Translate this page into:
Glochids microstructure and dew harvesting ability in Opuntia stricta (Cactaceae)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Liquid fresh water is scarce in arid and semi-arid regions. Some organisms in these stressful habitats are well adapted to the environmental conditions and obtain fresh water directly from the atmosphere. This ability of “moisture harvesting” relies on specific microstructural features organisms exhibit at their external surfaces. In this respect, the dew harvesting ability of the arid plant species Opuntia stricta (Cactaceae) has been investigated. Its tiny stem spines, known as glochids, have a unique microstructure, ordered in hierarchical manner with an anisotropic surface. The glochids are covered with cone-shaped barbs and have dense mats of hygroscopic trichomes at the base. Dew harvesting ability in O. stricta seems to be controlled by three driving forces, namely: Laplace pressure difference, Wenzel relation and differences in wettability. The formation of special external microstructural features supporting this ability represents an adaptive trait in O. stricta to dry arid environments. Studying such structures in detail will give insights on how biomimetic inspired devices may contribute to optimize moisture harvesting in dry regions.
Keywords
Opuntia stricta
Glochids
Microstructure
Dew harvesting ability
Scanning electron microscopy
1 Introduction
Of planet earth́s mainland, about one-third is classified as arid or semiarid (Whitford, 2002). Drought represents the prominent environmental condition of these areas, controlled largely by climatic factors, especially temperature and precipitation. In all arid or semiarid regions, annual rates of evaporation exceed that of precipitation, resulting in shortage or absence of surface fresh water. Organisms in such habitats must cope with water shortage, thus often morphological and metabolic adaptations enable them to acquire enough amounts of water (Cloudsley-Thompson, 1996; Batanouny, 2001). Most organisms obtain liquid water through absorption or imbibition, ingestion of hydrated solids, or metabolically from the food (O'Donnell and Machin, 1988). As liquid water is usually scarce in arid and semiarid habitats, many organisms inhabiting such environments are adapted to obtain water directly from the atmosphere (i.e. airborne moisture) (Henschel and Seely, 2008; Malik et al., 2014). These “moisture harvesters” include some animals like desert beetles, frogs, lizards and spiders (Parker and Lawrence, 2001; Zheng et al., 2010; Malik et al., 2014), and many plants (Ju et al., 2012; Malik et al., 2014, 2015; Azad et al., 2015; Sharma et al., 2016; Gürsoy et al., 2017).
Fog and dew are most prominent sources of airborne water that emerge from excess water vapour in the air, condensing on surfaces as water droplets. If droplets are suspended in the air and visible as shallow clouds, they are called fog (Fessehaye et al., 2014). Fog is formed through cooling of air below dew point that condenses as water drops on surfaces. Dew is formed when the humid air condenses on a surface as water drops (Beysens, 1995). In many arid and semiarid regions, fog and dew represent a constant water source depending on prevailing climatic conditions (Jacobs et al., 1999; Eckardt et al., 2013) and may even exceed the amount of annual rain during severe drought years (Evinari et al., 1971). Therefore, fog and dew play an important role in those environmentally demanding habitats.
To condensate atmospheric moisture to a dew-drop, preceding nucleation must progressively enhance. Nucleation means the formation and coalescence of minute, thermodynamically stable droplets from water vapour (Beysens, 1995). In a favorable microclimate, nucleation is largely dependent on the microstructure of the surface (Beysens, 1995; Agam and Berliner, 2006).
Important for drop formation on a solid surface is the contact angle (θc), the angle that forms at the three-phase boundary (where the liquid–vapour interface meets a solid surface (Koch and Barthlott, 2009)). When the contact angle is less than 90°, the surface is called hydrophilic, which means that wetting occurs and a drop will cover a large area on the surface. Whereas with a contact angle more than 90°, the surface is called hydrophobic with less wettability. A water drop on a hydrophobic surface tends to take a more spherical shape (Fig. 1a) and will only touch a small area on the surface. When the drop is moving on the surface, the contact angles of both sides of the drop tend to take a maximum (expanding) and a minimum (contracting) value, called advancing contact angle (θa) and receding contact angle (θr), respectively (Fig. 1b) (Yuan and Lee, 2013).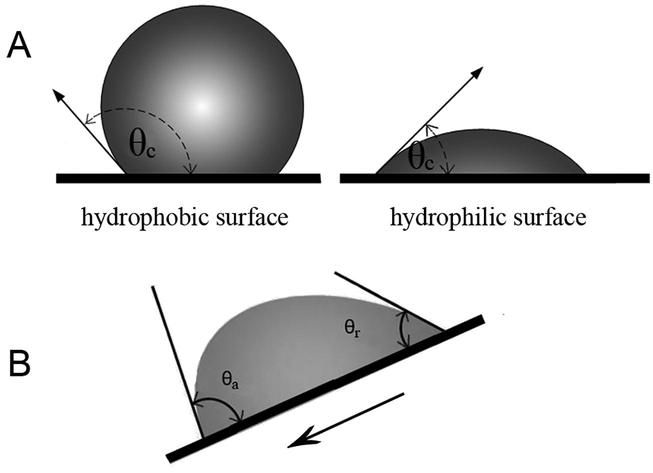
A. Drop behavior on the surface. Value of contact angle (θc) represents the measure of hydrophobicity or hydrophilicity of the surface. B. In the state of drop motion, the contact angles of both sides tend to take a maximum and minimum value, called advanced contact angle (θa) and receding contact angle (θr), respectively.
Few cacti inhabiting arid and semiarid regions are known to collect fog and dew with their spines or glochids (Mooney et al., 1977; Ju et al., 2012; Liu et al., 2015; Malik et al., 2015). Although there are hundreds of cacti species, their spines, and especially glochids vary greatly in surface microstructure (Gibson and Nobel, 1986; Mosco, 2009; Liu et al., 2015). Since dew condensation on a surface is controlled by air temperature, humidity and the surface microstructure (Beysens, 1995), spine microstructure determines the ability of a cactus species for effective dew harvesting under a suitable microclimate (Malik et al., 2015; Guo and Tang, 2015). To date, only a few cacti species have been confirmed to be efficient moisture harvesters (Malik et al., 2014, 2015; Guo and Tang, 2015).
Opuntia stricta (Haw.) Haw. is a spiny stem succulent of the Cactaceae family, native to southeast America, eastern Mexico and Caribbean islands, and is invasive in other regions including south and east Africa, south-western Saudi Arabia, Yemen, India and Australia (Masrahi, 2012; Masrahi and Sayed, 2017; Shackleton et al., 2017). So far, no studies describe this species as an atmospheric moisture harvester. Therefore, this study aims to investigate the microstructure of O. stricta glochids in relation to its dew harvesting ability. For this, SEM imaging was used to characterize microstructural features of glochid. Mist stream generator was used to simulate dew formation events on the glochids in the field. Glochid microstructural features and dew harvesting ability have been discussed with the applicable physical principles.
2 Materials and methods
2.1 Plant materials
The stem of O. stricta consists of segments. Every segment has scattered bud areas (areoles) bearing spines (2–3, each 10–25 mm) and many glochids (tiny spines) with dense minute trichomes at the base (Fig. 2a,b, Fig. 4a). Samples of stem segments were collected in May 2018 from rocky habitats east of Tihama of Jazan region (Saudi Arabia), at an altitude of 300–400 m, in which the plant invaded vast areas (Masrahi and Sayed, 2017). The areoles bearing spines and glochids were carefully selected for subsequent analyses. The spines of this cactus species are almost glabrous without any prominent structures on their surfaces, and did not show efficient dew collection ability. Thus, they were ignored in this study.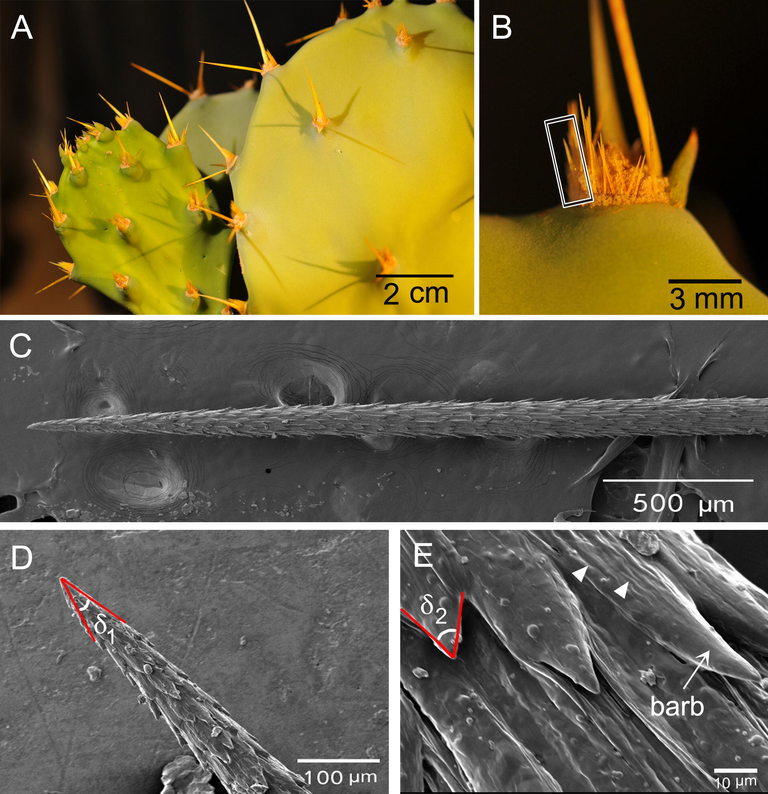
A. Stem segments of Opuntia stricta that bearing bud areas (areoles) with spines and glochids. B. One areole with its spines and glochids (one glochid enclosed by rectangle). C. SEM image of a single glochid with oriented barbs on the surface. D and E. Apex angle for glochid tip (δ1) and barb (δ2), respectively. In E, it's clear that tip of each barb is smooth whereas the base is rougher with microgrooves (arrowheads).
2.2 SEM imaging
Glochids and trichomes were examined with a scanning electron microscope (JSM-IT300 - Jeol, Japan) to investigate their microstructure in details. Dried glochids and trichomes were mounted on the stub with double side carbon tap, sputter-coated with gold and examined under high vacuum with an accelerating voltage of 5 keV using the secondary electron detector. Measurement of the microstructural features were conducted directly from SEM images.
2.3 Dew harvesting experiment
From the segment, one areole with dry spines and glochids was selected and put in front of a purifying mist stream. The stream was generated by a cold mist humidifier (BLACK + DECKER HM3000), at a distance of 15 cm from the mist outlet. Flow of the mist stream was adjusted by a control dial to low flow rate of ∼1 ml/h (lower than in other experiments, in which flow rate was 2–7 times higher (Azad et al., 2015; Sharma et al., 2016). The speed flow of the mist stream was ∼0.7 m s−1. This low flow rate was chosen to mimic realistic environmental conditions for dewy nights, and to obtain the efficiency of dew harvesting with higher precision. Three sets of observations of the dew harvesting ability were performed on three areoles, each for 1 min. and immediately photographed with a digital camera adjusted to a stereomicroscope (SONY FD Mavica 2.0 MP). The ability for dew harvesting is visualized by the ability of the glochids to capture tiny droplets, and gradual growth of the droplets to bigger drops, moving to the base of the glochids. The contact angle (θa) of droplets was calculated by direct optical method (Yuan and Lee, 2013) using a protractor on the enlarged images.
3 Results
3.1 Glochids surface microstructure
Fig. 2 illustrates the general habit of areoles bearing spines and glochids on the stem segments. Every areole has ∼80 glochids, each around 5 mm in length, and a diameter (in the middle portion) of 123.6 ± 5.1 µm (Table 1). Fig. 2c–e shows the glochids with more microstructural details of tip and surface. The surface of glochids is covered with oriented retrorse barbs, whereby grooves appear to be more prominent from the middle portion on until the base of the glochid. The tip of each barb is almost smooth with microgrooves on the base making the surface of the base rougher than the tip (Fig. 2e). The glochid has a conical shape with an apex angle (δ1) of 9.25 ± 2°, whereas the apex angle of the barb (δ2) is = 41.5 ± 8.6° (Fig. 2d, e; Table 1). A dense mat of trichomes is located on the areoles at the base of glochids and spines, whereby each trichome is a ribbon-like structure (Fig. 4a).
Character
Value
no. of spine
2–3
no. of glochids
79.7 ± 7.2
length of glochid (mm)
4.9 ± 0.8
diameter of glochid (in the middle portion, µm)
123.6 ± 5.1
cone-apex angle (δ1) of the glochid
9.25° ± 2
cone-apex angle (δ2) of the barb
41.5° ± 8.6
θa of growing droplet on the glochid
76.25 ± 16.8°
θr of growing droplet on the glochid
52.5 ± 12.6°
3.2 Dew harvesting observations
After one minute, glochids located in front of the mist stream show deposited water droplets on their surfaces. A progressive manner of droplet growth is observed at the glochids, whereby in the first step droplets nucleate at the tip of the barbs and also on the glochid tips (Fig. 3). Then small droplets move from the tip to the base of the glochid and coalesce together to form bigger droplets. The bigger droplets of ∼130 µm diameter start to move down to the glochid base (Fig. 3a–c). Analyses reveal a big advancing contact angle (θa) and relatively small receding contact angle (θr) (76.25 ± 16.8° and 52.5 ± 12.6°, respectively) for the “moving” droplets. Although most glochids in the experiment are vertical or semi-vertical, droplet deposition also happens at glochids with semi-horizontal orientation (tilt angle ∼ 0). The mean diameter of growing droplets that start to move towards the base of the glochids is = 131.25 ± 40.3 µm, which is appears as a critical size for droplet moving.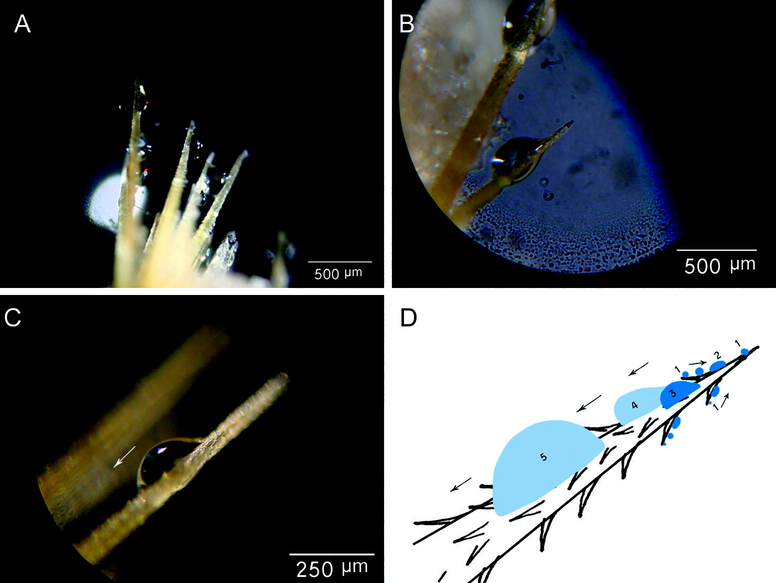
A-C. Optical microscopic images of water collection on the glochids. Each glochid reveals a progressive manner of droplets growing, which appeared to start at the tip of the barbs and also on glochid tips. D. Diagram of starting deposition of droplets and the gradually growing (coalesce with each other) to become a large drop moving to the base.
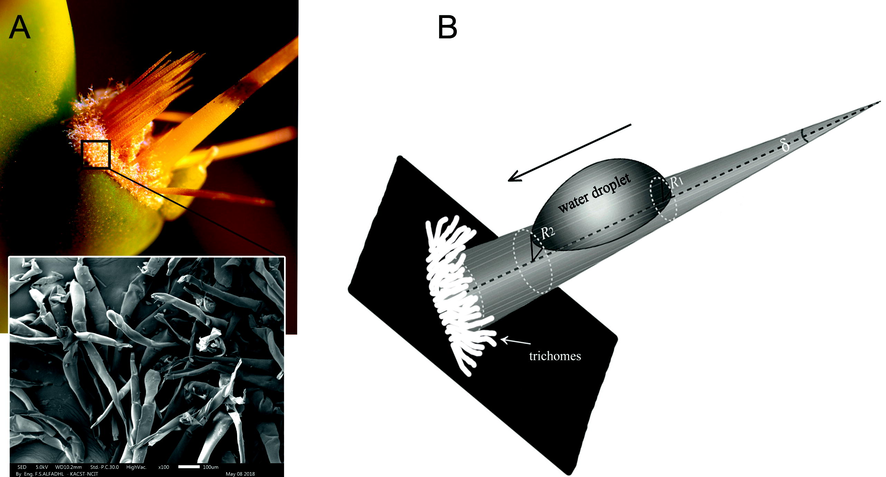
A. Optical image of areole with its glochids and dense mat of trichomes in the base. The insertion is SEM image of trichomes. B. Geometry of barb and glochid (cone-shape). In the cone shape, the difference between small radius (R1) and large radius (R2) generate Laplace pressure gradient that drives the drop to the base.
4 Discussion
The glochids of O. stricta have a unique microstructure ordered in a hierarchical manner, and organized into substructures that contribute to dew harvesting ability. The glochids and barbs covering them have a conical shape in the range of cone-apex angles of 9.25–41.5°. The conical shape of such morphological features produce a Laplace pressure gradient on the surface (Lorenceau and Quéré, 2004; Ma et al., 2015; Masrahi and Al Shaye, 2017). The tip of the cone (tip of the glochid or barb) has a larger Laplace pressure than the base of the cone (base of the glochid or barb). This difference is generated by the small radius-high curvature at the tip of the cone to the large radius-low curvature at the base of the cone (Fig. 4b), which can expressed by the Laplace theorem (Eggers and Villermaux, 2008):where R1 and R2 are the radii of curvature, γ is the surface tension of water droplet. The Laplace pressure gradient along the cone, from the tip to the base, and hence between the two sides of the droplet, represent one of the driving forces that lead to a spontaneous movement of the droplet from the tip of the glochid (and tip of the barb) to the base, but only when a critical size of ∼130 µm of the droplet is reached.
The glochid exhibits more prominent grooves towards the base. The same surface features also appear on the barbs, in which microgrooves increase toward the base. This increasing “roughness” of the surfaces of glochids and barbs from the tip to the base, may generate another driving force for droplet movement from the tip to the base of cone structures, according to the Wenzel relation (Quéré, 2008; Zhao et al., 2014; Guo and Tang, 2015):where r is surface roughness, θ* and θ are the apparent and intrinsic contact angles on rough and smooth surfaces, respectively.
In the principle of surface energy gradient, water droplets tend to be driven along a gradient of wettability, from low surface energy (less wettable) to high surface energy (more wettable) (Brochard, 1989; Chaudhury and Whitesides, 1992). In general, spines and glochids of plants from the family of Cactaceae have a similar character. They are waterproof due to cuticle and sclerified cells (Gibson and Nobel, 1986), while the trichomes in the base of the glochids have high wettability (Kim et al., 2017). The tips of glochids and barbs have a low surface energy, compared to the hygroscopic trichomes at the base of glochids that have high wettability. This gradient of surface energy seems to be the third driving force for the droplet movement, especially when droplets move and coalesce with each other to become a larger drop that gets absorbed by the highly wettable trichomes.
The experimental observations reveal the mechanisms behind dew harvesting of O. stricta enabled by a specialized microstructure of its glochids and barbs with anisotropic surfaces. Under favorable conditions, water molecules are captured as very small droplets on the tip of the cone structure (Cao et al., 2014). At the glochids and barbs, once the droplet is captured by the tip, it moves to the base, and all droplets from the barbs move by the same manner and coalesce together forming larger droplets, progressively forming larger drops that move towards the base where the trichomes are located (Fig. 3d). The trichomes then absorb the drops (Fig. 4). A big advancing contact angle (θa) and a small receding contact angle (θr) result in a “movement” of the droplets (Yuan and Lee, 2013). The droplet movement proceeds always towards the base of the glochids, even if the tilt angle is semi-horizontal. The mean volume of the growing droplets on the glochids is small (131 µm). When the droplet volume is small, the surface energy overcomes the gravitational force, and vice versa if the droplet volume is too large (>500 µm) (Ren et al., 2010; Chou et al., 2011). This range in size of growing droplets is preferential if the glochids was down or semi-down vertical in direction.
5 Conclusion
The dew harvesting ability of the glochids in Opuntia stricta was investigated and can be attributed to the microstructure of glochids with a hierarchical manner and an anisotropic surface. The water droplet is nucleating on the tip of the cone structure (tip of the glochid and tips of the barbs on the glochid surface) and starts to move towards the base. Tiny droplets coalesce with each other, becoming larger drops and become absorbed by trichomes at the base of glochids. This ability of dew harvesting appears to be controlled by three forces, Laplace pressure difference, Wenzel relation and differences in surface wettability. Ability of dew harvesting in O. stricta represents an adaptive trait in this plant that inhabits mostly arid land. On the other hand, the ability of dew harvesting in O. stricta with its glochid microstructure may act as template for biomimetic inspired designs and contribute to solve the problem of water shortage in dry regions.
Acknowledgement
The author is grateful to Mr. Fadhl Alfadhl, King Abdulaziz City for Science and Technology (KACST), Riyadh, for assistance in SEM.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dew formation and water vapor adsorption in semi-arid environments—A review. J. Ard. Environ.. 2006;65:572-590.
- [Google Scholar]
- Hierarchical surface architecture of plants as an inspiration for biomimetic fog collectors. Langmuir. 2015;31:13172-13179.
- [Google Scholar]
- Plants in the Deserts of the Middle East. Berlin: Springer; 2001.
- Motions of droplets on solid surfaces induced by chemical or thermal gradients. Langmuir. 1989;5:432-438.
- [Google Scholar]
- Facile and large-scale fabrication of a cactus-inspired continuous fog collector. Adv. Funct. Mater.. 2014;24:3235-3240.
- [Google Scholar]
- Equilibrium phase diagram of drop-on-fiber: coexistent states and gravity effect. Langmuir. 2011;27:3685-3692.
- [Google Scholar]
- Biotic Interactions in Arid Lands. Berlin: Springer-Verlag; 1996.
- The nature of moisture at Gobabeb, in the central Namib desert. J. Arid. Environ.. 2013;93:7-19.
- [Google Scholar]
- The Negeve, The Challenge of a Desert. Cambridge: Harvard University Press; 1971.
- The Cactus Primer. London: Harvard University Press; 1986.
- Experimental study on directional motion of a single droplet on cactus spines. Inter. J. Heat Mass Trans.. 2015;84:198-202.
- [Google Scholar]
- Bioinspired asymmetric-anisotropic (directional) fog harvesting based on the arid climate plant Eremopyrum orientale. Colloids Surf. A. 2017;529:959-965.
- [Google Scholar]
- Ecophysiology of atmospheric moisture in the Namib desert. Atmos. Res.. 2008;87:362-368.
- [Google Scholar]
- Dew deposition and drying in a desert system: a simple simulation model. J. Arid Environ.. 1999;42:211-222.
- [Google Scholar]
- A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun.. 2012;3:1247.
- [Google Scholar]
- Hydraulic strategy of Cactus trichome for absorption and storage of water under arid environment. Front. Plant Sci.. 2017;8:1777.
- [Google Scholar]
- Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Phil. Trans. R. Soc. A. 2009;367:1487-1509.
- [Google Scholar]
- Effective directional self-gathering of drops on spine of cactus with splayed capillary arrays. Sci. Rep.. 2015;5:17757.
- [Google Scholar]
- Bio-inspired humidity responsive switch for directional water droplet delivery. J. Mater. Chem. A. 2015;A3:15540-15545.
- [Google Scholar]
- Nature's moisture harvesters: a comparative review. Bioinspir. Biomim.. 2014;9:031002
- [Google Scholar]
- Dew harvesting efficiency of four species of cacti. Bioinspir. Biomim.. 2015;10:036005
- [Google Scholar]
- The Illustrated Guide of Wild Plants in Jazan Region. Jeddah: Sarawat Printers & Publishers; 2012.
- Microstructure of hygroscopic awns in three poaceae species. Int. Res. J. Plant Sci.. 2017;8(1):1-8.
- [Google Scholar]
- Invasive Opuntia stricta: case study in southwestern Saudi Arabia. Eco. Env. Cons.. 2017;23(4):2038-2043.
- [Google Scholar]
- Environmental adaptations of the Atacaman Desert cactus Copiapoa haseltoniana. Flora. 1977;166:117-124.
- [Google Scholar]
- Micro-morphology and anatomy of Turbinicarpus (Cactaceae) spines. Rev. Mex. Biodivers.. 2009;80:119-128.
- [Google Scholar]
- Water vapor absorption by terrestrial organisms. In: Gilles R., ed. Advances in Comparative and Environmental Physiology. Vol vol. 2. Berlin: Springer-Verlag; 1988. p. :47-90.
- [Google Scholar]
- Effects of gravity on the shape of liquid droplets. Opt. Commun.. 2010;283:3255-3258.
- [Google Scholar]
- Distribution and socio-ecological impacts of the invasive alien cactus Opuntia stricta in eastern Africa. Biol. Invasions. 2017;19:2427-2441.
- [Google Scholar]
- Investigations on the fog harvesting mechanism of Bermuda grass (Cynodon dactylon) Flora. 2016;244:59-65.
- [Google Scholar]
- Ecology of Desert Systems. San Diego: Academic Press; 2002.
- Contact angle and wetting properties. In: Bracco G., Holst B., eds. Surface Science Techniques. Berlin: Springer-Verlag; 2013. p. :3-34.
- [Google Scholar]
- Wetting failure of hydrophilic surfaces promoted by surface roughness. Sci. Rep.. 2014;4:5376.
- [Google Scholar]







