Translate this page into:
Glomerular filtration barrier in rat offspring exposed to maternal undernutrition
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Poor diet during pregnancy can increase blood pressure in offspring of human and laboratory animals. The present study examined the effects of moderate intrauterine undernutrition on the ultrastructure of the glomerular filtration barrier in 20-day-old rat fetuses and six-month-old rat offspring. Pregnant rats were provided with either ad libitum food during pregnancy (control group, C) or restricted to 50% of ad libitum food until delivery (food-restricted group, FR). Both groups were given free access to food after birth. The kidneys of embryonic day 20 and six-month-old rats were harvested. Transmission electron micrographs in glomeruli from both groups were obtained to study the ultrastructure of the glomerular filtration barrier. Blood pressure and glomerular filtration rate were measured in six-month-old rats. In comparison to the control group, the FR group had smaller body and kidney weights in both ages. Systemic blood pressure was significantly elevated in the FR group. The glomerular filtration rate was similar in both groups. A study of the glomerular ultrastructure showed a remarkable retardation in the development of the podocyte foot process in the FR group at embryonic day 20. Micrographs also showed remarkable changes in the glomerular filtration barrier of six-month-old rats including an increase in the thickness of the glomerular basement membrane and an increase in the width of filtration slits. These results suggest that maternal food restriction disturbs the development of the glomerular filtration barrier, which may contribute to hypertension in adult rat offspring. The long-term exposure to hypertension and glomerular hyperfiltration may have contributed to the damages observed in the glomerular filtration barrier of six-month-old rats exposed to intrauterine food restriction.
Keywords
Fetal programing
Kidney
Hyperfiltration
Podocyte
Glomerular barrier
Filtration slits
1 Introduction
Hypertension is a multifactor disease in which blood pressure is permanently elevated. More than 970 million people are affected by hypertension worldwide and this number is estimated to increase to 1.5 billion by 2025 (Kearney et al., 2005). The main function of the kidneys is to filter blood and to keep the body’s sodium and water at normal levels. The failure of the kidneys to control the sodium/water balance leads to hypertension (Brenner et al., 1988). Studies have linked impaired fetal development to adulthood hypertension in humans (Moore et al., 1996; Rich-Edwards et al., 1997) and experimental animals (Plagemann et al., 2000; Woods et al., 2001; Alwasel and Ashton, 2009; Xie et al., 2012; Vaccari et al., 2015). During the Dutch famine, the daily rations for the general adult population reduced from 1800 calories in December 1943 to 400–600 calories in April 1945 (Roseboom et al., 2001). The severe reduction in maternal caloric intake (−70%) during the Dutch famine was correlated with the increase in blood pressure in adult men (van Abeelen et al., 2011). Barker (1996) initiated the fetal programing hypothesis, which states that insults during fetal life result in fetal adaptations leading to adult diseases. One of the main maternal factors that induces fetal programing of hypertension in the next generation is poor diet during pregnancy (Barker, 1996; Langley-Evans 1997). Maternal malnutrition reduces the nutrient supply to the fetus, which negatively affects renal development. Investigators have used mild (30%), moderate (50%) or severe (70%) global food restriction in order to examine the programing effects (Woodall et al., 1996; Vickers et al., 2000; Vaccari et al., 2015).
There is an increasing body of evidence that maternal malnutrition impairs nephrogenesis and reduces nephron number, as estimated by counting mature glomeruli (Woods et al., 2001; Chou et al., 2008; Xie et al., 2012; Vaccari et al., 2015). The nephron is the basic structural and functional unit in the kidney. It consists of two parts: the glomerulus and the renal tubule. The nephron performs three major functions in order to separate the various components of blood: filtration, which occurs in the glomerulus, and reabsorption and secretion, which take place in the renal tubule (Skorecki et al., 2016). Glomerular filtration occurs when fluid in the blood is perfused across the glomerular filtration barrier. This barrier allows water and small- to medium-sized molecules to exit the glomerular capillary and enter the urinary space before they are passed into the renal tubule. In contrast, this highly specialized barrier prevents the filtration of proteins and other macromolecules (Brenner et al., 1988). Three major components form the glomerular filtration barrier: the fenestrated endothelial cell, the glomerular basement membrane, and the podocyte foot process with their filtration slits (Skorecki et al., 2016). The glomerular basement membrane is a selective permeable filter that consists of three layers: the lamina densa, a central electron-dense zone surrounded by a layer of lower electron density, the lamina rara interna toward the endothelia, and the lamina rara externa toward the podocytes (Miner, 2012). Abnormal thickness of the glomerular basement membrane is often linked to proteinuria and hematuria (Schwartz et al., 2007). The morphological development of the glomerular filtration barrier in rat fetuses exposed to maternal food restriction is unknown.
Some studies have postulated that the reduction in glomerular number could decrease the glomerular filtration rate and increase sodium retention leading to hypertension (Brenner et al., 1988). However, the glomerular filtration rate is not always reduced, in fact GFR of offspring exposed to 50% maternal food restriction was comparable to controls (Vaccari et al., 2015). This indicates that single glomerular filtration rates may be increased in order to maintain a normal filtration rate (Vaccari et al., 2015). The current study hypothesized that (A): maternal undernutrition may alter the development of the glomerular filtration barrier in fetal kidney. (B) The long-term exposure to hypertension and glomerular hyperfiltration may increase the risk of glomerular damages. Accordingly, the present study examined the effects of 50% maternal food restriction on the ultrastructure of the glomerular filtration barrier in 20-day-old rat fetuses and six-month-old rat offspring.
2 Materials and methods
2.1 Animal model
Ten-week-old female Wistar virgin rats (Rattus norvegicus) with body weights between 230 and 250 g, were purchased from the Experimental Animal Care Centre, King Saud University. All experiments were approved by The Ethics Committee of King Saud University. Animals were housed individually for three days at 24 °C and a 12 h light/dark cycle. During this time female rats were given free access to standard rat chow (food composition is shown in Table 1) and tap water. Cages with saw dust for bedding and nesting were provided. Each female (n = 32) was paired with a virgin male in a mating cage until mating was confirmed by the presence of a sperm plug. Pregnant rats were assigned into two groups: the first was the control group (C, n = 16), which was provided with ad libitum food from day 0 of pregnancy until delivery. Food consumption per 100 g body weight was calculated in each day of gestation. The second was the food-restricted group (FR, n = 16) which was given 50% of food consumed by control pregnant females. All animals were given free access to food and water from birth until 6 months of age where the blood pressure measurement and renal assessments were performed in male offspring.
Constituent
% Weight
Protein
20
Oil
10
Starch
42
Fibers
6
Sucrose
20
Minerals
1.5
Vitamins
0.5
2.2 Body weight and kidney weight
Eight pregnant rats from each group were anaesthetized at gestational day 20 with intraperitoneally dose of inactin (sodium thiobutabarbital, Sigma; 100 mg/kg of body weight). The uteri were exposed and fetuses were harvested. Male fetuses were dissected to obtain and examine fetal kidneys at embryonic day 20 (E20). After dissection, the fetal kidneys were weighed and preserved for electron microscopy. The rest of the dams (8 per group) were left for delivery. At birth, litter size and litter weight were recorded. Litter size was reduced to 8 pups, 4 male and 4 female, where possible. After a complete weaning, male offspring were separated and housed in small groups until the age of 6 months. Body weight was recorded and kidneys from six-month-old offspring were weighed and preserved for ultrastructural study.
2.3 Assessments of mean arterial blood pressure
Mean arterial blood pressure was measured in six-month-old male offspring (n = 8 per group). Blood pressure was recorded on three consecutive mornings using tail cuff non-preheating and non-invasive method (Muromachi MK-2000ST NP-NIBP, Muromachi Kikai Co. Ltd., Japan). All animals were trained to stay calm in restraint cages to avoid stress-related pressure.
2.4 Evaluation of the glomerular filtration rate
Six-month-old male offspring (8 per group) were individually housed in metabolic cages (Nalgene MC-8083, Tecniplast, Italy) for 24 h for acclimation. Urine was collected at the end of the next 24 h. Urine samples were measured, spun at slow speed and then stored in the refrigerator for later analysis. At the end of the experiment, rats were anaesthetized with inactin (100 mg/kg of body weight) to allow for heart blood collections and kidney dissections, after which an overdose was administrated. Plasma and urinary creatinine concentrations were determined using a colorimetric reaction (Bowers and Wong, 1980). Briefly, to prepare the working solution, three solutions were mixed: A (4.5 g of trisodium phosphate, 4.5 g of sodium tetraborate, and 2.5 g of sodium hydroxide in 200 ml distilled water), B (1% picric acid) and C (4% sodium dodecyl sulfate). Known and graded concentrations of creatinine were prepared as a standard curve. Ten microliters of samples and standards were transferred to cuvettes and mixed with 100 μl of working solution. The mixture was left to stand for 10 min at room temperature. The initial absorbance of each solution at 592 nm was read. Then, 10 μl of acetic acid was added to the cuvettes, and the samples were left at room temperature for five minutes. The absorbance at 592 nm was read again, and the difference between the two readings was calculated. The glomerular filtration rate was calculated as: (urinary creatinine [μmol/L] × volume of urine produced in 24 h [ml])/(plasma creatinine [μmol/l] × 1440 [min]).
2.5 Electron microscopy
Fresh kidneys of 20 day-old-fetuses (n = 8 per group) were fixed in Karnovsky’s fixative in 0.1 M sodium cacodylate buffer (pH 7.2) for four hours at 4 °C. For six-months-old rats, the left kidneys (n = 8 per group) were cut longitudinally into two halves. The medulla was removed and each half was cut into two pieces. One cubic centimeter tissue sample from each piece was fixed in Karnovsky’s fixative (4 samples per kidney). After washing in the same buffer, the samples were post-fixed in 2% osmium tetroxide for one hour. Then, the samples were embedded in Agar 100 proxy resin. Blocks were trimmed to trapezoid shaped pyramid for ultramicrotomy. Semi-thin sections were cut at 1 μm and stained with toluidine blue. Triplicates of ultra-thin sections (70–90 nm) from the same glomerulus were obtained at intervals of 20 μm using an ultramicrotome (Leica EM UC7, Germany). Ultrathin sections were double-stained with uranyl acetate and lead citrate. Photomicrographs were taken using JEOL 100 CX. Compartments of the glomerular filtration barrier were examined. Only mature glomeruli in the fetuses, confirmed by the presence of red blood cells in the capillaries, were examined. The thickness of the glomerular basement membrane and the width of the filtration slits were measured in four random spots in each photomicrograph (96 per group) at magnifications of 40,000× using image manager software (Leica IM 1500).
2.6 Statistical analyses
Results are expressed as mean ± standard error. Significant differences between control and FR animals were determined using Student’s t-test for equal variables. The two-way ANOVA with repeated measure was used to check the effects of diet and time on maternal body weight during pregnancy. Differences were considered significant when p < 0.05. All statistical analyses were performed using SPSS program (Version 20).
3 Results
3.1 Body weight and kidney weight
The maternal body weights in the FR group were significantly smaller than those in the control group throughout gestation except for the first three days. The maximum difference in maternal body weight was 78 g and occurred on gestational day 20, Fig. 1. The body weights of fetuses exposed to maternal food restriction were significantly reduced in comparison with the control group fetuses (C, 5.25 ± 0.17 vs FR, 4.72 ± 0.26 g, p < 0.05), Fig. 2A. In line with low body weights at embryonic day 20, FR fetal kidneys were significantly smaller than those in the control group (C, 0.046 ± 0.003 vs FR, 0.038 ± 0.002 g, p < 0.05), Fig. 2B. The kidney weight to body weight ratios of FR fetuses were smaller than the control group (C, 0.88 ± 0.02 vs FR, 0.81 ± 0.02%, p < 0.05). Litter size was comparable between the two groups (C, 11.73 ± 0.462 vs FR, 11.18 ± 0. 25 pup, p = 0.72, Fiet algure 3A), however, litter weight was significantly reduced in the FR group (C, 71.2 ± 0.64 vs FR, 61.6 ± 0.98, p < 0.05), Fig. 3B. A calculation of the mean body weight at birth showed that male FR pups were significantly smaller than their counterpart controls (C, 6.1 ± 0.42 vs FR, 5.5 ± 0.38, p < 0.05). Intrauterine food restriction had long-term effects on both body weight and kidney weight of FR offspring because both remained significantly smaller than the body (C, 584.6 ± 11.4 vs FR, 561.5 ± 16. 8, p < 0.05) and kidney weights (C, 1.88 ± 0.044 vs FR, 1.72 ± 0.025, p < 0.05) of the C offspring at 6 months, Fig. 4 A and B. A reduction in renal mass is an indicator of a decrease in glomerular number. The total number of glomeruli is not shown here but the FR offspring had a 27% reduction in glomerular number compared to the control offspring (data not shown).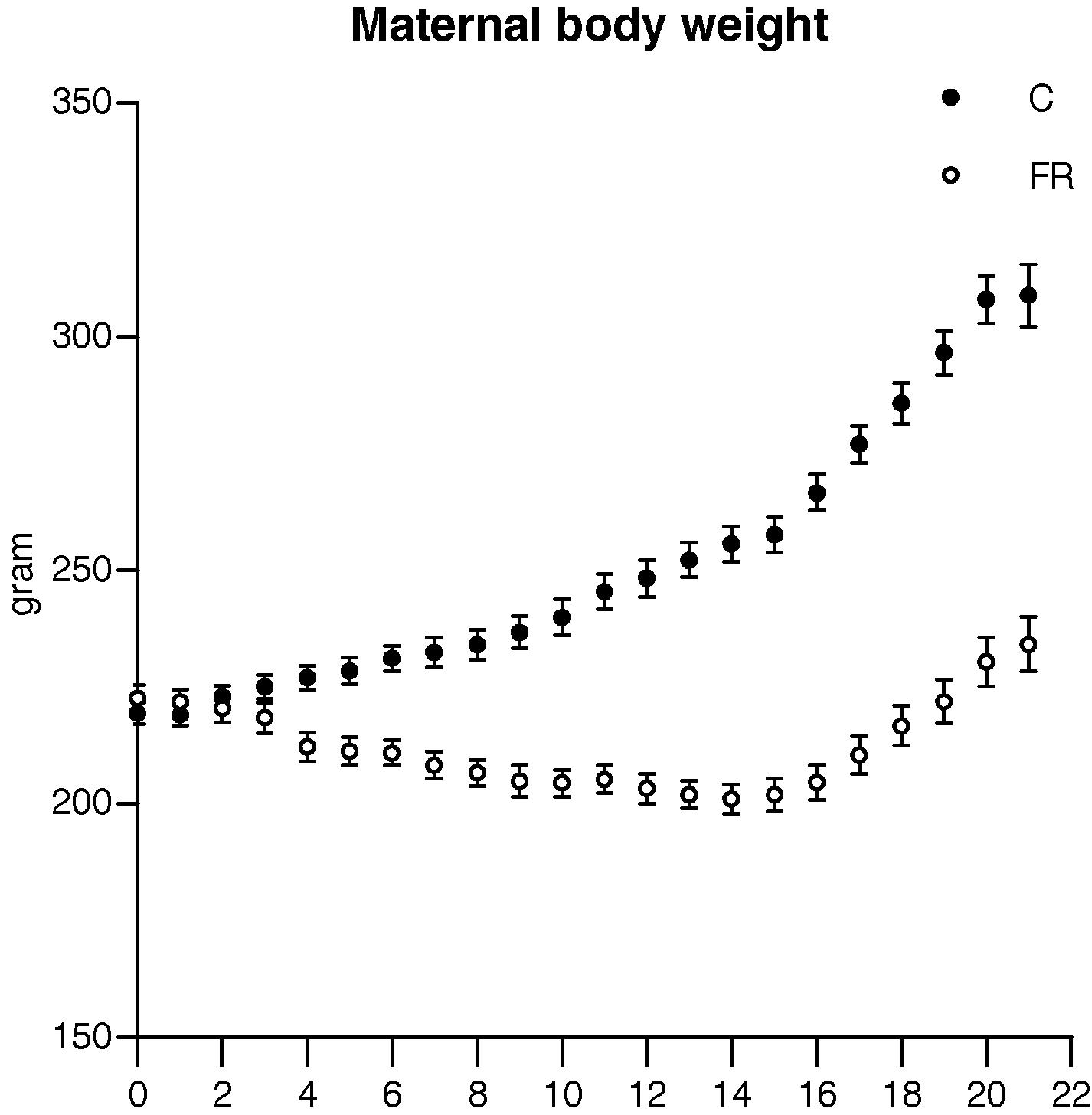
Body weight during gestation in control (C, n = 16) and food restricted (FR, n = 16) groups. The two-way repeated measure ANOVA revealed that both dietary regime and time had significant effects on maternal body weight of FR group.
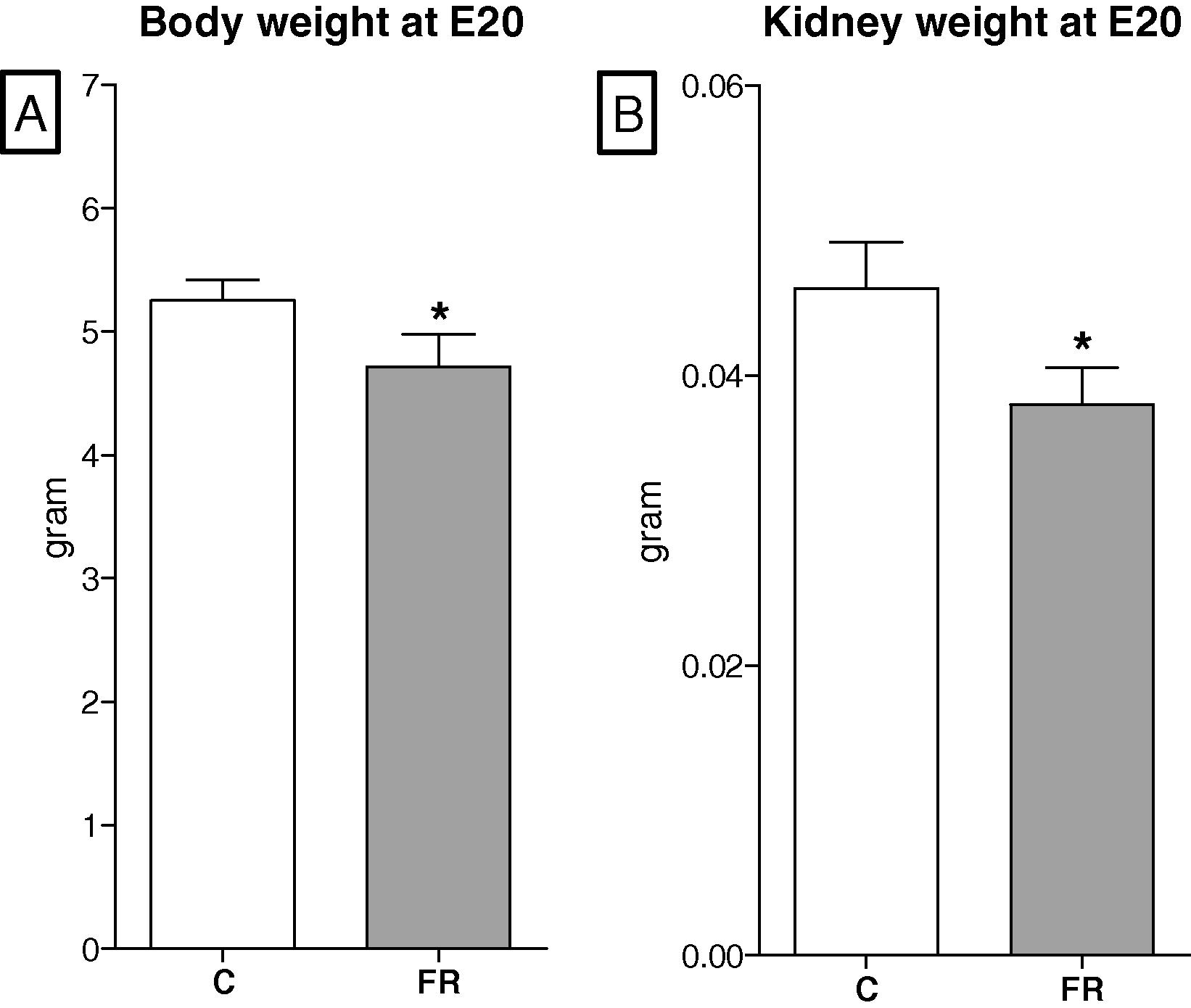
Effects of maternal food restriction on body weight (A) and kidney weight (B) at embryonic day 20. Both parameters were significantly decreased in FR fetuses (n = 8) compared to controls (n = 8).
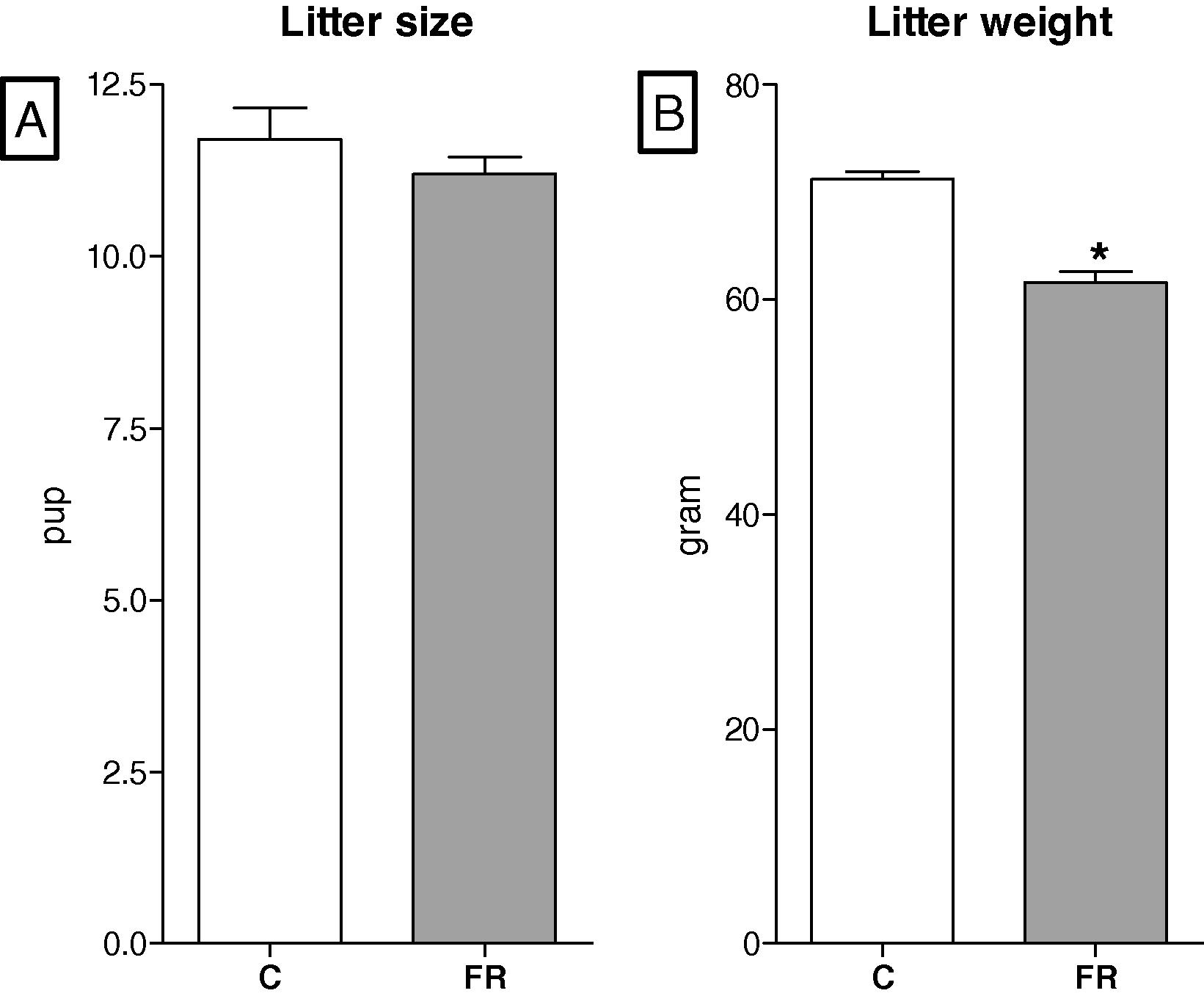
Litter size (A) was comparable between control (C, n = 8) and food-restricted groups (FR, n = 8). Litter weight (B) of FR group was significantly lower than control.
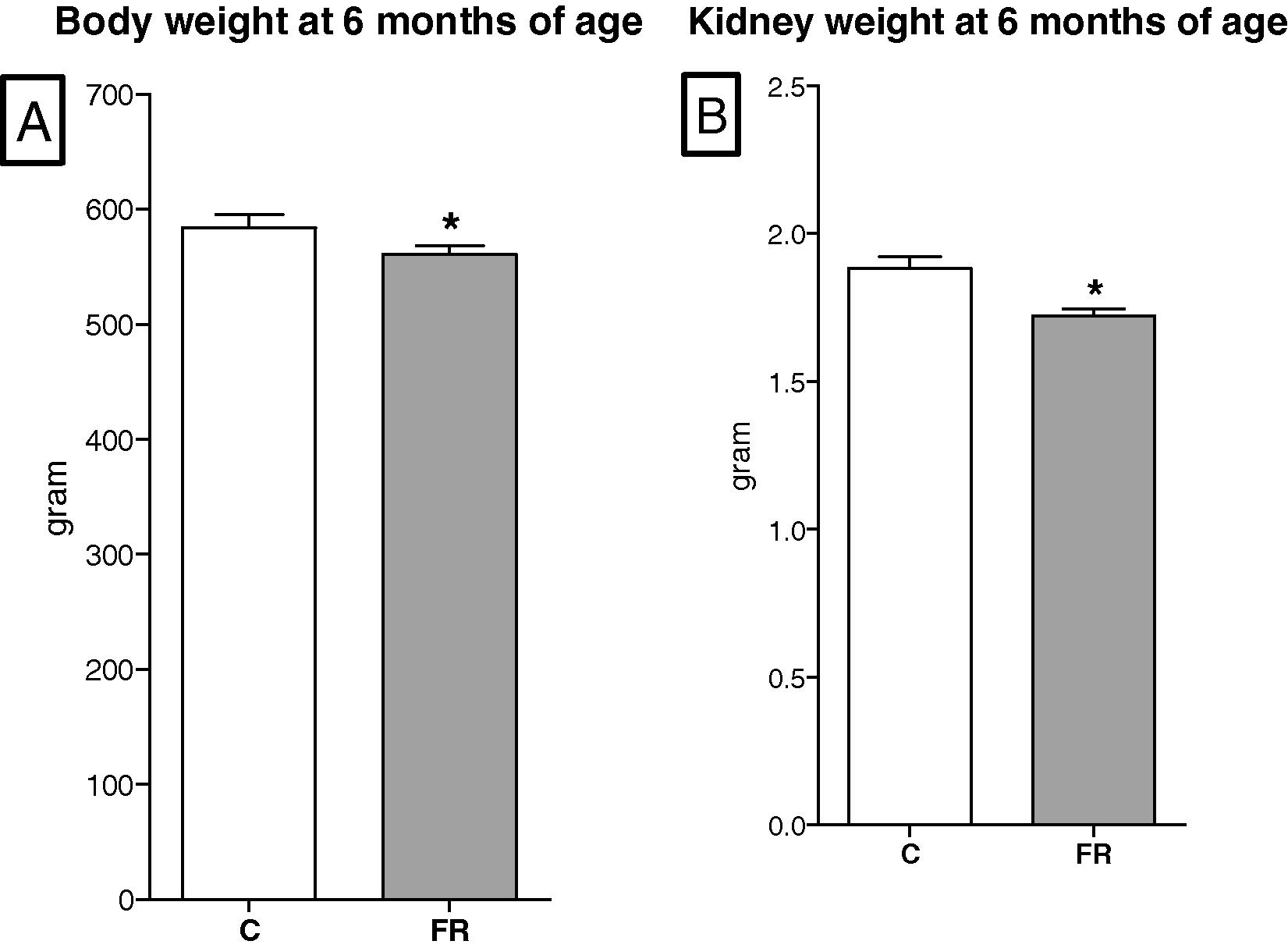
Long term effects of maternal food restriction on body weight (A) and kidney weight (B) at 6-months of age. Both parameters were smaller in the FR (n = 8) than the control offspring (n = 8).
3.2 Mean arterial blood pressure
The mean arterial blood pressure (8 per group) is shown in Fig. 5A. Blood pressure of 6-month-old FR offspring was increased by 14% compared with control offspring (C, 107.2 ± 1.24 vs FR, 124.22 ± 1.68, p < 0.05).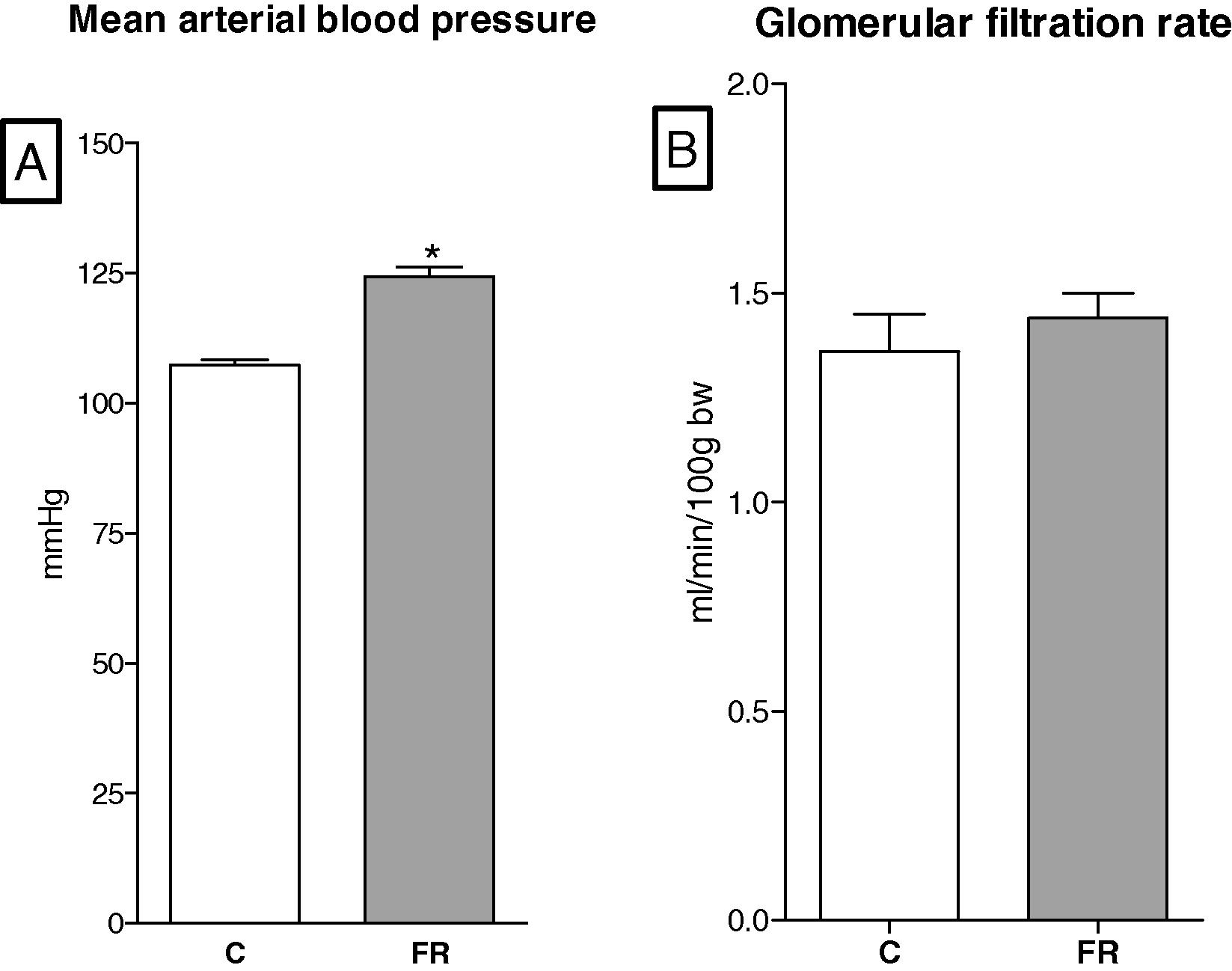
(A) Mean arterial blood pressure of food restriction rats (FR, n = 8) was significantly higher than controls (C, n = 8). (B) Glomerular filtration rate was comparable between groups in six-month-old rats.
3.3 Glomerular filtration rate
The glomerular filtration rates were comparable between the FR (n = 8) and control offspring (n = 8) at 6 months of age, Fig. 5B.
3.4 The ultrastructure of the glomerular filtration barrier
The ultrastructure of the glomerular filtration barrier was examined with different magnifications using a transmission electron microscope. In general, the development of the glomerular filtration barrier was impaired in FR fetuses (n = 8) at embryonic day 20 compared to control fetuses (n = 8). In addition, the ultrastructure of the glomerular filtration barrier in FR offspring (n = 8) was considerably altered at 6 months of age.
Fig. 6 shows segments of the glomerulus ultrastructure of control and FR offspring at embryonic day 20. The glomerular capillaries are lined by a single endothelial layer of cells. The formation of glomerular endothelial cell fenestrae (pores) had not been started at this stage in both groups. However, the formation of the podocyte foot process was present in the control fetuses at embryonic day 20 (Fig. 6A). The slits between the foot processes were open (Fig. 6B). In contrast to the control kidneys, the podocyte foot processes were less abundant in FR fetal kidneys (Fig. 6C) and some of the slits were not fully open (Fig. 6D).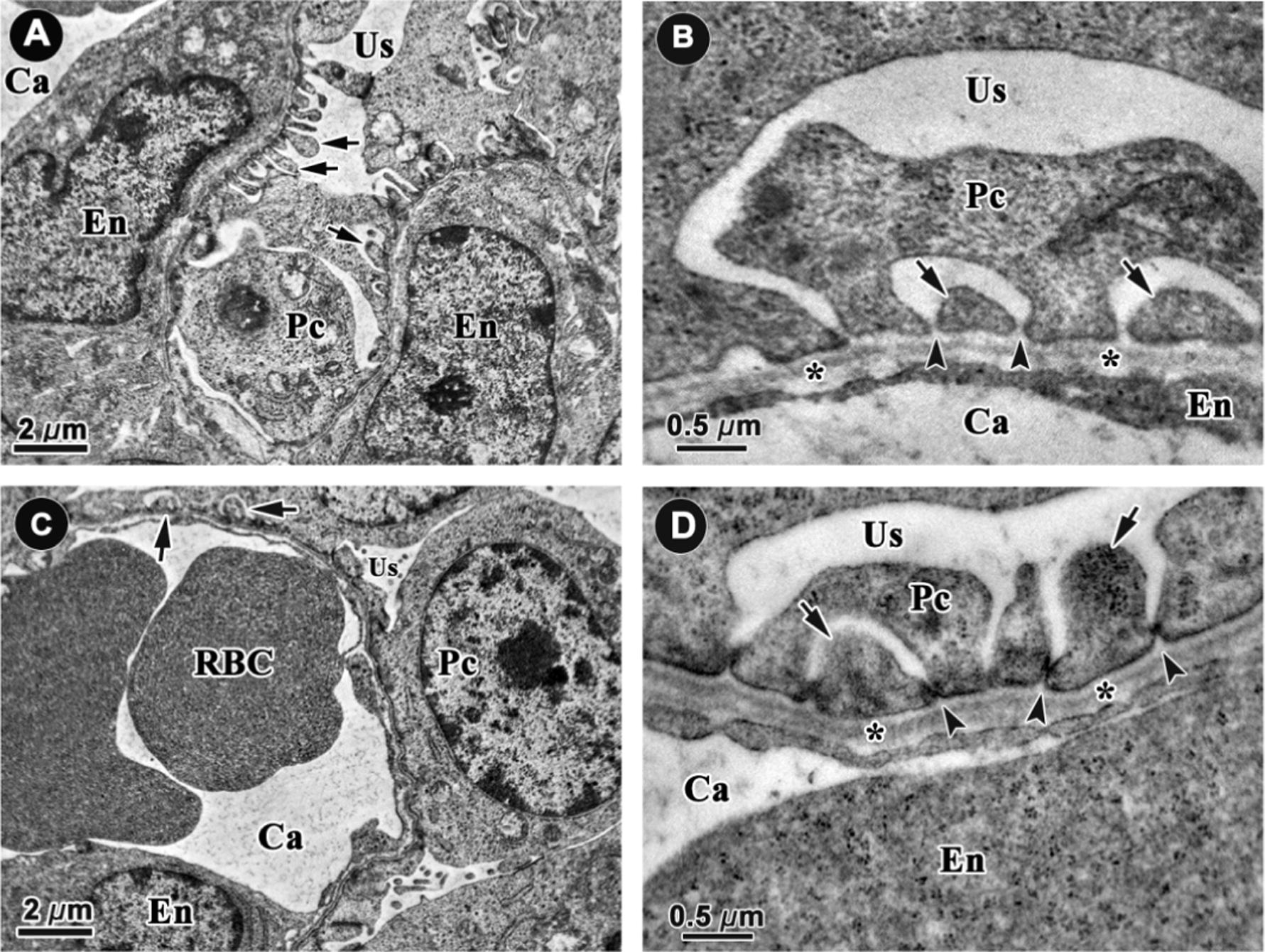
Representative electron microscope micrographs of glomerular capillaries in control (A, 6000×; B, 40,000×) and food restricted fetal kidneys (C, 6000×; D 40,000×) at embryonic day 20 (n = 8 per group). The capillaries (Ca) are lined by endothelial cells (En) which had no fenestrae (pores) at this stage in both groups. The podocyte foot processes (arrows) are present at this stage and slits (arrow heads) between them are open in control animals (A and B). The podocyte foot processes were hardly found and some of the slits were not fully open in the FR group. *: glomerular basement membrane. Pc: podocytes, RBC: red blood cell, Us: urinary space.
An examination at low magnification showed that the podocyte foot process in six-month-old rats exposed to maternal food restriction was shorter than those in the control offspring (Fig. 7A and B). A higher magnification showed that the slits were wider in the FR offspring than in the control offspring and that the glomerular basement membrane was thicker and less condensed in FR offspring compared with control offspring (Figs. 7 and 8).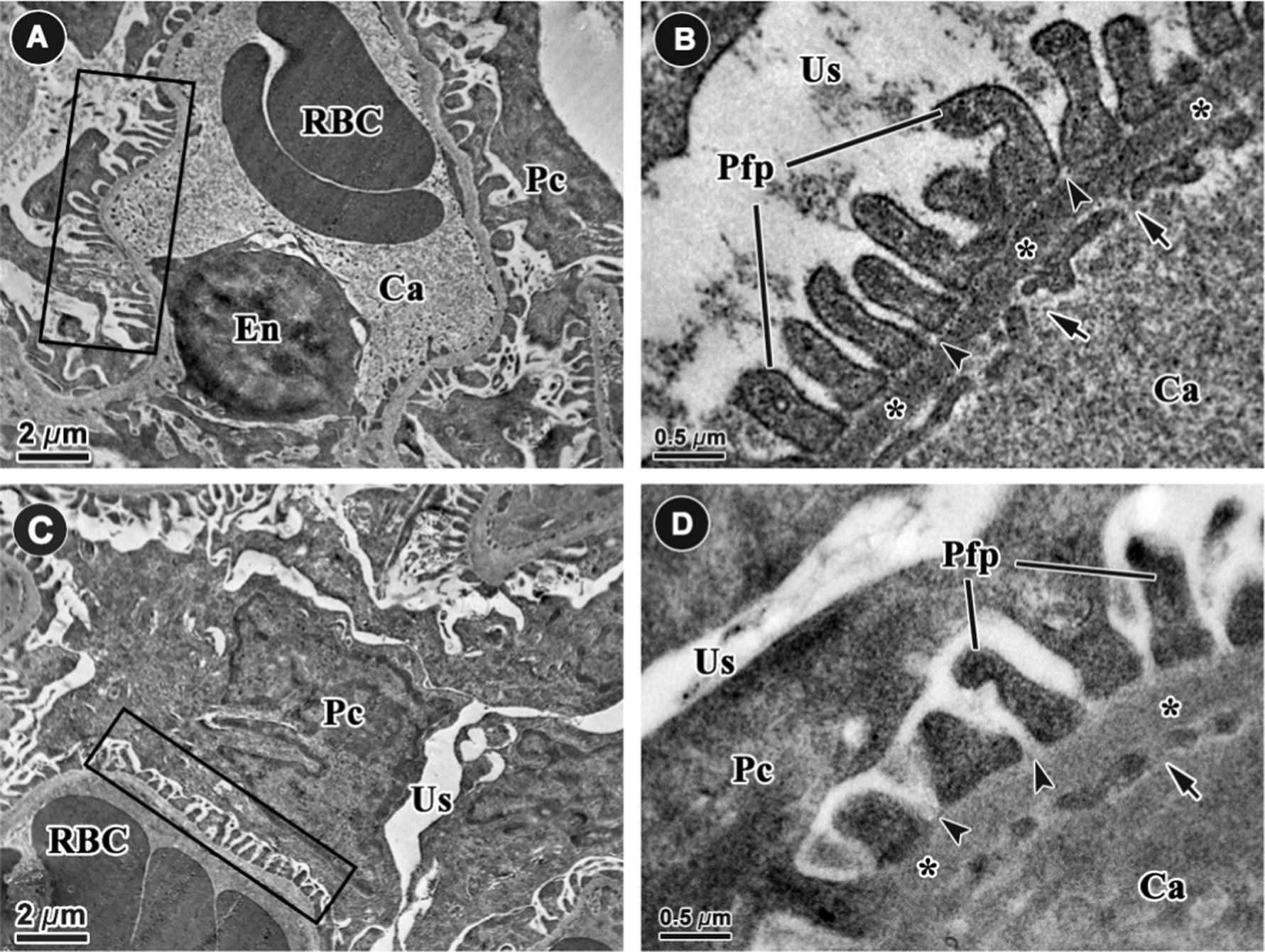
Representative electron microscope micrographs of the glomerular filtration barrier in the control (A and B) and food restricted offspring (C and D) at 6000× and 60,000× at six months of age (n = 8 per group). The podocyte foot processes (rectangles, Pfp) were shorter and thicker in FR offspring than in control offspring. The filtration slits (arrow heads) and glomerular capillary endothelial fenestrae (arrows) were wider in FR animals than controls. The thickness of the basement membrane (*) was increased in FR offspring compared to controls. Ca: capillary, En: endothelial cell, Pc: podocyte, RBC: red blood cell, Us: urinary space.
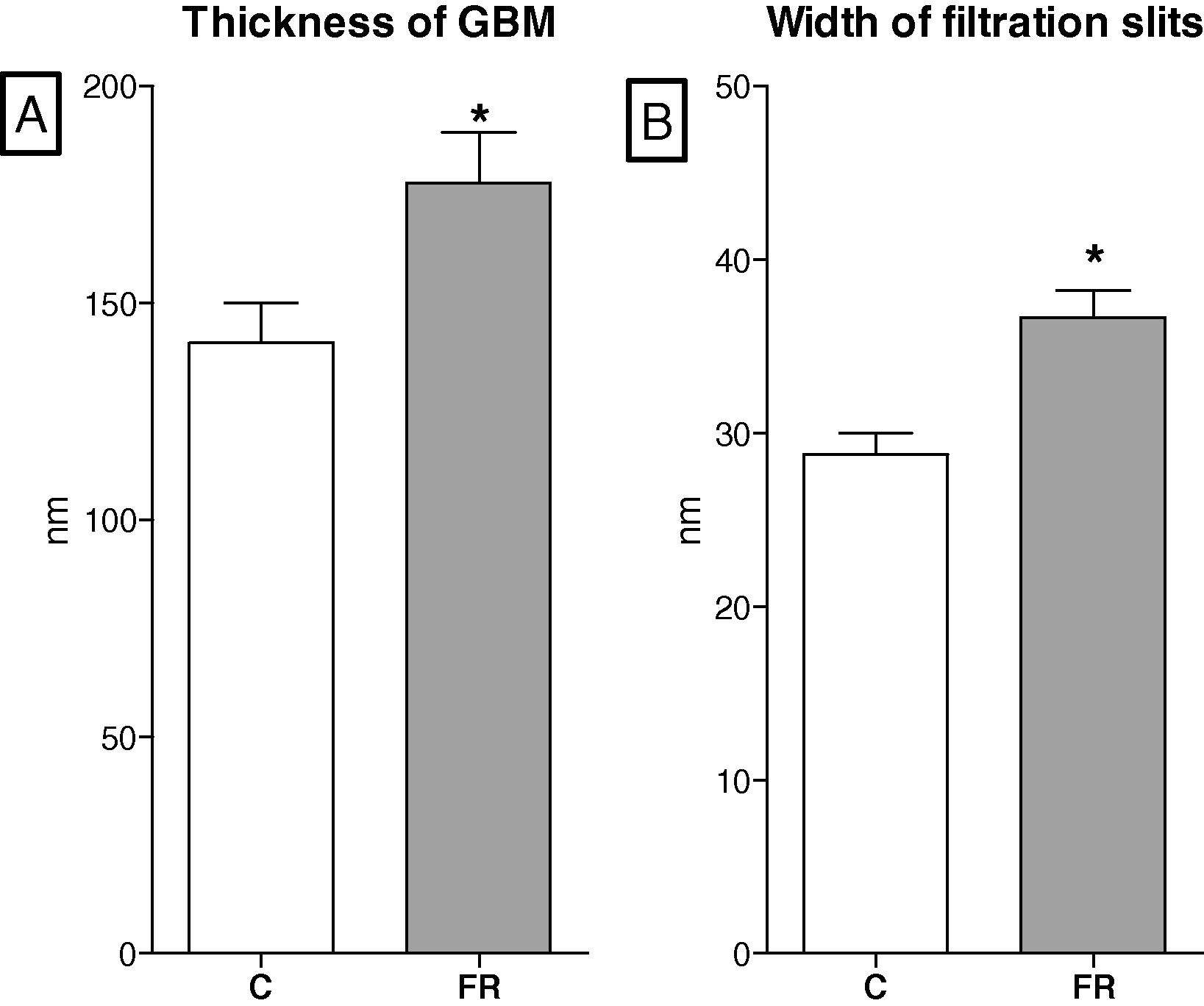
Long term effects of maternal diet on the thickness of glomerular basement membrane, GBM (A) and width of filtration slits (B) in six-month-old offspring. Maternal food restriction (FR, n = 8) increased the thickness of GBM and the width of slits in comparison to controls (C, n = 8).
4 Discussion
The current study investigated the effects of 50% maternal food restriction throughout gestation on the development of the glomerular filtration barrier at the ultrastructural level using transmission electron microscope micrograph. It also investigated the long-term effects of chronic hypertension and glomerular hyperfiltration on ultrastructure of the glomerular barrier.
The fetal programing hypothesis proposed that uterine insults stimulate the fetus to change developmental strategy in order to survive these conditions (Barker, 2004). For example, the undernourished fetus protects its brain development by diverting more blood to the brain at the expense of blood supply to other organ systems. Epidemiological studies have found a strong correlation between low birth weight and the risk of developing hypertension (Bagby, 2009). Low food intake during pregnancy and low maternal weight gain affect fetal growth and reduce birth weight (Abrams et al., 2000). In human populations, food shortages generally occur due to a complex combination of factors including natural disasters, poverty and wars. The reduction in daily food intake in such circumstances is classified into mild (30%), moderate (50%) or severe (70%). It is thought that the approach most representative of human famine is a global food restriction where nutrients are balanced but the quantity is reduced (Bertram and Hanson, 2001). In previous studies, investigators restricted pregnant rats to 30%, 50% or 70% of ad libitum to induce hypertension in adult offspring. It is important to acknowledge that food restriction can stimulate maternal stress. Evidence is available to show that the development of the major organs can be disturbed by nutritional factors and/or stress factors in fetal life (Langley-Evans, 1999).
Intrauterine growth retardation (IUGR), reflected by low birth weight, has been correlated to hypertension (Gunnarsdottir et al., 2002), diabetes (Eriksson et al., 2004), and kidney failure (Hoy et al., 2006). The present study showed that maternal weight gain was severely reduced in rats receiving 50% ad libitum food. This indicates that the nutrient supply to fetuses in the FR group may have been disturbed. The litter size in the FR group was unaffected indicating that the developmental strategy inside the rat uterus was to reduce fetal body weight rather than to reduce fetal number. Indirect evidence for this comes from the fetal weights at embryonic day 20 and also from the birth weights where both were significantly reduced in comparison to the control fetuses. Maternal weight gain in the control group was 90 g while the litter weight was 70 g. The control dams were able to added 20 g to the initial weight, perhaps as a preparation for lactation. In the FR group however, the mean gestational weight gain was 12 g and the litter weight was 62 g. This indicates that FR dams had lost 50 g of their initial weight. In such maternal food restriction, it is expected that maternal nutrient stores are catabolized to aid fetal growth (Lederman and Rosso, 1981).
Previous studies have shown that maternal malnutrition programmes hypertension in the offspring by altering kidney structure and function (Chou et al., 2008; Vaccari et al., 2015). Low birth weight has been linked to low glomerular number in humans and in animal models. In particular, a reduction in kidney mass before birth has been well correlated to a reduction in glomerular number (Hershkovitz et al., 2007). Impairment in nephrogenesis in humans and animals exposed to undernutrition in utero points to the kidney as a targeted organ.
Although it is clear that the number of glomeruli was affected by maternal malnutrition, the quality of the glomeruli that are present in the fetus has not been sufficiently investigated. The main functional part in the glomerulus is the glomerular filtration barrier which develops with the aid of capillary endothelial and podocyte cells. The effects of maternal food restriction on the development of the glomerular filtration barrier in late gestation remained unknown. Here, the ultrastructure of this barrier has been investigated at embryonic day 20. While microphotographs of control fetal kidneys showed well developed glomerular filtration barriers, micrographs of FR fetal kidneys showed a remarkable retardation in the development of this barrier. The number of podocyte foot processes was smaller and the filtration slits were either not formed or still not open. During nephrogenesis the epithelial cells, also known as podocytes, wrap around glomerular capillaries to form foot processes. A key protein involved in the formation of foot processes is α3β1 integrin (Kreidberg et al., 1996). The expression of this protein has not been measured in this model. Whether the expression of fetal α3β1 integrin has been disturbed by maternal food restriction or not, remained to be investigated.
Systematic blood pressure was significantly increased in rat offspring exposed to maternal food restriction, supporting other studies in the field of the fetal programing of hypertension (Marchand and Langley-Evans, 2001; Moritz et al., 2003; Vaccari et al., 2015). Brenner et al. (1996) suggested that low nephron numbers decrease glomerular filtration rates which increase sodium retention leading to high blood pressure. However, not all developmental programing studies found a reduction in GFR. The glomerular number in six-month-old FR rats was reduced, however, the glomerular filtration rate was similar to that of the control rats. This indicates that single glomerular filtration, a phenomenon called hyperfiltration, was increased (Brenner et al., 1996). The long-term effects of high blood pressure and glomerular hyperfiltration due to maternal food restriction on the glomerular filtration barrier are largely unknown. It has been hypothesized that chronic hypertension and long-term glomerular hyperfiltration may have negative effects on the glomerular filtration barrier. To examine these effects a transmission electron microscopic study was performed in 6 month control and FR rats. As expected, the ultrastructure study displayed remarkable changes in the glomerular filtration barrier of the FR rats compared with the controls. The thickness of the glomerular basement membrane in the FR group was significantly increased and its density was decreased compared with that in the control group. In addition, the podocyte foot processes in the FR offspring tended to be shorter and thicker. The slits between foot processes in the FR offspring were significantly wider than in the control offspring. These results are different from a previous study that found no changes in the ultrastructure of the glomerular filtration barrier (Chen and Chou, 2009). It is important to indicate that in the current study dams were food-restricted from day 0 of pregnancy until birth whereas in the Chen and Chou’s dams were food-restricted only in the last week of pregnancy. Maternal metabolism changes substantially during pregnancy. The first two weeks of gestation can be viewed as anabolic phase when an increase in the maternal nutrient stores occurs to meet placental, fetal and maternal demands of late pregnancy. The last week of gestation, however, is characterized as a catabolic phase where stored nutrients are being released to aid fetal growth (Lain and Catalano, 2007). It is thought that the maternal nutrient stores in the current study were diminished before the kidneys started to develop.
Abnormal thickness of the glomerular basement membrane was found in a patient with glomerular hematuria (Schwartz et al., 2007). The changes observed in the glomerular filtration barrier may facilitate hyperfiltration. However, it may have negative complications. Hyperfiltration is proposed to be a precursor of intraglomerular hypertension leading to albuminuria (Palatini, 2012). A further rise in albuminuria may lead, in the long run, to end-stage renal failure.
In conclusion, maternal food restriction impaired formation of the podocyte foot process in prenatal rat kidneys. The long-term exposure to hypertension and glomerular hyperfiltration may have attributed to the remarkable changes observed in the ultrastructure of the glomerular filtration barrier of old FR offspring.
Acknowledgements
The authors would like to extend his sincere appreciation to the Academic Council of King Saud University for funding this research.
References
- Prenatal programming of renal sodium handling in the rat. Clin. Sci. (Lond.). 2009;117:75-84.
- [Google Scholar]
- Developmental origins of renal disease: should nephron protection begin at birth? Clin. J. Am. Soc. Nephrol.. 2009;4:10-13.
- [Google Scholar]
- Developmental origins of adult health and disease. J. Epidemiol. Community Health. 2004;58:114-115.
- [Google Scholar]
- Animal models and programming of the metabolic syndrome. Br. Med. Bull.. 2001;60:103-121.
- [Google Scholar]
- Kinetic serum creatinine assays. 2. A critical-evaluation and review. Clin. Chem.. 1980;26:555-561.
- [Google Scholar]
- Glomeruli and blood pressure: less of one, more the other? Am. J. Hypertens.. 1988;1:335-347.
- [Google Scholar]
- The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int.. 1996;49:1774-1777.
- [Google Scholar]
- Effects of maternal undernutrition on glomerular ultrastructure in rat offspring. Pediatr. Neonatol.. 2009;50:50-53.
- [Google Scholar]
- Effects of maternal undernutrition on renal angiotensin II and chymase in hypertensive offspring. Acta Histochem.. 2008;110:497-504.
- [Google Scholar]
- Birth weight and cardiovascular risk factors in a cohort followed until 80 years of age: the study of men born in 1913. J. Intern. Med.. 2004;25:236-246.
- [Google Scholar]
- Relationship between size at birth and hypertension in a genetically homogenous population of high birth weight. J. Hypertens.. 2002;20:623-628.
- [Google Scholar]
- Fetal programming of adult kidney disease: cellular and molecular mechanisms. Clin. J. Am. Soc. Nephrol.. 2007;2:334-342.
- [Google Scholar]
- Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int.. 2006;70:104-110.
- [Google Scholar]
- Global burden of hypertension: analysis of worldwide data. Lancet. 2005;9455:217-223.
- [Google Scholar]
- Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537-3547.
- [Google Scholar]
- Maternal carbenoxolone treatment lowers birthweight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin. Sci. (Lond).. 1997;93:423-429.
- [Google Scholar]
- Effects of fasting during pregnancy on maternal and fetal weight and body composition in well-nourished and undernourished rats. J. Nutr.. 1981;111:1823-1832.
- [Google Scholar]
- Intrauterine programming of nephron number: the fetal flaw revisited. J. Nephrol.. 2001;14:327-331.
- [Google Scholar]
- Placental weight, birth measurements and blood pressure at age 8 years. Arch. Dis. Child.. 1996;74:538-541.
- [Google Scholar]
- Kidney development and the fetal programming of adult disease. BioEssays. 2003;25:212-220.
- [Google Scholar]
- Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol. Dial. Transplant.. 2012;1:1-7.
- [Google Scholar]
- Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams. J. Nutr.. 2000;130:2582-2589.
- [Google Scholar]
- Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. Br. Med. J.. 1997;315:396-400.
- [Google Scholar]
- Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol. Cell. Endocrinol.. 2001;20:93-98.
- [Google Scholar]
- Pathology of the Kidney (sixth edition). New York, USA: Lippincott Williams & Wilkins; 2007.
- Brenner & Rector’s the Kidney (tenth edition). Philadelphia, USA: Elsevier; 2016.
- Fetal kidney programming by severe food restriction: effects on structure, hormonal receptor expression and urinary sodium excretion in rats. J. Renin Angiotensin Aldosterone Syst.. 2015;16:33-46.
- [Google Scholar]
- The sex-specific effects of famine on the association between placental size and later hypertension. Placenta. 2011;32:694-698.
- [Google Scholar]
- Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab.. 2000;279:83-87.
- [Google Scholar]
- Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr. Res.. 1996;40:438-443.
- [Google Scholar]
- Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr. Res.. 2001;49:460-467.
- [Google Scholar]
- Effect of low birth weight on impaired renal development and function and hypertension in rat model. Ren. Fail.. 2012;34:754-759.
- [Google Scholar]







