Translate this page into:
Ginsenoside-Rg5 inhibits growth and metastasis of ovarian carcinoma via suppressing expression of fibroblast growth factor-8b (FGF8b)
⁎Corresponding author. lihongyu0929@sina.com (Hongyu Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ginsenoside-Rg5, a protopanaxadiol ginsenoside originates from ginseng, is known for its anti-cancer activities. This study aimed to explore the underlying mechanism of ginsenoside-Rg5 on ovarian carcinoma. We found that 10 µM–50 µM of ginsenoside-Rg5 could reduce viability, adhesive capacity and migration potential of ovarian cancer OCI-P9a culture cells in a dose dependent manner. Treatment of ovarian cancer mice using 25 mg/kg of ginsenoside-Rg5 for one month could significantly reduce tumor volume (165.5 ± 29.6 mm3 in treated group versus 979.2 ± 134.5 mm3 in control group). In contrast to control mice, no tumor metastasis was seen in ginsenoside-Rg5 treated mice. OCI-P9a cells were found to have a high level of fibroblast growth factor-8 (FGF8b) expression, and ginsenoside-Rg5 could significantly reduce the expression of FGF8b in these cells. Therefore, anti-cancer and anti-metastasis effects of ginsenoside-Rg5 may be related with FGF8b-associated pathways. This ginsenoside from ginseng can be a good therapy candidate for ovary cancer.
Keywords
Ginsenoside-Rg5
Fibroblast growth factor
Invasion
Metastasis
Tumor growth
Inhibition
1 Introduction
Ovarian cancer is one of the most common gynecologic cancers with high mortality rate. Studies have shown that its incidence may be associated with factors such as age, family history, hormone replacement therapy and obesity. Despite increasing knowledge of the risk factors involved, ovarian cancer remains as one of the fatal cancers for women worldwide due to limited access to early diagnosis and effective treatment (Bertone-Johnson, 2005). Clinically, advanced ovarian cancer is commonly associated with distant organic metastasis, such as lung, peritoneum, spleen, and liver (Jemal et al., 2011). Therefore, it is of urgent need to explore for new effective therapies for ovary cancer. Ginseng is a traditional Chinese medicine used for treatment of several diseases, including cancer (Kwon et al., 2001). The major constituent of ginseng, ginsenoside-Rg5 (Liang et al., 2015; Kim and Kim, 2015; Cho et al., 2015) is considered to play critical anti-cancer and anti-inflammation roles (Kim et al., 2007; Yun et al., 2001; Kang et al., 2007). Ginsenoside-Rg5, a member of protopanaxadiol ginsenosides, has been proven to exhibit a broad range of biological activities, such as anti-cancer (Nag et al., 2012; Lee et al., 1997), anti-dermatitic (Shin et al., 2006), anti-inflammatory, and neuroprotective effects (Kim et al., 2012, 2013). Although ginsenoside-Rg5 demonstrated some anti-tumor and anti-angiogenesis activities in previous studies (Nag et al., 2012; Lee et al., 1997), its effect on growth and metastasis of ovarian cancer remains generally unclear. In this study, we extensively revealed the effect of ginsenoside-Rg5 on development of ovarian cancer as well as the potential molecular target or pathway involved.
Fibroblast growth factors (FGFs) is a large signaling molecular family involved in cell patterning, mobilization, differentiation, and proliferation (David et al., 2015). FGF8 promotes tumor growth and metastasis and higher expression of FGF8b is usually associated with tumor formation and angiogenesis, including ovarian cancer (Mattila and Harkonen, 2007; Schwertfeger, 2009). Inhibition of FGF8b has been proven to be an appropriate strategy to reduce tumor growth and metastasis (Mattila and Harkonen, 2007; Hsieh et al., 2007). In the current study, the effect of ginsenoside-Rg5 on proliferation and metastasis of ovarian cancer was assessed in vitro in OCI-P9a cell line and in vivo in nude mice transplanted tumor model. Based on these investigations, we hope to provide some new insights for better understanding of mechanism and clinical treatment of ovarian carcinoma.
2 Materials and methods
2.1 Cells and chemicals
Ovarian carcinoma cell line OCI-P9a was purchased from ATCC and maintained in Dulbecco's-modified Eagle's medium (DMEM, Gibco, Waltham, MA, USA) contained 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA) and penicillin and streptomycin, in a 37 °C humidified incubator at 5% CO2 atmosphere. Ginsenoside-Rg5 was provided by the School of Life Sciences, Zhengzhou University. Extraction and preparation process of ginsenoside-Rg5 was carried out according to previous literature (Guo et al., 2017). Stock solution of ginsenoside-Rg5 was prepared by dissolving in dimethyl sulphoxide (DMSO) and stored at 10 °C.
2.2 Animals
Nude mice were obtained from the Shanghai Laboratory Animal Center (Shanghai, China) and raised in specific pathogen-free and temperature-controlled environment with 12 light/dark cycles in the Second Military Medical University. The animal experimental protocols obtained approval from the Committee of Experimental Animal Administration of the Second Military Medical University Laboratory (Shanghai, China).
2.3 MTT proliferation assay
MTT cell proliferation kit (Roche Applied Science, Beijing Puboxin Biotechnology Co., Beijing, China) was used to determine viability of OCI-P9a cells. Briefly, cells were seeded into 96-well microplate (2.0 × 105 per well) and cultured for 24 h. Ginsenoside-Rg5 of various concentrations (10 µM–50 µM) were added to different well. Subsequently, MTT solution (10 µl) was added to each well and the plate was incubated for another 4 h. DMEM was briefly discarded, followed by DMSO addition (150 µl/well). The plate was then incubated in a shaker at 37 °C for 15 min. Absorbance at 490 nm of each well was measured for three times using 3550-UV microplate reader (Bio-Rad, USA).
2.4 Adhesion assay
Matrigel (BD Biosciences, Shanghai, China) coated 96-well plates were blocked by using 1% BSA for one hour at 37 °C. After this, OCI-P9a cells treated with ginsenoside-Rg5 (2.5 × 105 per well) were transferred into the plates. One hour later, non-adherent cells in the plates were rinsed using cold PBS buffer and number of adherent cells were calculated with the aid of a light microscope.
2.5 Cell invasion
After 24 h of culture, OCI-P9a cells were harvested and re-suspended in serum-free DMEM at concentration of 2 × 106 per ml. Then, cells were seeded into the upper Matrigel coated compartment of Boyden chamber (Millicell, Millipore, Burlington, MA, USA) after treatment with various concentrations of ginsenoside-Rg5. DMEM medium with 20% FBS was added into the lower chamber. Following this, cells were cultured at 37 °C for one day. The cells were then fixed using methyl alcohol followed by hematoxylin-eosin (HE) staining. The cells were analyzed using a light microscopy.
2.6 Cell migration
The effect of ginsenoside-Rg5 on migration potential of OCI-P9a cells was assessed by using wound healing assay. Briefly, 2 × 105 cells/well were seeded into six-well plates and treated with ginsenoside-Rg5 for one day. Subsequently, cells were scratched using plastic scrapper and wells were rinsed twice using PBS to remove separated cells, the plate was then subjected to normal incubation. Decrease in diameter of scrapped cell zone was measured as for index of migration ability assessment.
2.7 Real-time PCR
After treatment with ginsenoside-Rg5 for 24 h, OCI-P9a cells were collected for RNA isolation using MirVana miRNA isolation kit (Ambion, through Shanghai Baili Biotechnology Co.). After quantification with NanoDrop ND-1000 spectrophotometer (Meigu Molecular Instruments (Shanghai) Co., Shanghai, China), reverse transcription assay was performed using TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems, Santa Clara, CA). Real-time PCR was carried out using TaqMan MicroRNA assay kit and ABI 7500 real time PCR system.
2.8 Preparation of mice model
Nude mice were administered with 2.5 × 105 OCI-P9a cells into dorsal portion of body. After cancerous mass formed on the back of the mice, the masses were extracted, dissected into thin sections and transplanted into right ovaries of the mice under anesthetization. The mice were then transferred to sterilized cages and gavaged with 25 mg/kg of ginsenoside-Rg5 daily for 30 days. Control mice received equal volume of saline. On Day 31, mice were sacrificed and their tissue samples including lungs, liver, spleen, peritonea, and ovaries were collected for subsequent analyses. Tumor volume was measured and the number of metastasis sites were counted. The extracted tissues were subjected to formalin fixing, embedded into paraffin and subsequently sectioned into 2 µm sections. The sections were de-paraffined by boiling in xylene and then stained using H&E.
2.9 Statistical analysis
Data in this study was analyzed using SPSS software (version 17.0; SPSS, Inc., Chicago, IL). Data analysis was also carried out by univariate analysis of variance. Data were presented in the form of mean ± standard deviation and significant difference was considered if P-value < 0.05.
3 Results
3.1 Ginsenoside-Rg5 suppresses growth and proliferation of OCI-P9a cells
To reveal the effect of ginsenoside-Rg5 on ovarian cancer, OCI-P9a cells were treated with indicated concentrations of ginsenoside-Rg5 and viability of the OCI-P9a cells was then assessed at 24 h and 48 h by MMT assay. Results revealed that ginsenoside-Rg5 could significantly inhibit viability of OCI-P9a cells in a dose-dependent manner at both 24 h and 48 h. Specifically, 10 µM–50 µM of ginsenoside-Rg5 could reduce cell viability by the range from 96% to 37% after 24 h (Fig. 1).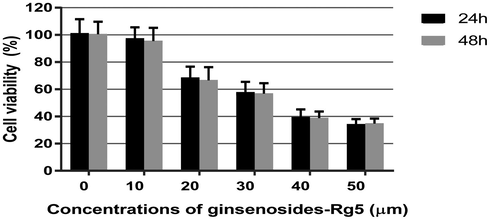
Effect of ginsenoside-Rg5 on viability of OCI-P9a cells. OCI-P9a cells were treated with ginsenoside-Rg5 of indicated concentration for 24 h and cell viability was analyzed by MTT assay. The experiment was performed in triplicates.
3.2 Ginsenoside-Rg5 reduces cell adhesion, invasion and migration
Effect of ginsenoside-Rg5 on cell properties of OCI-P9a was also determined using Matrigel, Matrigel coated-transwell and wound healing assays. The Matrigel assay showed that ginsenoside-Rg5 treatment could significantly reduce adhesion ability of OCI-P9a cells in a dose-dependent manner (P < 0.002; Fig. 2A). Specifically, adhesive ability of OCI-P9a cells reduced by 45% and 63% when treated with 30 µM and 50 µM of ginsenoside-Rg5 respectively. Moreover, the effect of ginsenoside-Rg5 on invasion of OCI-P9a cells was also determined in this study. Invasion assay showed that ginsenoside-Rg5 could significantly reduce invasion potential of OCI-P9a cells in a dose dependent manner (Fig. 2B). Furthermore, migration ability of OCT-P9a cells was determined by wound healing assay after treating with different concentration of ginsenoside-Rg5. The results demonstrated that ginsenoside-Rg5 treatment could discernibly attenuate migration of OCT-P9a cells as compared with controls (Fig. 2C).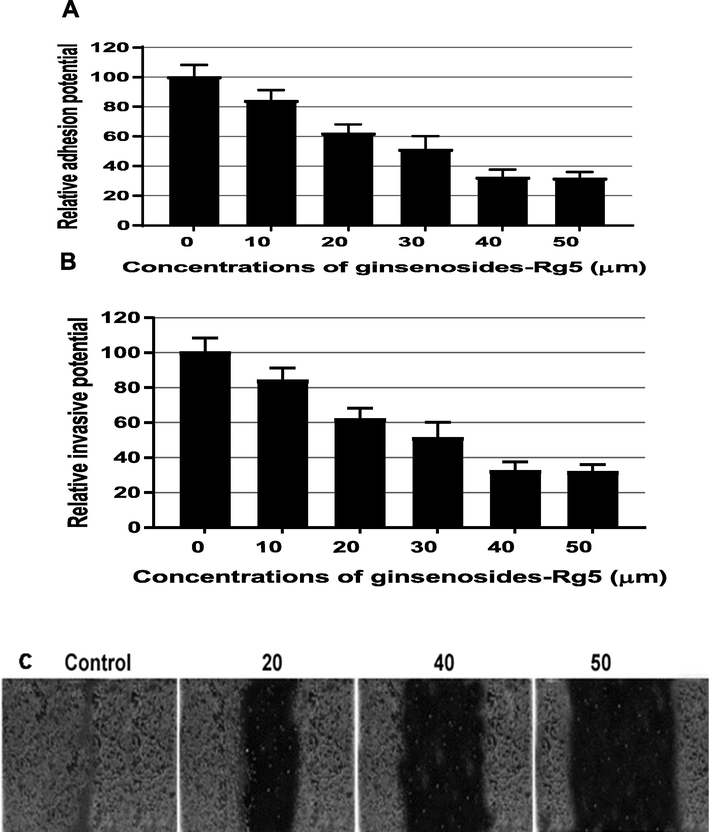
Effect of ginsenoside-Rg5 on properties of ovarian carcinoma cells OCI-P9a. (A) adhesion, (B) invasion, and (C) migration. OCI-P9a cells were incubated with indicated concentration of ginsenoside-Rg5 for 24 h, followed by adhesion, invasion and migration analyses. The experiments were performed three times for each used concentration.
3.3 Ginsenoside-Rg5 inhibits tumor growth and reduces metastasis
To further confirm the anti-cancer activity of ginsenoside-Rg5 on ovarian cancer, nude mice transplanted model was constructed with OCT-P9a cells; 25 mg/kg of ginsenoside-Rg5 was gavaged once a day for 30 days. Organ metastases in liver, lungs, spleen and peritonea and tumor volume were then analyzed among groups. The results showed that there were massive tumor metastases identified in liver, lungs, spleen and peritonea of the mice. However, there was no tumor metastasis observed in ginsenoside-Rg5 treated group (Fig. 3A, B, C, and D). In addition, tumor volume in the control group was 979.2 ± 134.5 mm3, which was significantly larger than that in the ginsenoside-Rg5 treated group 165.5 ± 29.6 mm3 (P < 0.05; Fig. 3E).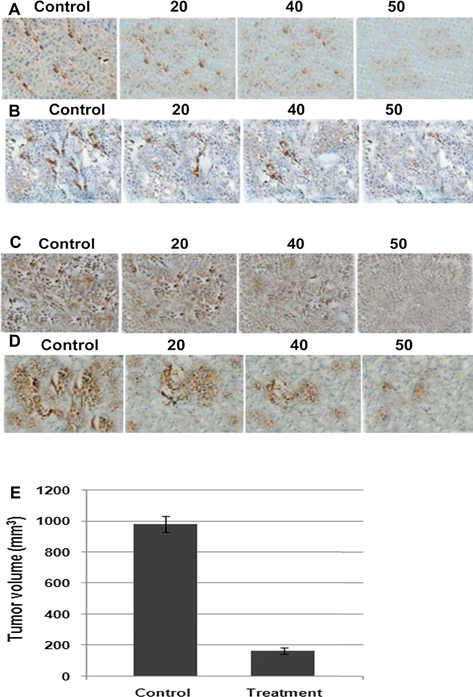
Effect of ginsenoside-Rg5 on growth and metastasis of ovarian cancer. Nude mice were transplanted with OCI-P9a cells and treated with 25 mg/kg of ginsenoside-Rg5 daily for 30 days. Then mice were sacrificed for tumor growth and metastasis analysis. Tumor metastasis sites in (A) liver, (B) lungs, (C) spleen and (D) peritoneum in the ginsenoside-Rg5 treated mice. (E) Tumor volume in ginsenoside-Rg5 treated group and control group.
3.4 Analysis of FGF8b expression in OCI-P9a cells
To further explore the mechanism of ginsenoside-Rg5 in treating ovarian cancer, expression of the underlying downstream target FGF8b was determined in this study. RT-PCR analysis revealed a relatively high expression of FGF8b in OCI-P9a ovarian cancer cells. Treatment of OCI-P9a cells with ginsenoside-Rg5 of concentrations range from 10 to 50 µM for 24 h led to suppression of FGF8b expression in OCI-P9a cells in a dose response manner (Fig. 4). Although the reduction of FGF8b expression was significant (P < 0.002) even at low concentration of ginsenoside-Rg5 (10 µM), the effect reached its maximum at 40 µM (Fig. 4). The degree of FGF8b expression reduction was dependent on the concentration of ginsenoside-Rg5 (Table 1).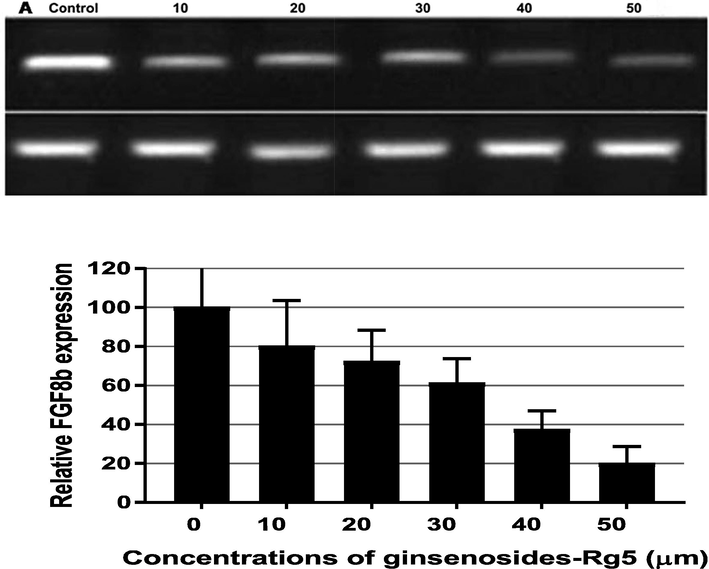
Ginsenoside-Rg5 attenuates expression of fibroblast growth factor-8 (FGF8b) in OCI-P9a cells. OCI-P9a cells were treated with different concentration of ginsenoside-Rg5 for 24 h and then analyzed using RT-PCR.
Ginsenoside-Rg5 concentration
FGF8b relative expression
0 µM
100.32 ± 32.21
10 µM
80.32 ± 23.27
20 µM
72.56 ± 15.78
30 µM
61.49 ± 12.29
40 µM
37.74 ± 9.34
50 µM
20.23 ± 8.38
4 Discussion
In the current study, ginsenoside-Rg5 was revealed to significantly attenuate cell viability of OCI-P9a in a dose-dependent manner. Moreover, it could also obviously reduce migration and metastasis potential of OCI-P9a cells. Further investigation revealed that ginsenoside-Rg5 could also markedly down-regulate the expression of FGF8b in OCI-P9a cells. These evidences indicated that ginsenoside-Rg5 may exert its anti-cancer activities via down-regulation of FGF8b expression.
Ginseng root is a common traditional Chinese medicine used for treatment of several diseases for a long time. Ginsenoside-Rg5 is the most abundant compound in steamed ginseng (Qi et al., 2011; Kim et al., 2012). Although the recent studies about the effect of ginseng on tumor mainly focused on function of ginsenoside-Rg2 and -Rh3 (Park et al., 2014; Huang et al., 2016), Rg5 has been reported to the main ingredient of ginseng root (Kim et al., 2015). Previous studies documented that ginsenoside-Rg5 could significantly decrease inflammation level in lipopolysaccharide-stimulated BV2 microglial cells (Lee et al., 2013) to serve a critical anti-inflammatory effect. Moreover, in vivo studies also reported that ginsenoside-Rg5 could obviously alleviate scopolamine-induced memory injury (Kim et al., 2013) and oxazolone-induced chronic dermatitis in mice (Shin et al., 2006). In addition, ginsenoside-Rg5 also suppressed cell transition from G1 to S phase in SK-HEP-1 cells via suppressing the activity of cyclin E-dependent kinase (Lee et al., 1997). Considering of these, ginsenoside-Rg5 may inhibit cell growth and metastasis of ovarian cancer through multipath mechanism. Thus, it may be useful to apply ginsenoside-Rg5 as cancer treatment to improve outcome of cancer patients (e.g, combined with chemotherapy drugs). Research by Lee YY et al. demonstrated that ginsenoside-Rg5 could significantly alleviate inflammation level in Bin BV2 microglial cells via several inflammatory-associated signaling pathways (Siddiqi et al., 2014). Siddiqi et al. reported stimulatory effect of ginsenoside-Rg5 on osteoblasts in mice (Mei et al., 2016). Our experiment demonstrated that ginsenoside-Rg5 could specifically inhibit the proliferation of ovarian cancer cells, which is consistent with the previous studies that reported that ginsenoside-Rg5 can induce apoptosis of tumor cells (Wang et al., 2013). Moreover, we also identified that ginsenoside-Rg5 could significantly decrease migration and invasion ability of ovarian cancer cells. Further in vivo tumor transplantation investigation also confirmed that ginsenoside-Rg5 could significantly inhibit growth. Taken together, these findings suggested that ginsenoside-Rg5 may play an important role in inhibiting progression and metastasis of ovarian cancer. Nevertheless, the exact mechanism of ginsenoside-Rg5 in treating ovarian cancer needs to be validated by further studies, which may be useful for improvement of ovarian cancer clinical outcome.
Abnormally high FGF8 expression is usually identified in several cancer cells, including ovary cancer. In addition, up-regulation of FGF8 is often associated with tumorigenicity and angiogenesis (Mattila and Harkonen, 2007; Schwertfeger, 2009). Down-regulation of FGF8b expression is considered an important good therapeutic marker during cancer chemotherapeutic treatment (Mattila and Harkonen, 2007; Hsieh et al., 2007). In the current study, expression of FGF8b was relatively high in OCI-P9a cells and ginsenoside-Rg5 could significantly reduce the expression level of FGF8b after 24 h of treatment. To further confirm the effect of ginsenoside-Rg5 on inhibition of FGF8b, OCI-P9a cells were treated with FGF8b antagonist K5-OS and it was found that K5-OS caused inhibition of FGF8b to the similar extent as that of ginsenoside-Rg5 (data not shown). These findings clearly demonstrated that ginsenoside-Rg5 inhibits expression of FGF8b in OCI-P9a cells. Immunohistochemical results showed that positive expression rate of FGF8b in ovarian cancer was significantly higher than that of normal ovarian tissue and benign ovarian tissue (P < 0.05). Expression of FGF8b in lymphatic metastasis of ovarian cancer was markedly higher than that in non-metastasis samples. The results are consistent with previous reports (Zhong et al., 2006; Cattaruzza and Perris, 2005; Zhang and Wang, 2006).
Migration and metastasis are two important properties of carcinoma cells as well as cancer development (Cattaruzza and Perris, 2005; Zhang and Wang, 2006). The present study revealed that ginsenoside-Rg5 significantly attenuated the viability of OCI-P9a cells in a dose-dependent manner. Ginsenoside-Rg5 treatment efficiently suppressed migration and invasion of OCI-P9a compared to the control cells. Advanced stage of ovarian carcinoma was associated with metastasis to distant organs, such as lungs, peritoneum, spleen, and liver (Jemal et al., 2011). In the migration of carcinoma cells, reduced adhesion is an essential prerequisite for carcinoma cells migrating to cells or different organs (Cattaruzza and Perris, 2005; Zhang and Wang, 2006). Thus, it is important to modulate adhesion ability of carcinoma cells to prevent development of carcinoma. This study showed that ginsenoside-Rg5 treatment for one month suppressed metastasis of ovarian cancer cells to adjacent as well as distant organs in the mice. None of the animals in the ginsenoside-Rg5 treatment group showed cancer metastasis to liver, spleen, lungs, or peritoneum. On the other hand, metastases from transplanted tumor to liver, spleen, lungs, and peritoneum were observed in all animals in control group. Our research group examined the effect of ginsenoside-Rg5 on suppressing proliferation of ovarian cancer cells by MTT method. The results showed that ginsenoside-Rg5 slowed down proliferation of OCI-P9a, and ginsenoside-Rg5 could reduce the adhesion rate of OCI-P9a cells. The results of cell invasion showed that invasion capacity of OCI-P9a was significantly decreased after treatment of ginsenosin-Rg5. Cells treated with ginsenoside-Rg5 had low migration capacity, and ginsenoside- Rg5 had a significant inhibitory effect on tumor growth in mice. The results are consistent with the previous reports (Wang et al., 2013; Zhong et al., 2006; Cattaruzza and Perris, 2005).
Conclusively, data of the present study suggested that ginsenoside-Rg5 significantly attenuates cell viability and inhibits migration and invasion of OCI-P9a ovarian cancer cells via down-regulation of expression of fibroblast growth factor-8b which plays an important role in pathophysiology of ovarian carcinoma. This suggests that ginsenoside-Rg5 may have anti-cancer activity which inhibits growth and metastasis of ovarian carcinoma. Therefore, ginsenoside-Rg5 can be a potential therapy for ovarian cancer. Results of this study will provide future reference to clinicians for treatment of ovarian carcinoma. Further studies on various pathways which play role in the pathophysiology of ovarian carcinoma are required for validation of the results in this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Proteoglycan control of cell movement during wound healing and cancer spreading. Matrix Biol.. 2005;24:400-417.
- [Google Scholar]
- Specific activation of insulin-like growth factor-1 receptor by ginsenoside-Rg5 promotes angiogenesis and vasorelaxation. J. Biol. Chem.. 2015;290:467-477.
- [Google Scholar]
- Study on the preparation process of ginsenoside-Rg5. Local Food Res. Dev.. 2017;20:42-46.
- [Google Scholar]
- Androgen-response elements in hormone refrac- tory prostate cancer: implications for treatment development. LancetOncol.. 2007;8:933-939.
- [Google Scholar]
- Ginsenoside-Rh2 inhibits proliferation and induces apoptosis in human leukemia cells via TNF-α signaling pathway. Acta Bioch. Bioph. Sin.. 2016;48:750-755.
- [Google Scholar]
- Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol. Pharm. Bull.. 2007;30:724-728.
- [Google Scholar]
- Simultaneous quantification of 14 ginsenosides in Panax ginseng C.A.Meyer (Korean red ginseng) by HPLC-ELSD and its application to quality control. J. Pharmaceut. Biomed.. 2007;45:164-170.
- [Google Scholar]
- Ginsenoside-Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int. Immunopharmacol.. 2012;12:110-116.
- [Google Scholar]
- Ginsenoside-Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int. Immunopharmacol.. 2012;12:110-116.
- [Google Scholar]
- Ginsenosides-Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J. Ethnopharmacol.. 2013;146:294-299.
- [Google Scholar]
- Ginsenosides-Rg5 and -Rh3 protect scopolamine-induced memory deficits in mice. J. Ethnopharmacol.. 2013;146:294-299.
- [Google Scholar]
- Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside-Rg5. J. Ginseng. Res.. 2015;39:125-134.
- [Google Scholar]
- Changes in the contents of prosapogenin in Red Ginseng (Panax ginseng) depending on the extracting conditions. J. Ginseng Res.. 2015;24:1-4.
- [Google Scholar]
- Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J. Chromatogr. A.. 2001;921:335-339.
- [Google Scholar]
- Ginsenoside-Rg5 suppresses cyclin E-dependent protein kinase activity via up-regulating p21Cip/WAF1 and down-regulating cyclin E in SK-HEP-1 cells. Anticancer Res.. 1997;17:1067-1072.
- [Google Scholar]
- Anti-inflammatory effect of ginsenoside-Rg5 in lipopolysaccharide-stimulated BV2 microglial cells. Int. J. Mol. Sci.. 2013;14:9820-9833.
- [Google Scholar]
- Ginsenoside-Rg5 induces apoptosis and DNA damage in human cervical cancer cells. Mol. Med. Rep.. 2015;11:940-946.
- [Google Scholar]
- Role of fibroblast growth factor 8 in growth and progression of hormonal cancer. Cytokine Growth Factor Rev.. 2007;18:257-266.
- [Google Scholar]
- Effect of ginsenoside-Rg5 on human esophageal cancer cells. Chin. Lab. Diagn.. 2016;20:1982-1984.
- [Google Scholar]
- Ginsenosides as anti-cancer agents: in vitro and in vivo activities, structure-activity relationships, and molecular mechanisms of action. Front. Pharmacol.. 2012;3:25-29.
- [Google Scholar]
- Stereospecific anti-cancer effects of ginsenoside-Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J. Ginseng. Res.. 2014;38:22-27.
- [Google Scholar]
- Isolation and analysis of ginseng: advances and challenges. Nat. Prod. Rep.. 2011;28:467-495.
- [Google Scholar]
- Fibroblast growth factors in development and cancer: insights from the mammary and prostate glands. Curr. Drug Targets. 2009;10:632-644.
- [Google Scholar]
- Inhibitory effect of ginsenoside-Rg5 and its metabolite ginsenoside Rh3 in an oxazolone-induced mouse chronic dermatitis model. Arch. Pharm. Res.. 2006;29:685-690.
- [Google Scholar]
- Inhibitory effect of ginsenoside-Rg5 and its metabolite ginsenoside-Rh3 in an oxazolone-induced mouse chronic dermatitis model. Arch. Pharm. Res.. 2006;29:685-690.
- [Google Scholar]
- Stimulative effect of ginsenosides-Rg5:Rk1 on murine osteoblastic MC3T3-E1 cells. Phytother. Res.. 2014;28:1447-1450.
- [Google Scholar]
- Screening a phage display library for a novel FGF8b-binding peptide with anti-tumor effect on prostate cancer. Exp. Cell. Res.. 2013;319:1156-1164.
- [Google Scholar]
- Anti-carcino genic effect of Panax ginseng C.A. Meyer and identification of active compounds. J. Korean Med. Sci.. 2001;16:6-18.
- [Google Scholar]
- Arsenic trioxide (As(2)O(3)) inhibits peritoneal invasion of ovarian carcinoma cells in vitro and in vivo. Gynecol. Oncol.. 2006;103:199-206.
- [Google Scholar]
- Cooperation between FGF8b overexpression and PTEN deficiency in prostate tumori-genesis. Cancer Res.. 2006;66:2188-2194.
- [Google Scholar]







