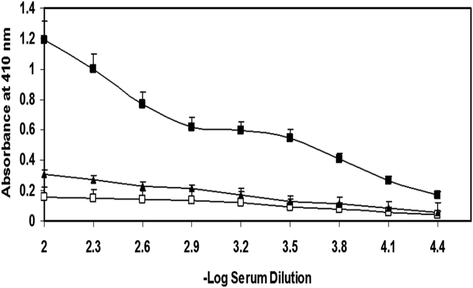
The ELISA spectrum of Mb antisera (▬▲▬), preimmune sera (▬□▬) and immune serum (▬■▬). The ELISA plate wells were coated with 3-DG-modified Mb (10 µg/mL). Each data point represents the mean-average of three experimental repeats.
Direct ELISA revealed 3-DG-modified Mb to be more immunogenic than native Mb (Fig. 2). Pre-immune sera did not show appreciable binding with either immunogen (native or glycated Mb). Similarly, competitive inhibition ELISA explored the specificity of 3-DG-modified Mb anti-serum. It demonstrated a reduction in antibody activity upon utilization of immunogen as an inhibitor (Fig. 3).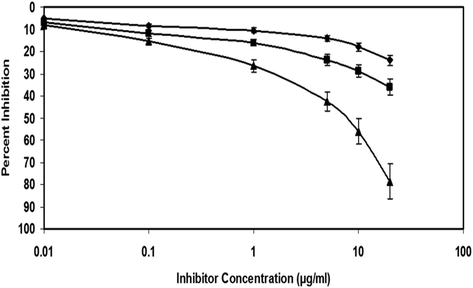
The competitive inhibition ELISA of native DNA antisera (▬■▬), preimmune sera (▬♦▬) and immune serum (▬▲▬). The microtiter wells were coated with 3-DG-modified Mb (10 µg/mL). Each datapoint represents the average of three experiments. The values represent the mean ± SD.
The hindrance in the action of the protein glycation delays the improvement of diabetic complications. Mb protein is predominantly found in the cardiovascular myocardium; it acts as an oxygen store that transfers its headed oxygen to the heart mitochondrial ATP during oxidative phosphorylation (Hendgen-Cotta, et al. 2014). Past reports suggest that GMb (with glucose or fructose) may generate harmful radicals, negatively affecting the pathophysiology of diabetic intricacies, such as atherosclerosis (Nabi et al., 2019).
Many investigations have underlined glucose-initiated protein glycation and ensuing AGEs arrangement. However, 3-DG-induced glycation is a highly probable event in hyperglycemic conditions as the formation of intermediate dicarbonyl compounds in glycation reaction. Along these lines, studies on glycation response between 3-DG and Mb are warranted.
The present study concludes that glycation of Mb with 3-DG has resulted in the physico-chemical modifications in the structures and functions of Mb macromolecule. This glycation has resulted into the formation of Amadori products (early glycation). The reaction of glycation has further led to the accumulation of AGEs in the reaction mixture. The antigenic impact of glycated Mb by 3-DG in the animal model is reflected by the presence of enhanced antibodies, and thus resulting and contributing to its immunogenic behaviour. The present examination also exhibited that these glycated molecules may act as antigenic determinants for eliciting immune response leading to antibody production against the glycated conformer.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Research Group Project No. RG-175.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preferential recognition of methylglyoxal-modified calf thymus DNA by circulating antibodies in cancer patients. Indian J. Biochem. Biophys.. 2011;48:290-296.
- [Google Scholar]
- Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2+. Biochem. Biophys. Res. Commun.. 2011;407(3):568-574.
- [CrossRef] [Google Scholar]
- Physicochemical studies on glycation-induced structural changes in human IgG. IUBMB Life. 2012;64(2):151-156.
- [CrossRef] [Google Scholar]
- Bio-physical characterization of ribose induced glycation: a mechanistic study on DNA perturbations. Int. J. Biol. Macromol.. 2013;58:206-210.
- [CrossRef] [Google Scholar]
- Preferential recognition of Amadori-rich lysine residues by serum antibodies in diabetes mellitus: role of protein glycation in the disease process. Human Immunol.. 2009;70:417-424.
- [CrossRef] [Google Scholar]
- Physicochemical analysis of poly-L-lysine: an insight into the changes induced in lysine residues of proteins on modification with glucose. IUBMB Life. 2011;63:26-29.
- [CrossRef] [Google Scholar]
- 3-Deoxyglucosone: a potential glycating agent accountable for structural alteration in H3 histone protein through generation of different AGEs. PLoS One. 2015;10(2)
- [CrossRef] [Google Scholar]
- Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-β: implications for diabetic atherosclerosis. FASEB J.. 2007;21(12):3096-3106.
- [CrossRef] [Google Scholar]
- Implication of CO inactivation on myoglobin function. Am. J. Physiol. Cell Physiol.. 2006;290:C1616-C1624.
- [CrossRef] [Google Scholar]
- Human DNA damage by the synergistic action of 4-aminobiphenyl and nitric oxide: an immunochemical study. Environ. toxicology.. 2014;29(5):568-576.
- [CrossRef] [Google Scholar]
- Attenuation of glucose-induced myoglobin glycation and the formation of advanced glycation end products (AGEs) by (R)-α-lipoic acid in vitro. Biomolecules. 2018;1:9.
- [CrossRef] [Google Scholar]
- Myoglobin functions in the heart. Free Radical Biol. Med.. 2014;73:252-259.
- [CrossRef] [Google Scholar]
- Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl. Res.. 2012;159(5):355-365.
- [CrossRef] [Google Scholar]
- Immunoglobulin glycation with fructose: a comparative study. Clin. Chima. Acta. 2007;378:86-92.
- [CrossRef] [Google Scholar]
- Myoglobin: just an oxygen store or also an oxygen transporter. News Physiol. Sci.. 2000;15:269-274.
- [CrossRef] [Google Scholar]
- Hypoxia reprograms calcium signaling and regulates myoglobin expression. Am. J. Physiol. Cell Physiol.. 2009;296(3):393-402.
- [CrossRef] [Google Scholar]
- Immunochemical studies on native and glycated LDL–an approach to uncover the structural perturbations. Int. J. Biol. Macromol.. 2018;115:287-299.
- [CrossRef] [Google Scholar]
- Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: an emerging biological factor in cancer onset and progression. Mol. Nutr. Food Res.. 2016;60(8):1850-1864.
- [CrossRef] [Google Scholar]
- Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42(4):1163-1170.
- [CrossRef] [Google Scholar]
- Glycation and HMG-CoA reductase inhibitors: implication in diabetes and associated complications. Curr. Diabetes Rev.. 2019;15(3):213-223.
- [CrossRef] [Google Scholar]
- Myoglobin: an essential hemoprotein in striated muscle. J. Exp. Biol.. 2004;207:3441-3446.
- [CrossRef] [Google Scholar]
- The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a congress series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab. Res. Rev.. 2001;17:189-212.
- [CrossRef] [Google Scholar]
- In vitro nonenzymatic glycation enhances the role of myoglobin as a source of oxidative stress. Free Radic. Res.. 2004;38(2):139-146.
- [CrossRef] [Google Scholar]
- Hydroxyl radical modification of collagen type II increases its arthritogenicity and immunogenicity. PLoS ONE. 2012;7:e31199
- [CrossRef] [Google Scholar]
- Acquired immunogenicity of human DNA damaged by N-hydroxy-N-acetyl–4-aminobiphenyl. IUBMB Life. 2012;64:340-345.
- [CrossRef] [Google Scholar]
- Glycation of hemoglobin leads to the immunogenicity as a result of neo-epitope generation. Int. J. Biol. Macromol.. 2019;123:427-435.
- [CrossRef] [Google Scholar]
- Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol.. 2014;18:1-14.
- [CrossRef] [Google Scholar]
- Protein & nucleotide damage by glyoxal & methylglyoxal in physiological systems-role in ageing & disease. Drug Interact.. 2008;23(1–2):125-150.
- [CrossRef] [Google Scholar]







