Translate this page into:
GCF cytokine profile comparison between patients with lingual fixed appliances and aligners
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Remodelling of alveolar bone in orthodontics is through optimal force application producing tissue reactions, thus releasing cytokines in gingival crevicular fluid (GCF). The present study compared the cytokine profile amid the treatments with lingual fixed appliances and the aligners.
Patients and methods
80 participants with the requirement of orthodontic treatment were considered for the study. While 40 participants were treated with lingual fixed appliance, others were treated with the aligners. The gingival crevicular fluid specimens were collected pre-treatment and post 3 weeks of treatment from all the participants using a microcapillary pipette and subjected to enzyme-linked immunosorbent assay (ELISA) for cytokine profile assessment comprising of interleukins IL-1α, 1β, 2, 6, 8 and tumor necrosis factor (TNF)-α.
Results
Paired t test expressed a significant (p < 0.001) augmentation of interleukins 1α, 1β, 2, 6, 8 and tumour necrosis factor at baseline in the study groups. Independent sample t test displayed a statistically significant higher TNF-α, IL-1α and IL-1β levels in GCF of teeth treated by lingual appliance than aligners (p = 0.001).
Conclusion
Lingual fixed appliance showed higher cytokines levels than aligners.
Keywords
Aligners
Biomarkers
Bone remodelling
Cytokines
Gingival crevicular fluid
Interleukins
Lingual fixed appliances
1 Introduction
Alveolar bone modelling along with remodelling takes place due to the orthodontic force application producing tissue reactions from the perturbation generated by these orthodontic appliances (Storey, 1973). On application of orthodontic force for an extended time period, initiation of inflammatory process is observed. The osteoclasts appear and bone resoprtion takes place, initiating tooth movement (Proffit and Fields, 2000; Storey, 1973; Teitelbaum, 2000). The disease of periodontium is considered as a condition which is multifactorial involving host factors, microbiological and environmental. Orthodontic appliances performs a salient role in complex etiology of this disease (Verna, 2012). Biologically active substances responsible for osteoclast activation and tissue remodelling are induced in the cells of periodontium from the mechanical stresses from orthodontic appliances. The monitorization of these materials in humans could be elicited non-invasively by examining the variations in the composition of GCF (Hoshino-Itoh et al., 2005).Table 1Table 2.
N
Mean
SD
t Value
P Value
Lingual Appliances
TNF Alpha
Follow-up
20
951.69
212.27
19.83
0.001*
Baseline
20
10.47
1.09
IL-8
Follow-up
20
1.68
0.10
26.001
0.001*
Baseline
20
0.48
0.01
IL-6
Follow-up
20
2.34
0.18
55.865
0.001*
Baseline
20
0.67
0.02
IL-2
Follow-up
20
5.37
0.60
58.316
0.001*
Baseline
20
1.28
0.09
IL-1
Follow-up
20
0.10
0.01
40.967
0.001*
Baseline
20
0.03
0.00
IL-1
Follow-up
20
41.56
1.29
51.249
0.001*
Baseline
20
0.27
0.09
Aligners
TNF Alpha
Follow-up
20
61.6311
8.8963
30.102
0.001*
Baseline
20
9.7811
0.62337
IL-8
Follow-up
20
1.6593
0.08919
37.677
0.001*
Baseline
20
0.4841
0.01293
IL-6
Follow-up
20
2.4114
0.14955
46.044
0.001*
Baseline
20
0.6788
0.02222
IL-2
Follow-up
20
5.46
0.48482
52.35
0.001*
Baseline
20
1.3297
0.07278
IL-1
Follow-up
20
0.0878
0.00501
143.275
0.001*
Baseline
20
0.0256
0.00176
IL-1
Follow-up
20
4.8955
0.17935
114.024
0.001*
Baseline
20
0.3095
0.01356
Factors
Group
N
Mean
SD
Mean Diff
t value
P Value
TNF Alpha
Lingual Appliances
20
941.23
212.68
889.3735
18.685
0.001*
Aligner
20
51.85
8.80
IL-8
Lingual Appliances
20
1.21
0.10
0.03
1.013
0.318
Aligner
20
1.18
0.09
IL-6
Lingual Appliances
20
1.66
0.18
−0.067
−1.299
0.202
Aligner
20
1.73
0.15
IL-2
Lingual Appliances
20
4.08
0.65
−0.0485
−0.266
0.791
Aligner
20
4.13
0.49
IL-1 Alpha
Lingual Appliances
20
0.07
0.01
0.013
5.465
0.001*
Aligner
20
0.06
0.01
IL-1 Beta
Lingual Appliances
20
41.30
1.26
36.7085
129.113
0.001*
Aligner
20
4.59
0.18
Now a days, effective and faster orthodontic treatments are expected from the patients. Varieties of orthodontic brackets are available in the market. Irrespective of the type of appliance, prominent changes in the periodontal tissue homeostasis is promoted by the orthodontic appliance which demonstrates chemical mediators within the gingival sulcus (Čelar et al., 2013). Cytokines induces inflammatory response in the periodontal tissues in a chronic way (Marcaccini et al., 2010). There is increase in the demand of aesthetically comfortable replacement to fixed labial appliances in the very recent years due to which aligners and lingual orthodontics have grown rapidly (Rossini et al., 2015a). As there are varying properties for pro and anti-inflammatory mediators, we assessed the cytokine profile in participants with different orthodontic appliances. The alteration in cytokine levels was demonstrated due to different bracket designs’ (Darveau, 2010).
After the orthodontic force is applied, there is regulation of bone remodelling which takes place by the cytokines produced by the inflammatory cells migrating from periodontal ligament cappillaries which are dilated. (Davidovitch et al., 1988). Cytokines are nothing but protiens that help in signalling between the cells of the immune system. The cytokines (IL-1, IL-6, TNF-α, etc) have multiple activities such as bone resorption, bone remodelling, new bone deposition, etc (Sternberg, 1989, Canalis et al., 1988, Baylink et al., 1993). The key mediator for variety of activities in immune and acute phase inflammatory responses is IL-1. (Dinarello, 1989). During orthodontic tooth movement, there is increase in IL-1β in human gingival fibroblasts (Ngan et al., 1988). At the inflammatory sites, the immune response is regulated by IL-6 having autocrine/ paracrine activity that stimulates osteoclastic activities (Kurihara et al., 1990, Roodman et al., 1991). IL-1β and TNF-α have shown their roles in implicating bone remodelling (Lowney et al., 1995).
Limited information is available in the literature on release of mediators in GCF of humans during the orthodontic treatment with no study comparing cytokine levels in the current study groups. Hence, the study as formulated.
2 Materials and methods
The present study considered participants indicated for orthodontic treatment with lingual fixed appliance and aligners. To avoid the bias due to treatment, patients having clinical presentations which are same (mild malocclusion between 2.1 and 4.0 mm) were included in the study. Random allocation of the included cases into two treatment groups was done to avoid selection bias.(Gelgör et al., 2007; Sidlauskas and Lopatiene, 2009) Forty patients (20 were considered for lingual fixed appliance and 20 with aligners) with an age range of 12–32 years were included. The inclusion criteriae were that the patients should have good general health, healthy periodontium, no history of anti-inflammatory drugs/ antibiotics in the past 6 months, absence of radiographic evidence of periodontal bone loss. Exclusion criteria included history of smoking, presence of gingivitis and periodontitis, and systemic disease. The institutional ethics committee approval (IEC No. LE/IECAPR-18/3) was obtained for the study. Participants signed the informed consent. Maintenance of good oral health was advised for all the included patients. There was periodic checking of oral hygiene levels of the participants.
Lingual metallic brackets (Ormco Corporation, Glendora, CA, USA) were used for bonding in twenty patients using orthodontic light-cured adhesive (Transbond XT) in both the gnathic bones. The initial alignment and levelling was done using 0.012-inch nickel titanium maxillary and mandibular archwires. Twenty other patients were undergoing treatment with Invisalign aligners (Align Technology, San Jose, Calif.).
2.1 GCF sampling
Site of collecting the samples was based on comfort and absence of significant gingival inflammation. Standardization of sample sites for all the subjects was done to the proximal region of canines in the maxillary arch (Gelgör et al., 2007; Gujar et al., 2019; Jamesha et al., 2018; Lerner, 2012; Sidlauskas and Lopatiene, 2009; Yadav et al., 2014). Isolation of the area with sterile gauze was done to prevent the contamination at the sampling site. A micro-capillary pipette (Sigma- Aldrich, Inc., St. Louis, Missouri, United States) was placed at the gingival sulcus entrance to collect the GCF (Bergamo et al., 2018). A standarsized volume of 1 µL of the sample was collected using a calibrated color-coded (0.2–2 µL. Blood and saliva contaminated samples were discarded. These collected samples were transferred to Eppendorf tubes (0.5 mL). The tubes with the samples were centrifuged at 3000 rpm for 10 min. The centrifuged samples were stored until further procedure at −80 °C (Başaran et al., 2006). Samples collection was done at two time points: before initiating the orthodontic treatment –T0 and 21 days after the initiation of treatment –T1. The orthodontic treatment induced indirect resorption starts at 21st day and hence 21st day was selected as the second collection point (T1) (Alfaqeeh and Anil, 2014; Almeida et al., 2015; Başaran et al., 2006; Canavarro et al., 2013; Drummond et al., 2012; Nassrawin, 2018). Using the enzyme linked immunosorbent assay (ELISA), a total of six cytokine molecules were to be evaluated. Thus, six samples (one for each cytokine) from each patient were collected at T0 and T1. Samples were coded to prevent patients identification.
2.2 GCF analysis using ELISA
The stored samples were thawed before being subjected to ELISA. Each sample was diluted ten times in phosphate buffered saline. Commercially testing systems by Raybiotech, Inc. Norcoss, GA, USA were used to analyse the cytokine levels in the samples. In total, the samples collected from the lingual fixed appliance treated cases were 240 and those from the aligner cases were 240, resulting in a total of 480 samples. From each group, out of the 240 samples, a single cytokine molecule was evaluated by collecting 40 samples (20 cases and 20 controls). Hence from a single appliance type, 240 samples were collected for evaluating six cytokine molecules.
2.3 Data analysis
Statistical package for Social sciences (SPSS) version 22.0 was employed. The mean values of interleukins α, 1β, 2, 6, 8 and TNF-α levels (pg/ml) in each group were calculated and compared between the baseline and follow-up using Paired t-test. The mean cytokine levels between the lingual fixed appliances and aligners were compared using Independent sample t-test keeping the p < 0.05 as significance limit.
3 Results
3.1 Comparative analysis
Paired t test showed augmented interleukins 1a, 1β, 2, 6, 8 and TNF-α (p < 0.001) which was statistically significant in the GCF of teeth treated with study groups at the follow-up (Table 1).
3.2 Most active cytokine
Independent sample t test displayed a statistically significant higher TNF-α levels in the GCF of teeth treated with lingual fixed appliance compared to those treated with aligners. (P = 0.001). Similarly, IL-1α and IL-1β levels also displayed statistically significant higher in lingual appliance compared to aligners (P = 0.001) (Table 2 and Figs. 1-3).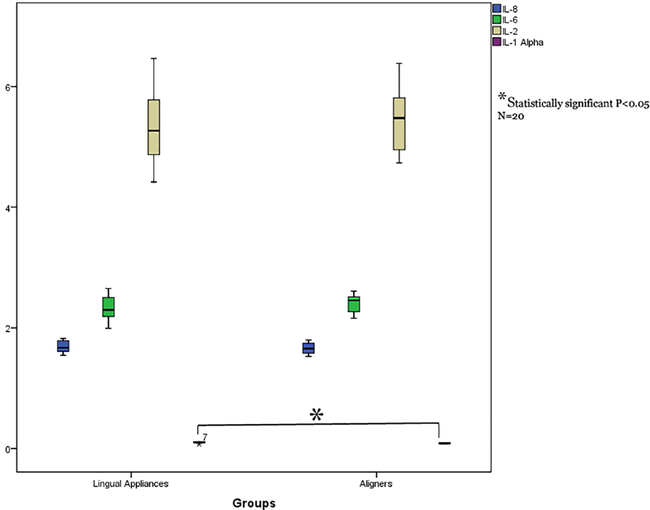
Comparison interleukin 1α, 2, 6, 8 levels in GCF of study groups (lingual fixed vs aligners).
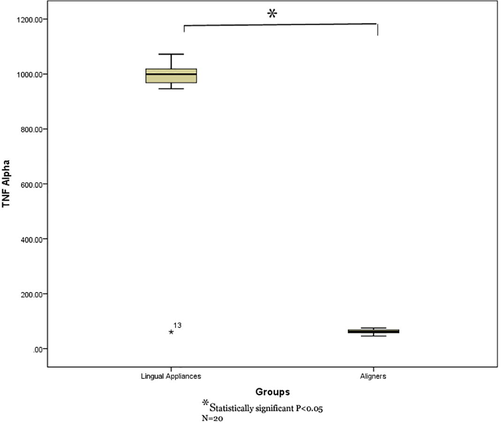
Comparison of TNF-α levels in GCF of study groups (lingual fixed vs aligners).
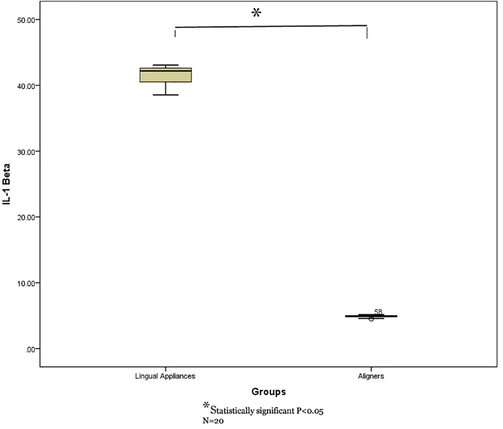
Comparison of IL-1β levels in GCF of study groups (lingual fixed vs aligners).
3.3 Least active cytokines
No significant variation was noted in interleukins 2, 6 and 8 in study groups. (Table 2 and Figs. 1-3).
4 Discussion
Alveolar bone remodelling shows tissue reactions due to the forces produced by orthodontic appliances. The osteoclasts’ appearance and bone resorption are considered as the crucial factors for tooth movement (Proffit and Fields, 2000). This orthodontic tooth movement causes inflammatory response which produces and releases varieties of cytokines.(Verna, 2012) The initial reactions of periodontal tissues to the mechanical stress is caused by the forces induced by the orthodontic appliances responsible for several metabolic changes that leads to the tooth movement. These mechanical stresses induces cells in periodontium to express different cytokines and enzymes which are responsible for connective tissue remodelling and be studied non-invasively through evaluating vatiations in GCF composition in humans during the orthodontic treatment.(Hoshino-Itoh et al., 2005).
Due to an increase in demand for aesthetic yet comfortable alternative to labial fixed orthodontic appliances, there is increased in usage of aligners in the recent years.(Rossini et al., 2015a) The maintenance of oral hygiene in better with aligners in comparison to fixed orthodontic appliances.(Karkhanechi et al., 2013; Luca et al., 2015; Rossini et al., 2015b) Limited studies are available explaining the metabolism of bone caused due to it. The most important factors in plaque accumulation is the design of this appliance. (Rody et al., 2014) Despite early innovation, only after Invisalign system was launched by Align technology (Santa Clara, CA, USA) and introducing CAD/CAM to orthodontics, did the technology flourish (Lombardo et al., 2017). Using a software programme, polymer based aligner series were made for virtually positioning the tooth (Abbate et al., 2015). There is combination of removable and fixed appliances in this system where the clear splints made up of plastic covering all teeth including gingival margins moving the teeth gradually into its ideal positions (Miethke and Vogt, 2005).
Cytokines act as biomarker and initiate osteoclast formation and bone resorbing activities (Al-Ghurabi et al., 2014; Rody et al., 2014). The cytokine IL-1 plays a pivotal role in osteoclast fusion, survival and activation. IL-1 and IL-1β are secreted chiefly by the monocytes and partially by macrophages, fibroblasts and endothelial cells (Stylianou and Saklatvala, 1998; Hofbauer et al., 1999). Majority of the studies demonstrate activation of inflammatory cytokine activation through mechanical stimuli (Sandy et al., 1993; Alhashimi et al., 2001). Bone metabolism is affected directly by IL-1β and TNF-α. Even at exceptionally minimal levels, IL-1β and TNF-α have been seen involved in the bone remodelling procedure by the action of the specific receptors present on bone cell populace (Bertolini et al., 1986; Gowen et al., 1983; Saito et al., 2009).
Studies have recorded that IL-6 levels increased during the initial stages of orthodontic treatment only. This could perhaps describe the minimal fluctuations, rather than a steady rise of IL-6 in the present study (Saito et al., 1990). IL-8 receptors were precisely localized in noninflamed human gingiva and in periodontitis suggesting it to play multifunctional role in diseases of periodontium (Sfakianakis et al., 2002). In an earlier study it was shown that IL-8 levels increased after orthodontic treatment (Tuncer et al., 2005).
Gujar et al observed an elevated expression of interleukins 1α, 1β, 2, 6, 8 and TNF-α about 21 days post treatment with the current study groups with augmentation being greater in interleukin-1β and TNF-α than others (Gujar et al., 2019).
Increase in the use of lingual fixed appliance is seen due to increasing demand for esthetics. There is greater risk of plaque accumulation due to their site of placement as compared to labial fixed appliance (Serra et al., 2003). Also, there is increased risk of microbial accumulation and damage to the periodontium due to lingual fixed appliance compared to labial orthodontic therapy according to Demling et al. (Demling et al., 2009).
Another study done by Gujar et al showed the cytokine levels were higher in lingual fixed appliance treated cases in comparison to the labial appliance, indicating that the lingual appliance could potentially be exerting greater mechanical stress on the periodontium than the labial fixed appliance. Based on their data, increased expressions of TNF–α and IL–1β noted in the lingual fixed appliance could be the result of greater mechanical stress applied on the periodontium by lingual fixed appliance (Gujar et al., 2020).
Taking into consideration the various factors and biological responses in the periodontal ligament and GCF, the limiting factor in the present study could be the lower sample size and shorter observation time (21 days). Further studies should therefore be carried out considering these limitations.
5 Conclusion
Interleukins 1α, 1β, 2, 6, 8, and TNF–α were augmented in the study groups at 21 days following post treatment. TNF–α, interleukins 1α and 1β showed significant higher levels in lingual fixed appliances than aligners.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Periodontal health in teenagers treated with removable aligners and fixed orthodontic appliances. J. Orofac. Orthop./Fortschritte der Kieferorthopädie. 2015;76(3):240-250.
- [Google Scholar]
- Evaluation of salivary levels of proinflammatory cytokines (IL- 1$α$, IL-8 and GM-CSF) in adult orthodontic patients. IOSR J. Dent. Med. Sci.. 2014;13(3):75-78.
- [Google Scholar]
- Gingival crevicular fluid flow rate and alkaline phosphatase level as potential marker of active tooth movement. Oral Health Dent. Manag.. 2014;13:458-463.
- [Google Scholar]
- Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am. J. Orthod. Dentofac. Orthop.. 2001;119:307-312.
- [CrossRef] [Google Scholar]
- Levels of gingival crevicular fluid matrix metalloproteinases in periodontally compromised teeth under orthodontic forces. Angle Orthod.. 2015;85:1009-1014.
- [CrossRef] [Google Scholar]
- Interleukine-1beta and tumor necrosis factor-alpha levels in the human gingival sulcus during orthodontic treatment. Angle Orthod.. 2006;76:830-836.
- [CrossRef] [Google Scholar]
- Growth factors to stimulate bone formation. J Bone Miner Res. 1993;8(suppl):S565-S575.
- [Google Scholar]
- Cytokine profile changes in gingival crevicular fluid after placement different brackets types. Arch. Oral Biol.. 2018;85:79-83.
- [Google Scholar]
- Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516-518.
- [CrossRef] [Google Scholar]
- Growth factors and the regulation of bone remodeling. J Clin Invest. 1988;81(2):277-281.
- [Google Scholar]
- Matrix metalloproteinases -1, -2, -3, -7, -8, -12, and -13 in gingival crevicular fluid during orthodontic tooth movement: a longitudinal randomized split-mouth study. Eur. J. Orthod.. 2013;35:652-658.
- [CrossRef] [Google Scholar]
- Systematic review on self-ligating vs. conventional brackets: initial pain, number of visits, treatment time. J. Orofac. Orthop. / Fortschritte der Kieferorthopädie. 2013;74(1):40-51.
- [Google Scholar]
- Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol.. 2010;8:481-490.
- [CrossRef] [Google Scholar]
- Neurotransmitters, cytokines and the control of alveolar bone remodeling in orthodontics. Dent Clin North Am. 1988;32:411-435.
- [Google Scholar]
- Influence of lingual orthodontic therapy on microbial parameters and periodontal status in adults. Eur. J. Orthod.. 2009;31:638-642.
- [CrossRef] [Google Scholar]
- Interleukin 1 and its biologically related cytokines. Adv Immunol. 1989;44:153-205.
- [Google Scholar]
- The monitoring of gingival crevicular fluid volume during orthodontic treatment: a longitudinal randomized split-mouth study. Eur. J. Orthod.. 2012;34:109-113.
- [CrossRef] [Google Scholar]
- Prevalence of malocclusion among adolescents in central anatolia. Eur. J. Dent.. 2007;01(03):125-131.
- [Google Scholar]
- An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983;306:378-380.
- [CrossRef] [Google Scholar]
- Comparison of biochemical markers of bone metabolism between conventional labial and lingual fixed orthodontic appliances. Niger. J. Clin. Pract.. 2020;23:568-573.
- [CrossRef] [Google Scholar]
- Cytokine levels in gingival crevicular fluid during orthodontic treatment with aligners compared to conventional labial fixed appliances: a 3-week clinical study. Acta Odontol. Scand.. 2019;77:474-481.
- [CrossRef] [Google Scholar]
- Interleukin-1β and tumor necrosis factor-α, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255-259.
- [CrossRef] [Google Scholar]
- Levels of t-PA and PAI-2 in gingival crevicular fluid during orthodontic tooth movement in adults. Aust. Orthod. J.. 2005;21:31-37.
- [Google Scholar]
- Comparison of gingival crevicular fluid periostin levels in healthy, chronic periodontitis, and aggressive periodontitis. J. Indian Soc. Periodontol.. 2018;22:480.
- [CrossRef] [Google Scholar]
- Periodontal status of adult patients treated with fixed buccal appliances and removable aligners over one year of active orthodontic therapy. Angle Orthod.. 2013;83:146-151.
- [CrossRef] [Google Scholar]
- Interleukin-6 stimulates osteoclast-like multinucleated cell formation in long-term human marrow cultures by inducing IL-1 release. J Immunol. 1990;144:426-430.
- [Google Scholar]
- Osteoblasts, osteoclasts, and osteocytes: unveiling their intimate-associated responses to applied orthodontic forces. Semin. Orthod.. 2012;18(4):237-248.
- [Google Scholar]
- Predictability of orthodontic movement with orthodontic aligners: a retrospective study. Prog. Orthod.. 2017;18:35.
- [CrossRef] [Google Scholar]
- Orthodontic force increases tumor necrosis factor-α in the human gingival sulcus. Am J Orthod Dentofacial Orthop. 1995;108:519-524.
- [Google Scholar]
- Scanning electron microscopy analysis of the growth of dental plaque on the surfaces of removable orthodontic aligners after the use of different cleaning methods. Clin. Cosmet. Investig. Dent.. 2015;125
- [CrossRef] [Google Scholar]
- Myeloperoxidase activity is increased in gingival crevicular fluid and whole saliva after fixed orthodontic appliance activation. Am. J. Orthod. Dentofac. Orthop.. 2010;138:613-616.
- [CrossRef] [Google Scholar]
- A comparison of the periodontal health of patients during treatment with the Invisalign® system and with fixed orthodontic appliances. J. Orofac. Orthop./Fortschritte der Kieferorthopädie. 2005;66(3):219-229.
- [Google Scholar]
- Detection of ostecalcin in gingival crevicular fluid in a group of orthodontic patients. J. Int. Soc. Prev. Community Dent.. 2018;8:168.
- [CrossRef] [Google Scholar]
- Immunohistochemical assessment of the effect of chemical and mechanical stimuli on cAMP and prostaglandin E levels in human gingival fibroblasts in vitro. Arch Oral Biol. 1988;33(3):163-174.
- [Google Scholar]
- Proffit, W.R., Fields, H.W., 2000. Contemporary Orthodontics, 3rd ed.
- Differences in the gingival crevicular fluid composition between adults and adolescents undergoing orthodontic treatment. Angle Orthod.. 2014;84:120-126.
- [CrossRef] [Google Scholar]
- Interleukin-6: a potential autocrine/paracrine factor in Paget’s disease of bone. J Clin Invest. 1991;89(1):46-52.
- [Google Scholar]
- Efficacy of clear aligners in controlling orthodontic tooth movement: A systematic review. Angle Orthod.. 2015;85:881-889.
- [CrossRef] [Google Scholar]
- Periodontal health during clear aligners treatment: a systematic review. Eur. J. Orthod.. 2015;37:539-543.
- [CrossRef] [Google Scholar]
- Interactive effects between cytokines on PGE production by human periodontal ligament fibroblasts in vitro. J. Dent. Res.. 1990;69:1456-1462.
- [CrossRef] [Google Scholar]
- Bone-resorbing activity and prostaglandin E produced by human periodontal ligament cells in vitro. J. Bone Miner. Res.. 2009;5:1013-1018.
- [CrossRef] [Google Scholar]
- Recent advances in understanding mechanically induced bone remodeling and their relevance to orthodontic theory and practice. Am. J. Orthod. Dentofac. Orthop.. 1993;103:212-222.
- [CrossRef] [Google Scholar]
- Lactate dehydrogenase activity in gingival crevicular fluid during orthodontic treatment. Am. J. Orthod. Dentofac. Orthop.. 2003;124:206-211.
- [CrossRef] [Google Scholar]
- Localization of the chemokine interleukin-8 and interleukin-8 receptors in human gingiva and cultured gingival keratinocytes. J. Periodontal Res.. 2002;37:154-160.
- [CrossRef] [Google Scholar]
- The prevalence of malocclusion among 7–15-year-old Lithuanian schoolchildren. Medicina (Kaunas).. 2009;45:147-152. 19289905
- [Google Scholar]
- Levels of interleukin-8 during tooth movement. Angle Orthod.. 2005;75:631-636.
- [CrossRef] [Google Scholar]
- Verna C, M.B., 2012. Adult Orthodontics, in: Tissue Reaction. pp. 78–98.
- Effect of periodontal therapy on lactoferrin levels in gingival crevicular fluid. Aust. Dent. J.. 2014;59:314-320.
- [CrossRef] [Google Scholar]







