Translate this page into:
Genotypic variability in salt tolerance of Vicia faba during germination and early seedling growth
⁎Corresponding author. tmsoliman2000@yahoo.co.uk (Taha Mohamed El-Katony),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Salt tolerance of four Egyptian Vicia faba L. cultivars – Nubaria 1, Nubaria 2, Sakha 1 and Giza 3 – was investigated at germination in relation to seed size. The variability in seed size was wide as seed index of Giza 3 was about half that of Nubaria 1. The four investigated cultivars can withstand up to 150 mM NaCl; with marginal reductions in germination capacity but with marked reductions in speed of germination and embryo growth. The vague genotypic variability among the four cultivars in germination parameters emerged more clearly under salt stress. The response of early emergence of the embryo to salt stress seems to differ from that of the subsequent extension of embryonic axis; since based on magnitude and speed of germination Nubaria 1 was the most salt sensitive cultivar but exhibited relatively high salt tolerance on the basis of embryo growth. The salt-sensitive Nubaria 1 produced smaller embryo, with shorter and thicker radicles than the other three cultivars. Germination speed and embryo growth were maximum but lag period was minimum for the medium-sized seeds. Length and thickness of the emerging radicles were affected more by the genotype than by salinity stress and nutrient supply during germination. The beneficial effects of nutrients on seed germination and embryo growth were more evident in the salt-tolerant Nubaria 2 than in the salt-sensitive Nubaria 1.
Keywords
Embryo growth
Germination speed
Salinity
Seed index
Vicia faba
1 Introduction
The task of utilization of problem soils can be achieved either by modulating the plant to fit the soil or by improvement of the soil to meet the plant demand. The first approach involves selection of the most efficient species or cultivars and introduction of novel lines of plants that are more tolerant to the problem soils. The second approach involves fixing the soil problems and implementation of new, more efficient agricultural practices that allow great areas of abandoned or barren lands to become productive. A significant fraction of agricultural crops are cultivated on low quality soils, mostly salt-affected soils in the arid regions of the world. According to George et al. (2012), soil is considered saline when the electrical conductivity of the soil saturation extract (ECe) exceeds 4 dS m−1 (>40 mM NaCl). Salt tolerance differs according to plant genotype, developmental stage, climatic conditions and soil fertility. Seed germination is a critical stage, limiting the establishment of plants under saline conditions because seeds usually reside near the soil surface where salt levels are usually high. The diminished germination of seeds under salt stress may lead to uneven plant cover and reduction in crop yield. Therefore, emergence of the embryo is expected to be highly salt tolerant relative to the subsequent extension of embryo and vegetative plant growth. This trend has been reported for the psammophytic grass Elymus farctus (El-Katony et al., 2015), Oryza sativa (Walia et al., 2005) and Zea mays (Carpýcý et al., 2009). Nevertheless, salt response during germination can be manipulated as a reliable quick test for salt response of the plant. Generally, assessment of plant response to salinity is based on two parameters: the threshold and the critical salinity levels.
Legumes come second only to cereals in their importance to humans (Graham and Vance, 2003). They account for 27% of the world’s primary crop production, with grain legumes alone contributing 30%–60% of the dietary protein nitrogen needs of humans (Vance et al., 2000). Moreover, the dietary importance of legume seeds is expected to grow in accordance with the increasing demand on economic and healthy high-protein foods; particularly in light of the increasing concern about the risk of consumption of animal food sources. Among the consumable legumes faba bean (V. faba) comes after common bean (Phaseolus vulgaris), pea (Pisum sativum) and chickpea (Cicer arietinum) as the fourth primary dietary legume worldwide (Graham and Vance, 2003). In Egypt, V. faba is one of the most important leguminous crops, where it represents a major constituent of the daily food for most of the population. In addition to its high nutritive value, V. faba has outstanding therapeutic potentialities through impacting lipid profiles in human body and providing a good supply of antioxidants and chemo-preventive factors (Madar and Stark, 2002). In addition, Rabey et al. (1992) demonstrated marked activity of V. faba as a therapeutic agent for Parkinsonian features through increasing plasma levodopa values. Interestingly, response of V. faba to abiotic stress was employed to increase the level of Levo-dihydroxy phenylalanine (l-DOPA) in the plant (Randhir and Shetty, 2004).
The benefits of V. faba extend to improving soil characteristics which has positive consequences on the succeeding crops; since it has the highest reliance on N2 fixation among the winter grain legumes. As a consequence, the savings in N fertilizer required to maximize the yield of succeeding crops approach 100–200 kg N ha−1 (Jensen et al., 2010).
A large genetic variability has been identified in V. faba in terms of seed size and composition as well as tolerance to several biotic and abiotic stresses (Duc et al., 2010; Khoufi et al., 2013). More research is therefore needed to characterize the traits of different V. faba cultivars related to environmental adaptation and stress tolerance. The present work was conducted to explore the role of genotypic variability among four Egyptian V. faba cultivars in relation to salt tolerance at germination stage. Further, the salinity × fertility interaction was investigated in two contrasting cultivars in salt tolerance.
2 Materials and methods
2.1 Germination conditions
Seeds of four cultivars of faba bean (V. faba L.): Nubaria 1, Nubaria 2, Sakha 1 and Giza 3 were obtained from the Experimental Station of Agricultural Research at Giza, Egypt. Seeds of the four cultivars reflected appreciable variability in size; therefore seed index (g seed−1) was estimated. Homogeneous seeds of each cultivar were used in the experiments. First, salt tolerance of the four cultivars at germination stage was evaluated by monitoring seed germination and embryonic growth in 150 mM NaCl compared with a non-salinized control. Seeds were germinated in the dark between layers of soft tissue saturated with the test solutions in 25 × 12 cm plastic trays at temperature of 25/17 °C day/night. Each tray contained 20 seeds of the same cultivar. The experiment was factorial with two factors and three replications in a completely randomized design. The main factors were: 1) cultivar with four levels (Nubaria 1, Nubaria 2, Sakha 1 and Giza 3; and 2) salinity with two levels (0 and 150 mM NaCl).
Most of the germination parameters and embryo growth measures of the preliminary screening test revealed mild effect of 150 mM NaCl but marked genotypic variability in salt tolerance; with Nubaria 1 and Nubaria 2 exhibiting the most contrasting responses. Therefore, seeds of Nubaria 1 and Nubaria 2 were selected for further investigation involving salinity × fertility interaction on germination in a second experiment. Seeds of Nubaria 1 and Nubaria 2 were germinated in graded salinity levels (0, 50, 100 and 200 mM NaCl) prepared either in distilled water or superimposed on a complete nutrient solution under the same conditions of the first experiment. The nutrient solution contained the following macro-nutrients (mM): N (as NO3−) 5, K 2.5, P 0.5, Ca 1.5, Mg 0.5 and S 3 and the micronutrients (µM): Fe (FeEDTA) 25, Mn 5, Zn 0.5, Cu 0.5, B 25, Na 50, Cl 50, Mo 0.1 and Co 0.1. The second experiment was factorial with three factors and four replications in a completely randomized design. The main factors were: cultivar with 2 levels (Nubaria 1 and Nubaria 2), nutrient supply with two levels (distilled water and nutrient solution) and salinity with four levels (0, 50, 100 and 200 mM NaCl).
In both experiments, seeds were frequently transferred to new trays with fresh paper tissue saturated with test solutions to prevent buildup of salt. Seeds were considered germinated with the emergence of radicle up to 2 mm and the number of germinated seeds was monitored daily for a period of 4 days in the first experiment and 7 days in the second experiment.
2.2 Definitions, calculations and statistical analysis
According to Ranal and Santana (2006) seed germination was assessed in terms of germinability as well as speed and lag of germination.
-
Germinability or germination capacity is the final cumulative germination percentage.
-
Rate or speed of germination was estimated in terms of the following indices:
-
Peak value (PV) is the maximum mean daily germination.
-
where G1, G2, G3 and Gn are the cumulative germination% at the first, second, third and final time respectively and T is the total germination period.
-
The germination index (GI), is a measure of both percentage and speed of germination and assigns maximum arithmetic weight to early germinating seeds and less weight to those germinating later.
-
The coefficient of velocity of germination (CVG), was calculated using the following formula:
-
-
T10 or time to 10% germination is a measure of the lag period between imbibition and onset of germination
The threshold salinity of a particular germination parameter was defined as the highest salinity level leading to non-significant reduction; and the critical salinity as the salinity level leading to 50% reduction below the control.
Final cumulative germination percentage was arcsine transformed before performing statistical analysis to ensure homogeneity of variance. Data were analyzed using SPSS version 22 and the effects of main factors: cultivar and salinity (in the first experiment) or cultivar, nutrients and salinity (in the second experiment) and their interaction were assessed using two-way and three-way ANOVA respectively. Mean separation was performed using the Duncan's multiple range test at p < 0.05. Coefficient of variation (CV) was used to compare the relative dispersion of the different germination parameters that had divergent means and variances.
Where SD is the sample standard deviation and s the sample mean.
3 Results
The four V. faba cultivars exhibited marked variability in seed index (Table 1). Seed index was highest in Nubaria 1, followed by Nubaria 2 and Sakha 1 and lowest in Giza 3 (about 60% of that of Nubaria 1). Time course of seed germination of the four cultivars exhibited similar patterns, with minor variation between cultivars in absence of salinity, but with appreciable lag (except Sakha 1) under salt stress (Fig. 1). Table 2 reveals significant (p < 0.05) to highly significant (p < 0.01) effect of the main factors (cultivar and salinity) on seed germination and embryo growth with non-significant interaction of the two factors.
Cultivar
Seed index (g seed−1)
Nubaria 1
1.215 ± 0.020a
Nubaria 2
0.838 ± 0.024bc
Sakha 1
0.870 ± 0.016b
Giza 3
0.762 ± 0.029d
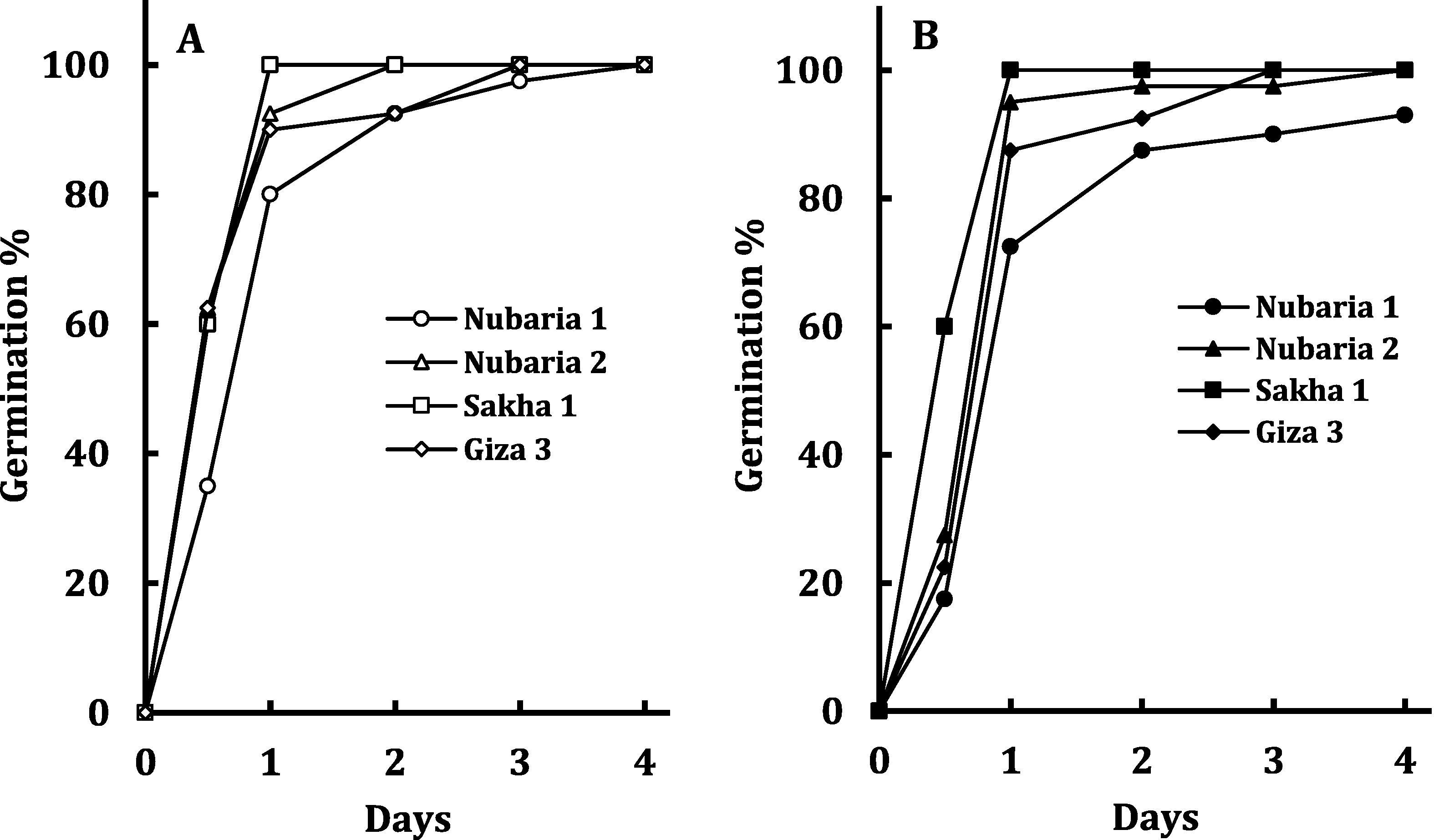
Time course of seed germination of four cultivars of V. faba at zero salinity (A) and 150 mM NaCl (B). Each value is the mean of three replicates.
Source of variation
df
F
P
F
P
Germinability (deg.)
Plumule FW
Cultivar
3
1.833
0.181
21.32
0.000
Salinity
1
4.500
0.049
26.52
0.000
Cultivar × Salinity
3
1.833
0.181
21.32
0.000
Germination index
Radicle length
Cultivar
3
11.38
0.000
6.388
0.005
Salinity
1
46.32
0.000
4.376
0.053
Cultivar × Salinity
3
2.200
0.128
0.265
0.850
Timson index
Radicle/Plumule FWratio
Cultivar
3
12.57
0.000
6.030
0.006
Salinity
1
52.40
0.000
1.489
0.240
Cultivar × Salinity
3
2.941
0.065
1.149
0.360
Radicle FW
Radicle FW/Radicle length ratio
Cultivar
3
8.337
0.001
5.588
0.008
Salinity
1
29.74
0.000
1.130
0.304
Cultivar × Salinity
3
2.718
0.079
0.801
0.512
Speed of germination -in terms of germination index (GI) and Timson index- was affected by salinity stress to a greater extent with greater genotypic variability than the final germination percentage. The final germination percentage was maintained at 100% in the four cultivars in absence of salinity and was hardly affected by 150 mM NaCl where a significant reduction of 7% below the control was found only in Nubaria 1 (Table 3). By contrast, speed of germination showed marked genotypic variability as well as more severe inhibition under salt stress. In absence of salinity, speed of germination was slower in Nubaria 1 than in the other three cultivars. The genotypic variability emerged more clearly under salt stress where the speed of germination was slowest in Nubaria 1 and fastest and least affected by salinity in Nubaria 2. The effect of salt stress in maximizing the genotypic variability is evident from the high coefficients of variation (CV) among the four cultivars for the different indices of germination under salt stress compared with the low CVs of the control.
Parameter
V. faba cultivar
Nubaria 1
Nubaria 2
Sakha 1
Giza 3
CV (%)
0 mM NaCl
Germinability (%)
100 ± 0.0a
100 ± 0.0a
100 ± 0.0a
100 ± 0.0a
0.00
Germination index (% d)
310 ± 10a
350 ± 5.8bc
363 ± 8.8bcd
340 ± 15.3b
6.65
Timson index (% d−1)
102 ± 2.5a
112 ± 1.4b
116 ± 2.2bc
110 ± 3.8b
5.14
T10 (d)
0.150
0.080
0.075
0.075
38.7
Radicle FW (mg plant−1)
130 ± 20a
249 ± 8.6b
257 ± 21bc
249 ± 25b
27.4
Plumule FW (mg plant−1)
97.2 ± 16a
354 ± 4.3bc
299 ± 38b
270 ± 50b
43.5
Radicle length (cm)
1.85 ± 0.24a
4.43 ± 0.27b
3.03 ± 0.61ab
4.36 ± 0.94b
35.9
Radicle/Plumule
FW ratio1.34 ± 0.37a
0.70 ± 0.08abcd
0.86 ± 0.04abc
1.02 ± 0.28ab
28.5
Radicle FW/Radicle
length ratio70.2 ± 5.6abc
56.4 ± 2.1a
91.0 ± 18c
61.38 ± 10ab
18.7
150 mM NaCl
Germinability (%)
93.3 ± 3.3b*
100 ± 0.0a
96.7 ± 3.3ab
100 ± 0.0a
3.27
Germination index (% d)
265 ± 2.9a*
323 ± 3.3c*
293 ± 11b*
306 ± 8.8bc*
8.30
Timson index (% d−1)
89.2 ± 0.8a*
106 ± 0.8c
96.7 ± 3.0b*
102 ± 2.2bc*
7.29
T10 (d)
0.300
0.18
0.150
0.230
69.3
Radicle FW (mg plant−1)
118 ± 12a
178 ± 17bc*
147 ± 26ab*
138 ± 20ab*
17.3
Plumule FW (mg plant−1)
56.6 ± 13a
238 ± 24c*
182 ± 33bc*
128 ± 19ab*
51.1
Radicle length (cm)
1.58 ± 0.21a
3.17 ± 0.48ab
1.98 ± 0.53a
3.45 ± 0.88b
35.5
Radicle/Plumule
FW ratio2.38 ± 0.67a*
0.75 ± 0.04bcd
0.80 ± 0.03bc
1.14 ± 0.29b
52.4
Radicle FW/Radicle
length ratio74.9 ± 5.1b
57.3 ± 4.3ab
77.3 ± 6.6bc
43.17 ± 6.8a
31.2
In addition to affecting magnitude and speed of germination, salinity delayed onset of germination (leading to longer T10) with the expression of more genotypic variability compared with the control treatment. In absence of salinity, the value of T10 was longer for Nubaria 1 than for the other cultivars; whereas under the impact of 150 mM NaCl it was longest for Nubaria 1, followed by Giza 3 and least for Nubaria 2 and Sakha 1.
Embryo growth- in terms of fresh weights of radicle and plumule as well as radicle length- was in general lowest in the heaviest and slowest germinating seeds of Nubaria 1 and was variably higher in the other three cultivars. The reductions in embryo biomass due to salinity were significant in the four cultivars (except the non-significant reduction in Nubatia 1) whereas those in radicle length were generally non-significant (Table 3). The extent of reduction in embryo biomass under salt stress was comparable in radicle and plumule, with almost unaffected radicle/plumule ratio, except Nubaria 1 in which salinity led to doubling of the radicle/plumule ratio. The radicle fresh weight/length ratio (a measure of radicle thickness) was affected by genotype to a greater extent than by salinity; and was greater in Nubaria 1 and Sakha 1 than in Nubaria 2 and Giza 3.
Fig. 2 presents the interaction of salt stress (up to 200 mM NaCl) and nutrients on time course of germination of two selected V. faba cultivars (Nubaria 1 and Nubaria 2). The two cultivars exhibited contrasting behavior either in absence or in the presence of salinity but with limited effect of nutrients. In absence of salinity, full germination was achieved by Nubaria 1 within 6 days from sowing but in Nubaria 2 this was reached in only 4 days. In addition, the inhibitory effect of 200 mM NaCl on germination percentage was evident only in Nubaria 1. Table 4 revealed non-significant effect of nutrients on germination parameters of the two cultivars but highly significant effects of cultivar, followed by salinity.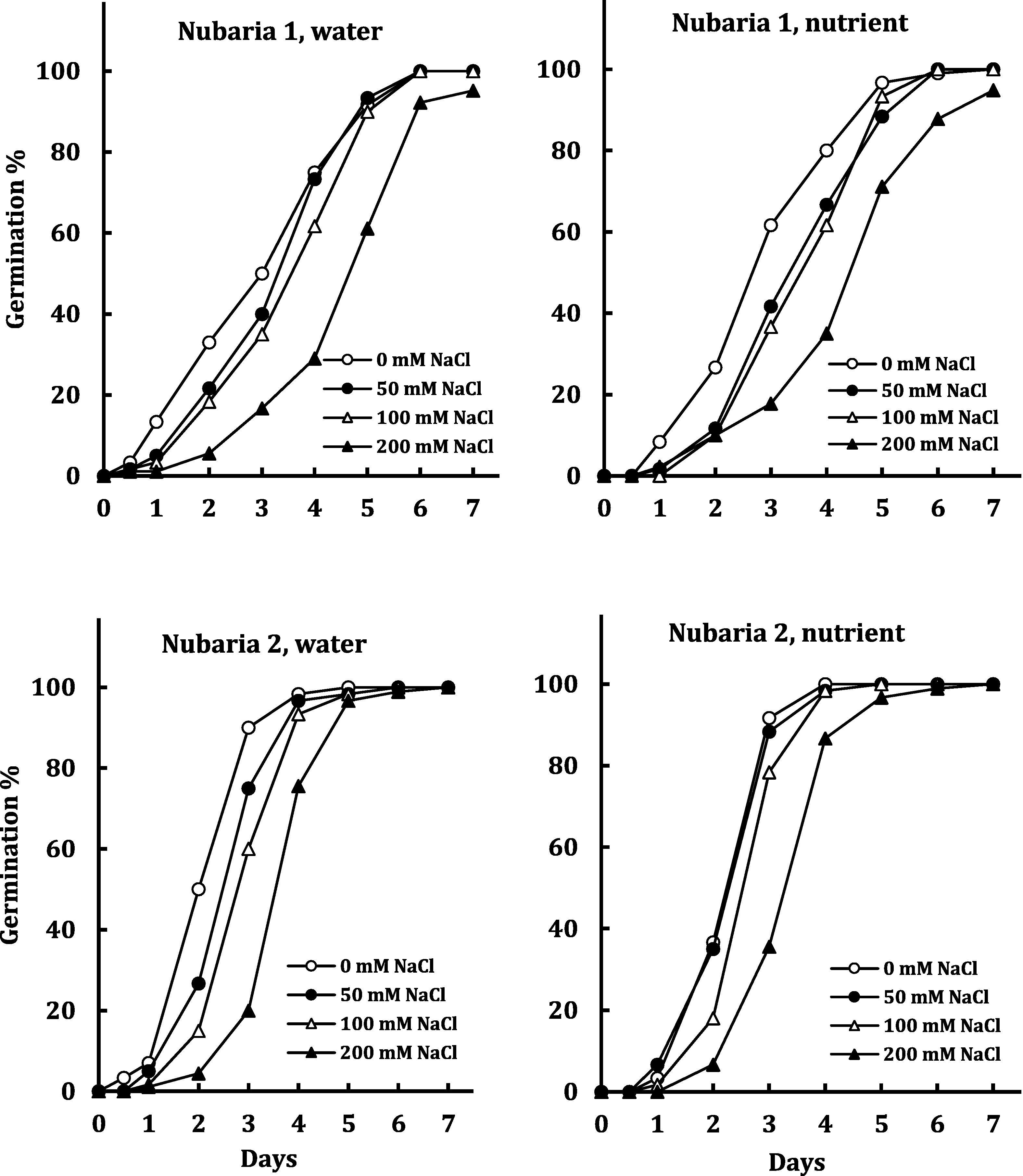
Effect of nutrients on time course of seed germination of two selected cultivars (Nubaria 1 and Nubaria 2) of V. faba under the impact of NaCl salinity. Each value is the mean of 4 replicates.
Source of variation
df
F
P
F
P
Germinability (deg.)
Radicle length
Cultivar
1
16.88
0.000
377.1
0.000
Nutrients
1
0.012
0.914
170.5
0.000
Salinity
3
16.88
0.000
831.1
0.000
Peak value
Radicle DW
Cultivar
1
160.8
0.000
0.826
0.365
Nutrients
1
1.549
0.222
22.84
0.000
Salinity
3
33.93
0.000
47.96
0.000
Germination index
Plumule DW
Cultivar
1
90.25
0.000
105.3
0.000
Nutrients
1
0.261
0.613
24.07
0.000
Salinity
3
50.58
0.000
78.15
0.000
Coefficient of velocity of germination
Radicle/Plumule FW ratio
Cultivar
1
92.83
0.000
26.64
0.000
Nutrients
1
0.438
0.513
0.007
0.932
Salinity
3
43.28
0.000
0.992
0.410
Speed of germination was significantly higher in Nubaria 2 than Nubaria 1 but was non-significantly affected by presence of nutrients (Fig. 3). In Nubaria 1 the reduction in speed of germination in terms of PV, GI and CVG was comparable and averaged around 28% as salt level increased from 0 to 200 mM NaCl; but in Nubaria 2, the reduction in GI amounted to 21% and that in PV and CVG was 33% as an average of the two nutrient treatments.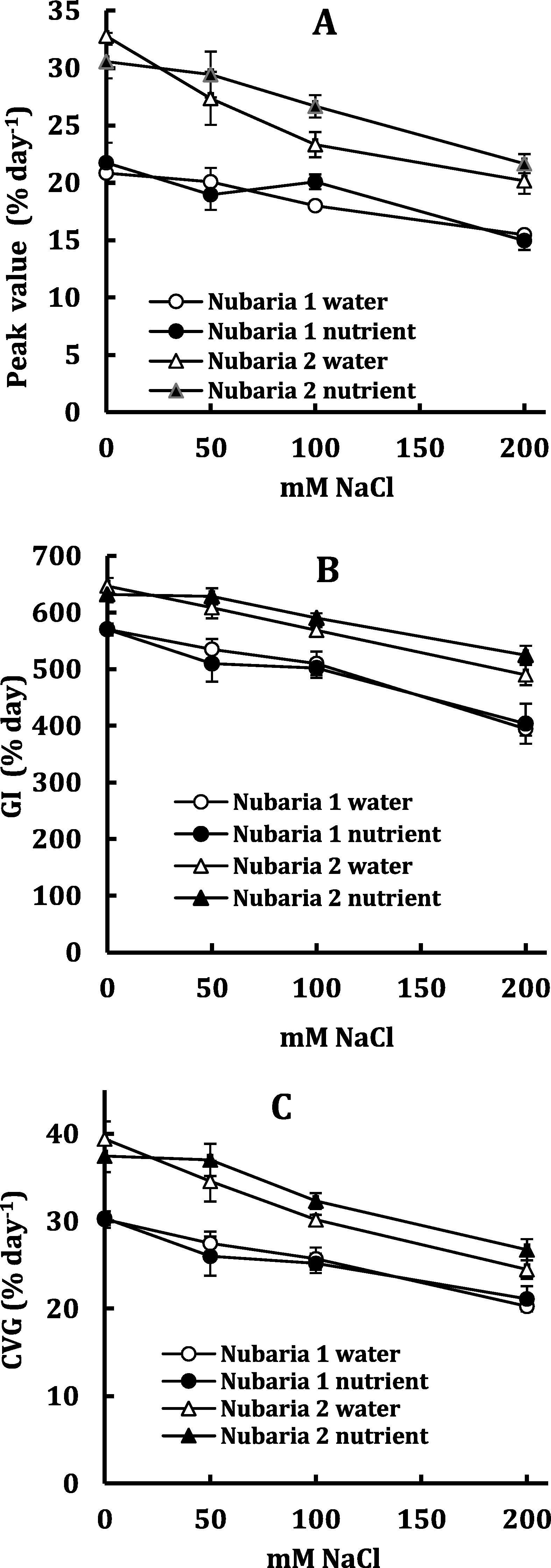
Peak value (A) germination index (GI, B) and coefficient of velocity of germination (CVG, C) of Nubaria 1 and Nubaria 2 cultivars of V. faba in response to salinity and nutrients. Each value is the mean of four replicates ± SE.
Table 4 reveals an overall highly significant effect of the main factors (cultivar, nutrients and salinity) on embryo growth of V. faba, particularly the plumule. The interaction of the three factors was further evident in Fig. 4, where the genotypic variability (the superior growth of Nubaria 2) was more evident in the presence of nutrients than in their absence and the beneficial effect of nutrients was more evident in Nubaria 2 than in Nubaria 1. The increase in plumule fresh weight of Nubaria 2 over Nubaria 1 amounted to 90% and 170% in water and nutrient solution respectively; likewise, the increase due to presence of nutrients amounted to 30% and 87% in Nubaria 1 and Nubaria 2 respectively. The effects of genotype and nutrients were less evident on radicle growth as the increase in radicle fresh weight of Nubaria 2 over Nubaria 1 was 32% and 60% in water and nutrient solution respectively and the increase due to presence of nutrients was 43% and 73% in Nubaria 1 and Nubaria 2 respectively.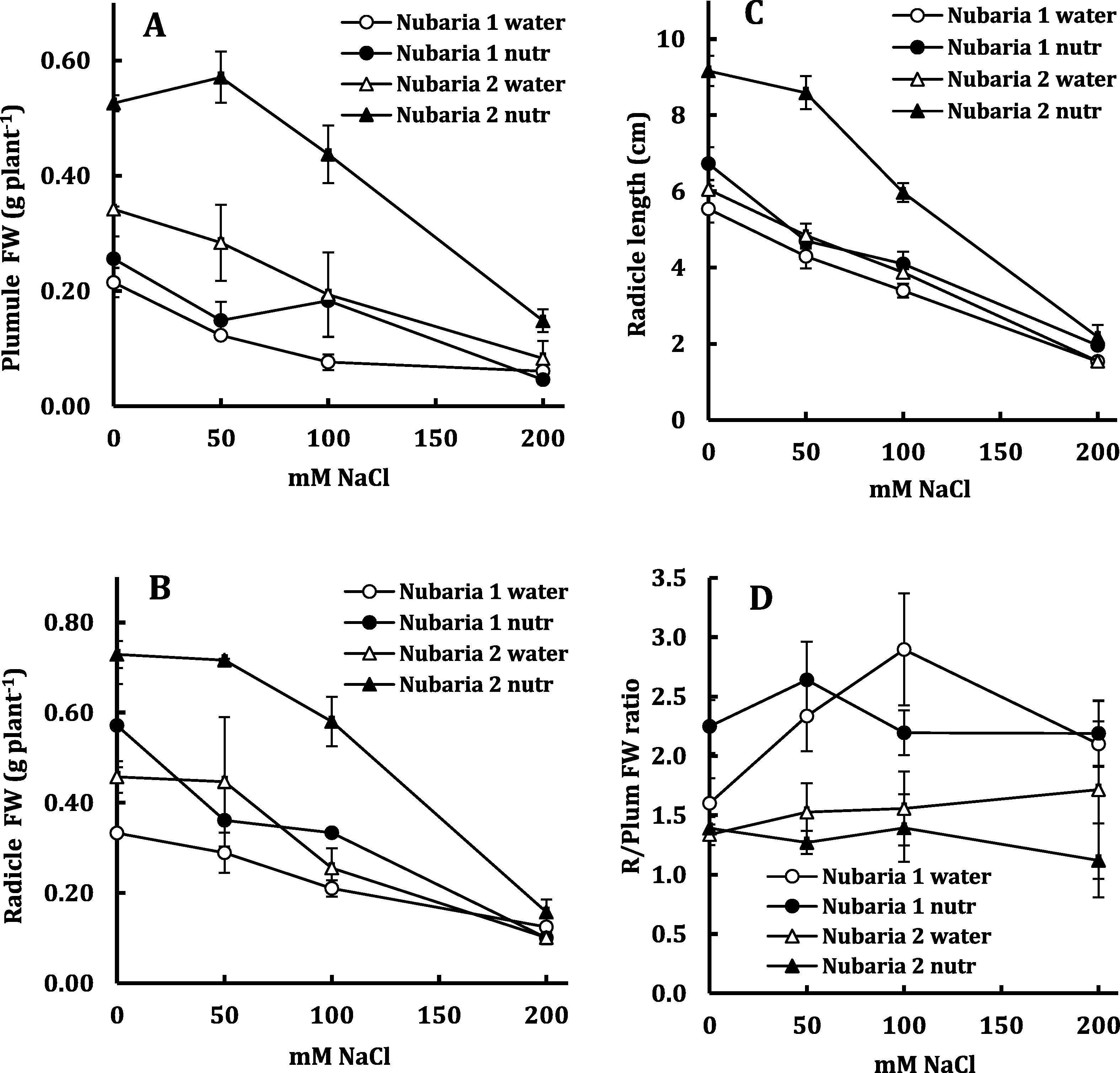
Plumule FW (A), radicle FW (B), radicle length (C) and radicle/plumule fresh weight ratio (D) of Nubaria 1 and Nubaria 2 cultivars of V. faba in response to salinity and nutrients. Each value is the mean of four replicates ± SE.
Salinity adversely affected embryo growth of the two V. faba cultivars; and the magnitude of reduction differed according to the cultivar and presence of nutrients. In Nubaria 1, increasing salinity level from 0 to 200 mM NaCl progressively reduced plumule fresh weight by 72% and 84% in water and nutrient solution respectively. In Nubaria 2, the 75% reduction in plumule fresh weight was progressive over the whole range of salinity in water but appeared beyond a threshold of 50 mM NaCl in the nutrient solution. The fresh weight of radicle of Nubaria 1 was progressively reduced by 63% and 82% as salt level increased from 0 to 200 mM NaCl in water and nutrient solution respectively; but in Nubaria 2 the reductions averaged around 78% for the two nutrient treatments as salt level exceeded a threshold of 50 mM up to 200 mM NaCl.
Radicle length was about 30% higher in Nubaria 2 than Nubaria 1; and in both cultivars it was higher in the presence of nutrients than in their absence. However, the beneficial effect of nutrients on radicle length was greater in Nubaria 2; in which nutrients increased radicle length by about 60% above water in comparison with only 15% increase in Nubaria 1. The reduction in root length amounted to 75% (as an average for the two cultivars and the two nutrient treatments) as salt level increased from 0 to 200 mM NaCl. The radicle/plumule fresh weight ratio of the seedlings was 60% higher in Nubaria 1 than Nubaria 2 and was non-significantly affected by nutrients and salinity, except with the marked increase in the ratio of Nubaria 1 at 100 mM NaCl which was followed by a slight reduction with further increase in salinity up to 200 mM NaCl.
4 Discussion
The four V. faba cultivars expressed marked variation in seed size where seeds index of the smallest seeds approached about 60% of that of the largest seeds. This wide variability is quite expected in crop species due to the growing concern about production of new cultivars; and it was demonstrated in V. faba with regard to seed traits and tolerance to biotic and abiotic stresses (Duc et al., 2010; Khoufi et al., 2013). Vicia faba exhibited appreciable salt resistance during germination and early seedling growth since germination capacity was hardly affected by as high salinity levels as 200 mM NaCl. The genotypic variability in germination capacity and in time course of germination was very subtle in absence of salinity, where the four cultivars approached full germination with comparable speed. But, the genotypic variability emerged more clearly under the impact of salt stress where the values of CV for most of the germination parameters among the four cultivars were consistently higher under salt stress above those of the control. Thus, salt stress might represent a screening tool aiding to increase resolution of the rather vague genetic potentiality of the four cultivars. In this respect, genotypic variability in growth among two Oryza sativa cultivars (Gao et al., 2013) and three Phaseolus vulgaris cultivars (Kaymakanova, 2009) growing under normal conditions was non-significant but emerged quite clearly under the impact of salt stress.
The magnitude of germination seems to be a less sensitive measure of salt response and also a poor measure of genotypic variability compared with the time-dependent germination parameters (such as speed or lag of germination) and embryo growth. Whereas final germination percentage was the same in the four cultivars in absence of salinity with limited genotypic variability under salt stress, germination speed as well as embryo growth reflected better both genotypic variability and more precise salt response. Greater salt susceptibility of germination rate compared to magnitude of germination has been encountered in several plant species including Atriplex halimus (Bajji et al., 2002), triticale (Atak et al., 2006), Helianthus annuus (Mostafavi and Heidarian, 2012), Oryza sativa (Ologundudu et al., 2014) and Chenopodium quinoa (Panuccio et al., 2014).
In agreement with our results, distinct genotypic variability in salt response has been reported during germination and vegetative growth among 5 natural populations of the halophyte Atriplex halimus (Haddioui and Baaziz, 2006; Haddioui et al., 2008) as well as during germination among 4 cultivars of red clover (Trifolium pratense) (Mandić et al., 2014) and five Zea mays cultivars (Khayatnezhad and Gholamin, 2013). Nevertheless, the present work suggests that embryo growth is a more reliable measure of salt response than the mere emergence of the embryo; and that the two phases of plant development might exhibit contrasting responses since the ranking of the four cultivars according to salt tolerance based on embryo growth varied from that based on seed germination. Based on embryo growth, Giza 3 seemed the most salt-sensitive cultivar but Nubaria 1 the most salt-tolerant, but the reverse was evident on the basis of seed germination parameters. Similar trend has been demonstrated in rice (Oryza sativa), where the ranking of cultivars according to salt sensitivity was different during germination than the subsequent vegetative growth (Ologundudu et al., 2014).
The different measures of seedling growth such as fresh weight of the emerging embryo and radicle length can be affected differentially by salt stress. It seems also that some traits of seedling growth such as radicle length, radicle/plumule weight ratio and the weight/length ratio of radicle (a measure of radicle thickness) were determined primarily by the genotype with limited effect of salinity stress since Nubaria 1 exhibited an overall smaller embryo, with shorter but thicker radicles than the other three cultivars irrespective of salinity treatment. Although salinity might affect plumule growth of V. faba to a greater extent than radicle growth (as in Nubaria 1) or affect the two organs similarly (the other three cultivars), greater salt injury has been reported on roots than on shoots in different cultivars of Oryza sativa (Ologundudu et al., 2014) and Phaseolus vulgaris (Kaymakanova, 2009).
The genotypic variability in germination parameters and embryo growth of V. faba can be correlated to seed vigor. Germination speed and embryo growth exhibited close dependence on seed index in a curvilinear pattern with maximum values for the medium-sized seeds and slow speed of germination and low embryo growth of the largest seeds. By contrast, germination lag (in terms of T10) and the radicle/plumule fresh weight ratio exhibited a different pattern of minima for the medium-sized seeds and higher values in the large seeds.
The role of nutrients in salt tolerance during germination was further investigated in two contrasting cultivars of V. faba: Nubaria 1 (salt-sensitive) and Nubaria 2 (salt-resistant) at higher salt levels (up to 200 mM NaCl). Again, speed of germination proved to be a more precise measure of plant performance (exhibiting greater response to cultivar, salinity and nutrients) than germination capacity. The better salt tolerance of Nubaria 2 was manifested as higher germination capacity and speed compared with the less tolerant cultivar Nubaria 1. In addition, the present work suggests that the germination pattern of V. faba, particularly speed of germination, seems to be determined primarily by the genetic make-up of the plant judged from the very high F ratio of cultivar, followed by environmental conditions (the fairly high F ratio of salinity and the non-significant F ratio of nutrients).
The beneficial effect of nutrients in alleviating the toxic effect of NaCl salinity was very weak in V. faba; in contradiction with the substantial improvement of salt tolerance during germination by nutrients in the psammophytic grass Elymus farctus (El-Katony et al., 2015). This differential response to nutrients of the two species might be related to the greater seed size and amount of stored nutrients in the seeds of V. faba. This conclusion can be supported by the fact that the smaller seeds of Nubaria 2 benefited more from application of nutrients particularly under salt stress than did the larger seeds of Nubaria 1. This was manifested as moderate reductions in germination parameters and seedling growth of Nubaria 2 under salt stress with the emergence of a 50 mM NaCl threshold, compared with greater and progressive reductions in germination parameters of Nubaria 1.
5 Conclusion
V. faba can be considered salt resistant during germination where the seeds can withstand up to 200 mM NaCl with limited effect on germinability but with marked effect on germination speed. Germination and early seedling growth is strongly determined by genotype, followed by salinity with mild effect of nutrients. Germination speed rather than germination capacity is a more useful tool to screen genotypes according to salt response and the genotypic variability emerged more clearly under salt stress.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Effects of NaCl on the germination, seedling growth and water uptake of triticale. Turkish J. Agric. For.. 2006;30:39-47.
- [Google Scholar]
- Osmotic and ionic effects of NaCl on germination, early seedling growth, and ion content of Atriplex halimus (Chenopodiaceae) Can. J. Bot.. 2002;80:297-304.
- [Google Scholar]
- Effects of salt stress on germination of some maize (Zea mays L.) cultivars. Afr. J. Biotechnol.. 2009;8:4918-4922.
- [Google Scholar]
- Diversity maintenance and use of Vicia faba L. genetic resources. Field Crops Res.. 2010;115:270-278.
- [Google Scholar]
- Nutrients alleviate the deleterious effect of salinity on germination and early seedling growth of the psammophytic grass Elymus farctus. Botany. 2015;93:559-571.
- [CrossRef] [Google Scholar]
- Comparative transcriptional profiling of two contrasting barley genotypes under salinity stress during the seedling stage. Int. J. Genomics. 2013;2013:1-19.
- [CrossRef] [Google Scholar]
- Adaptation of plants to adverse chemical soil conditions. In: Marschner P., ed. Marschner's Mineral Nutrition of Higher Plants (third ed.). Academic Press; 2012. p. :409-472.
- [Google Scholar]
- Legumes: importance and constraints to greater use. Plant Physiol.. 2003;13:872-877.
- [Google Scholar]
- Effect of salinity on seed germination and early growth of five natural populations of Atriplex halimus L. in Morocco. Physiol. Mol. Biol. Plants. 2006;12:247-251.
- [Google Scholar]
- Effect of salinity on growth of five natural populations of Atriplex halimus L. in Morocco. J. Agron.. 2008;7:197-201.
- [Google Scholar]
- Effect of salinity on germination and seed physiology in bean (Phaseolus vulgaris L.) Biotechnol. Biotechnol. Equipment. 2009;23(sup1):326-329.
- [Google Scholar]
- Effects of salt stress levels on five maize (Zea mays L.) cultivars at germination stage. Afr. J. Biotechnol.. 2013;10:12909-12915.
- [Google Scholar]
- Assessment of diversity of phenologically and morphologically related traits among adapted populations of sunflower (Helianthus annuus L.) Helia. 2013;36:29-40.
- [Google Scholar]
- Genetic variability of red clover seedlings in relation to salt stress. Biotechnol. Animal Husbandry. 2014;30:529-538.
- [Google Scholar]
- Effects of salinity different levels on germination indices in four varieties of sunflower (Helianthus annuus L.) Int. Res. J. Appl. Basic Sci.. 2012;3:2043-2051.
- [Google Scholar]
- Effect of salt stress on germination and growth parameters of rice. Notulae Scientia Biologicae. 2014;6:237-243.
- [Google Scholar]
- Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants. 2014;6:1-18. plu047
- [CrossRef] [Google Scholar]
- Broad bean (Vicia faba) consumption and Parkinson's disease. Adv. Neurol.. 1992;60:681-684.
- [Google Scholar]
- How and why to measure the germination process? Revista Brasileira de Botânica. 2006;29:1-11.
- [Google Scholar]
- Microwave-induced stimulation of L-DOPA, phenolics and antioxidant activity in fava bean (Vicia faba) for Parkinson’s diet. Process Biochem.. 2004;39:1775-1784.
- [Google Scholar]
- Biological nitrogen fixation: phosphorus a critical future need? In: Pederosa F.O., Hungria M., Yates G., Newton W.E., eds. Nitrogen fixation: from molecules to crop productivity. Dordrecht, The Netherlands: Kluwer Academic; 2000. p. :509-514.
- [Google Scholar]
- Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol.. 2005;139:822-835.
- [Google Scholar]







