Translate this page into:
Gastrodia elata extract exerts anti-obesity effects on obese mice caused by a high-starch diet by suppressing SREBP-1 and ChREBP
⁎Corresponding author at: Ilwonbio Co., Ltd, & Department of Physiology, College of Korean Medicine, Wonkwang University, 460 Iksandaero, Iksan, Jeonbuk 54538, South Korea. desson@wku.ac.kr (Kang-Beom Kwon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Gastrodia elata (GE), a natural treatment, is extensively used in traditional Korean medicine. The study's goal was to look into how GE affected obese mice that had been fed a high-starch diet (HSD).
Methods
The C57BL/6 J mice used in this investigation were given HSD to make them obese. Gastrodia elata extract (GEE) was administered orally to the mice throughout the course of the next five weeks in doses of 100, 200, and 500 mg/kg. When the experiment was over, we measured the animals body weight, liver and fat weight, blood biochemical parameters, and gene expression that is related to obesity.
Results
The overall body weight of animals was dramatically lowered via GEE oral treatment. In comparison to the HSD group, the serum and liver TG levels were considerably lower in the GEE-treated group. Additionally, regional fatty tissues and liver weight markedly dropped in the GEE-treated batch in comparison to the HSD batch. We observed considerably lower levels of PPAR-γ, C/EBP-α, and ChREBP mRNA expression level in the GEE-treated group in addition to lower levels of SREBP-1, ACC, and FAS protein expression.
Conclusion
The studies show that GEE has anti-obesity advantages in the mouse model of obesity caused by HSD. Despite the fact that we utilized mice as a model for the experiment rather than people, who have different biochemical pathways, the finding has significant implications for how GEE generally functions.
Keywords
Gastrodia elata
Anti-obesity
High starch diet
SREBP-1
ChREBP
1 Introduction
Since its discovery in 1980, non-alcoholic fatty liver disease (NAFLD) has shared pathological characteristics with hepatic disease caused by alcoholics, with the exception of alcohol usage. The cause of this illness still hasn't fully been understood despite of forty years of research. The prevalence of sufferers of NAFLD is rising quickly, and greater than a quarter of the world's population suffers from it. The main contributors to NAFLD include a lack of physical activity and a diet loaded with of sugar and cholesterol. Consumers frequently substitute high-sugar diets, primarily starchy carbs, for foods high in fat in order to lower calorie intake and achieve satiety in order to treat or prevent NAFLD. Yet, compared to investigations on fat consumption, there are significantly fewer investigations that focus on the impact of dietary carbs on liver function with NAFLD.
There are 3 phases to the origins of carbs eating in human history: (1) As rice became a staple food, it gradually took the place of foods like animal flesh, fruit, and vegetables that were acquired through prehistoric hunter-gatherer lifestyles. (2) The carbohydrate became a widely available consumer good thanks to the large production of sugar cane. Beginning to arrive were cookies, fruit preserves, and packaged meals. The manufacturing of carbohydrates encouraged consumers to switch from natural foods to processed ones and to consume more carbs. (3) Following the Second World War, sugar syrup and other ultra-processed carbs became available all over the place, while the cost of unrefined carbs fell sharply (Kroemer et al., 2018). After that time forward, usage of sugars has spread across society and became essential to daily life.
Three substances, oxygen, hydrogen, and carbon make up sugars. Following ingestion, sugars breakdown into smaller molecules including cellulose, lactose, and monosaccharides (such as fructose and glucose). These less complex types of sugars have comparable constituents in common, yet they perform somewhat differently and go through distinct processes of metabolism in the human system. Fructose, glucose, sucrose, and other sugars are examples of mono- and disaccharides. The two basic types of polysaccharides are cellulose and starch. Based on their structural differences, starchy sugars are commonly classified as resistant starch, amylose, and amylopectin. Amylopectin has the greatest impact on postprandial blood sugar within starchy carbs then resistant starch and amylose. The majority of the processed starchy foods which are frequently consumed in everyday circumstances, like steamed buns, white noodles, and white rice, contain a substance called amylopectin the “culprit” behind the sudden rise in blood sugar levels following dinners. A whole-grain food has a less impact on weight gain and widespread inflammation than a food high in processed grain has on insulin sensitivity or the composition of the gut flora (Roager et al., 2019).
A common achlorophyllous orchid known as Gastrodia elata (family Orchidaceae) is highly prized for its medicinal and culinary properties. Gastrodia elata is mostly found in Oceania, Southeast Asia, and East Asia (Chen et al., 1999; Liu et al., 2015). In Asian nations like China, Korea, and Japan, its tuber (mature rhizome) is a well-liked medicinal remedy (Ahn et al., 2007; Tang and Eisenbrand, 1992). Additionally, the root has been consumed as foodstuff and is frequently prepared in soups. Extracts of G. elata or its compounds that are active have pharmacological and health-improving properties, such as neuroprotective and anticancer actions (Liu et al., 2018; Heo et al., 2007; Park et al., 2015; Xian et al., 2016). This medication has been used extensively to treat paralysis, dizziness, epilepsy, and hypertension, particularly in Asia (Ojemann et al., 2006; Xiong et al., 2013). In this region, G. elata has also been used to address cognitive impairments and prevent neurodegeneration, vascular dementia and Parkinson’s disease (Liu et al., 2018; Xian et al., 2016; Shi et al., 2020).
A variety of phenolic molecules, organic acids and sterols have been found in G. elata through earlier phytochemical examinations. These substances possess a range of health-improving actions including anticonvulsant, neuroprotective, anti-inflammatory, and antioxidant properties (Ojemann et al., 2006; Lee et al., 2006; Huang et al., 2006; Jung et al., 2007). It has been shown, for instance, that a number of phenolic compounds, including vanillin, gastrodin, and gastrodigenin, protect neuronal cells and enhance neurological function by minimizing damage brought on by oxidative stress and inflammatory responses (Jang et al., 2015). However, there hasn't been any research done on how Gastrodia elata extract (GEE) affects obesity brought on by a high starch diet. As a result, we looked into GEE's anti-obesity effects in a mouse model.
2 Materials and methods
2.1 Plant material preparation of crude GE extract
The plant was recognized and verified by Dr. Chang-Su Kim of the Jeollabuk-Do Agricultural Research & Extension Services in Jinan, Jeonbuk, South Korea. At the Department of Herbology, Wonkwang University Korean Medical School, South Korea, a voucher specimen was deposited. 1 fold of dried GE rhizome has been boiled for 6 h at 90°Celsius in 15 folds of deionized water to produce GEE. The filter paper was used to filter the final product to produce the crude extract. The remaining rhizome was then mixed with an additional 15 folds of deionized water; the combination was once more boiled for 6 h at 90 °C in order to obtain the additional extract. The two extracts were mixed together, concentrated, and then the end result was spray-dried before being kept for future use. The yield was 30.2%.
2.2 Mouse model of HSD-induced obesity
Male C57BL/6N mice aged 8 weeks (20 ± 2 g) were bought from Seongnam, Korea's Orient Bio, Inc. In this study, 40 perfectly normal mice have been utilized as the study's test subjects. These animals spent a week in a lab environment before the study properly began. Following this, mice (n = 8) were randomized into any of the five distinct groups: the 22% low starch-diet group (LSD), the 56.87% high starch-diet group (HSD), and the GEE-treated groups (GEE 100, 200, or 500 mg/kg p.o.). Both water and food were available to the mice at all times. All mice other than those in the LSD group were given an HSD. The GEE-treated groups received GEE orally, whereas the LSD and HSD groups received physiological saline as a treatment. Animals were kept in a 12/12-hour-dark environment with a humidity level of 50 ± 5% and a temperature of 22 ± 2 °C. Records of body weight were recorded on a weekly basis. The mice were fed for six hours after an overnight fast, after which they were euthanized, and blood was drawn through a heart puncture. The specimens of blood have been spun at 2,000 × g for fifteen minutes at 4˚C after being allowed to clot for 30 min at room temperature. Laboratory animal care and use is governed by the Ethical Committee of Wonkwang University (reg. no. WKU21-84) gave its approval to all procedures, which followed the guidelines provided by the National Institutes of Health. The nutritional makeup of mice's diet is displayed in Table 1.
Low starch diet
(LSD)High starch diet
(HSD)
Corn starch, g%
22
56.869
Sucrose, g%
2
2
Protein, g%
17.7
12.39
Fat, g%
4.16
4.16
2.3 Biochemical analysis
Using the proper test kits and following the manufacturer's instructions (Asan Pharmaceutical Co. Ltd., Seoul, Korea), the serum and liver TG levels were determined.
2.4 Western blot analysis
In order to extract protein from tissue, RIPA buffer was utilized. A Bradford assay kit (Bio-Rad Laboratories, Hercules, California) was used to measure the protein concentration. Electrophoresis on polyacrylamide gels with sodium dodecyl sulfate at a concentration of 8–12% separated exactly same amounts of protein, which were subsequently moved to Polyvinylidene difluoride (PVDF) membranes. The primary antibody was incubated on the blot for the overnight period at 4 °C after blocking solution (5% skim milk) was added to the blot for 1 h at room temperature. After being washed 3 times with Tween 20/Tris-buffered saline (T/TBS), blots were incubated with a secondary antibody coupled to horseradish peroxidase for two hours at room temperature. T/TBS was used once again to wash the blots three times before being developed using enhanced chemiluminescence solution and the Imaging system (Tokyo, Japan: AE-9300 Ez-capture MG/AE-9160 Ez-capture ST) was used to capture images of the protein bands.
2.5 Quantiative Reverse-Transcription polymerase chain reaction (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from liver tissue in line with the manufacturer's recommendations to analyze the genes linked with adipogenesis. A kit for cDNA synthesis from TaKaRa in Shiga, Japan was used to make complementary DNA (cDNA). According to the instructions provided by the manufacturer, qRT-PCR was carried out using a Power SYBR Green PCR Master Mix (Applied Biosciences, CA, USA). The following were the gene-specific primers for the genes in liver tissues that are linked to obesity: Forward 5′– CGTCCCGTAGACAAAATGGT–3′ and reverse 5′– TTGATGGCAACAATCTCCAC –3′ for mouse GAPDH, forward 5′– GTACTGTCGGTTTCAGAAGTGCC–3′ and reverse 5′– ATCTCCGCCAACAGCTTCTCCT–3′ for mouse PPAR-γ, forward 5′– GCGAGCACGAGACGTCTATAGA–3′ and reverse 5′– GCCAGGAACTCGTCGTTGAA–3′ for mouse C/EBP-α, and forward 5′– GCTTTGACCAGATGCGGGACA–3′ and reverse 5′– AGTGCTGAGTTGGCGAAGGGA–3′ for mouse ChREBP. The expression levels of each group were expressed as fold changes in comparison to the HFD group, and relative expression levels were standardized to mouse GAPDH expression.
2.6 Statistical evaluation
Version 26.0 of the SPSS Software Package (IBM, NY, USA) was used for the statistical analysis, which included Duncan's multiple comparison evaluation. The data were provided as mean standard deviation (SD), with p < 0.05 being the significance level.
3 Results
Every week, the body weights of the animals were recorded. Comparing the mice fed with HSD and LSD at the end of the fifth week, it was clear that the HSD-fed mice had significantly more visible body fat. GEE was administered orally at doses of 100, 200, or 500 mg/kg, resulting in a decrease in body weight compared to the HSD group (Fig. 1). After the fifth week, the HSD-fed mice showed considerably more visible increases in liver and fat mass as compared to the LSD-fed mice. The liver and fat mass were decreased when GEE was given orally at a dose of 500 mg/kg in contrast to the HSD group (Fig. 2).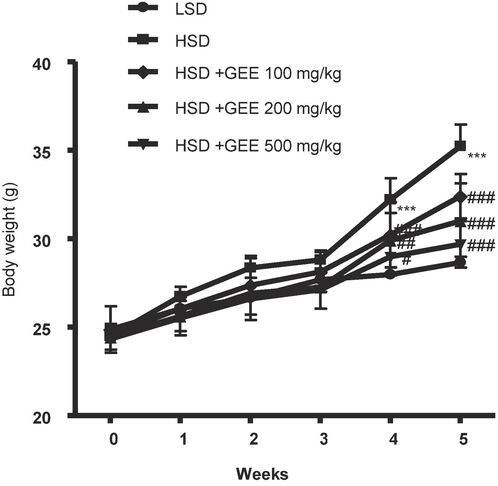
Effect of Gastrodia elata extract (GEE) on body weight in high starch diet (HSD)-fed mice. ***p < 0.001 vs. LSD group, #p < 0.05, ##p < 0.01, ###p < 0.001 vs. HSD group. n = 8.
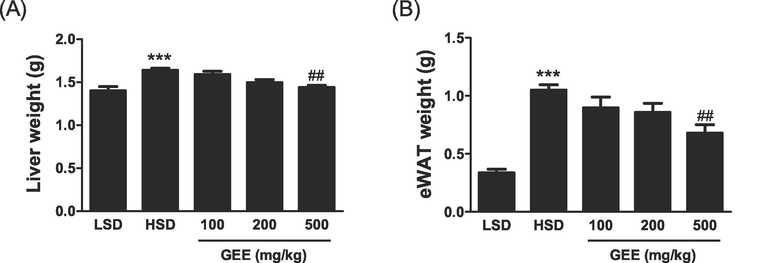
Effect of Gastrodia elata extract (GEE) on the liver (A) and epididymal fat (B) weights in high starch diet (HSD)-fed mice. ***p < 0.001 vs. LSD group, ##p < 0.01 vs. HSD group. n = 8.
In comparison to the LSD group, the HSD group's serum TG concentrations were noticeably higher. In mice given an HSD, the level of serum TG was 125.84 ± 8.68 mg/dL; however, when given 500 mg/kg of GEE, the serum TG level was lowered to 103.36 ± 9.32 mg/dL (Fig. 3A). The HSD group had significantly greater liver TG levels than the LSD group. When 500 mg/kg of GEE was administered to mice, the liver's TG level dropped to 23.03 ± 2.16 mg/g proteins from 28.56 ± 2.91 mg/ g proteins in HSD-treated mice (Fig. 3B).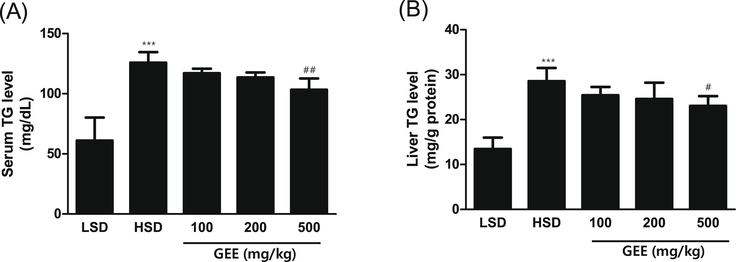
Serum TG (A) and liver TG (B) levels in high starch diet (HSD)-fed mice with effect of Gastrodia elata extract (GEE). ***p < 0.001 vs. LSD group, #p < 0.05, ##p < 0.01 vs. HSD group. n = 8.
SREBP-1, ACC, and FAS levels of protein expression were higher in the livers of the HSD group compared to the LSD group and decreased in the groups that had undergone GEE treatment (Fig. 4). When GEE was administered, the hepatic PPAR-γ, C/EBP-α, and ChREBP mRNA expression levels were noticeably suppressed. Comparing the HSD group to the LSD group, these levels were up-regulated (Fig. 5).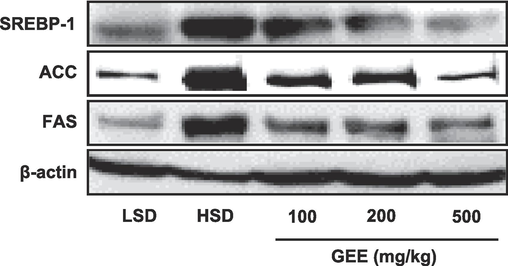
Effects of Gastrodia elata extract (GEE) on liver obesity-related protein expression in high starch diet (HSD)-fed mice. SREBP-1, ACC, FAS protein expressions were determined by western blot analysis.
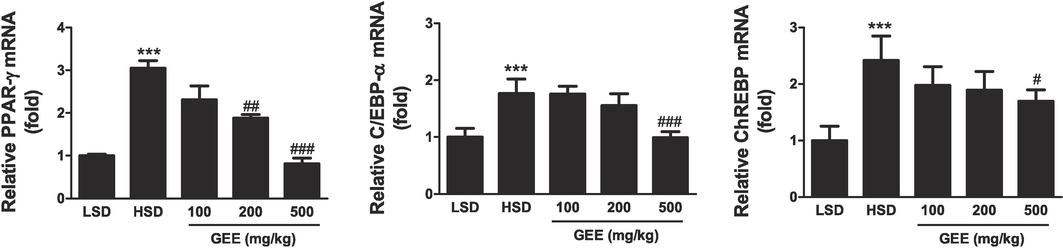
Effect of Gastrodia elata extract (GEE) on Hepatic mRNA expression level of obesity-related gene in high starch diet (HSD)-fed mice. Hepatic mRNA expressions were determined by Real-time PCR analysis. (A) Quantitative analysis of PPAR-γ mRNA expression. (B) Quantitative analysis of C/EBP-α mRNA expression. (C) Quantitative analysis of ChREBP mRNA expression. ***p < 0.001 vs. LSD group, #p < 0.05, ##p < 0.01, ###p < 0.001 vs. HSD group. n = 8.
4 Discussion
The majority of animals, including humans, rely on starch as a source of nutrition because of its unique flavor and low cost. Even some of them choose to eat a lot of starchy meals. According to studies (Lee et al., 2011; Vilà et al., 2014; Feng et al., 2015), chronic consumption of high-starch diets has been linked to a range of diseases, including obesity, non-alcoholic fatty liver disease (NAFLD) and hyperlipidemia. Hoekstra et al. (2013) state that the liver performs vital physiological tasks such nutrition metabolism, detoxification, and absorption in the animal body. Thus, liver dysfunction from an excess of nutrients is a simple consequence. According to research done on rats, a high-fat diet led to severe steatosis, enlarged hepatocytes, and a markedly greater rate of apoptosis (BedÊ et al., 2020). Panchal et al. (2011) and Ip et al. (2014) found that rats on a high-carb diet were more likely to grow fat, suffer hepatitis, and experience oxidative stress in their livers. These ailments can damage the liver and potentially result in liver cancer. Consequently, consuming too many carbohydrates is bad for the liver.
A study by Greenberg and Obin (2006), an imbalance between the consumption and use of energy is what leads to obesity, and the extra energy builds up as TG in fat tissue. To test GEE's ability to prevent obesity, we utilized mice that had been made obese by HSD. We verified that a 5-week GEE therapy reduced body weight increase without altering calorie intake which is consistent with earlier research (Gao et al. 2023). Additionally, eWAT (epididymal white adipose tissue) weights in GEE plus HSD fed mice were considerably lower than those in the HSD fed group. These findings imply that GEE treatment affects body weight growth reduction and is probably connected to decreased eWATs weight. Additionally, our findings showed that mice given HSD and a GEE supplement had considerably lower serum and liver TG levels. These findings imply that GEE may lessen hyperlipidemia brought on by HSD.
This study shows that an HSD can worsen NAFLD by enhancing the influx of fatty acids into the liver, which is brought on by the enhanced expression of PPAR-γ, C/EBP-α, ChREBP, and SREBP-1 mediated molecular pathways. The levels of the lipogenic enzymes Acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), as well as their transcription factors carbohydrate-responsive element binding protein (ChREBP) (Dentin et al., 2005) and sterol regulatory element binding protein 1 (SREBP-1), are all influenced by a diet high in carbohydrates, according to research (Bray et al., 2004; Dentin et al., 2005). Based on studies, ACC, FAS, SREBP-1, and ChREBP levels rise on a carbohydrate-rich diet compared to fasting levels (Dentin et al., 2004).
In order to comprehend the mechanism underlying the decrease in body fat brought on by GEE, we looked at the levels of expression of genes and proteins linked to adipogenesis in liver tissue in HSD-induced obese mice. Vidal-Puig et al. (1996) claim that PPAR-γ is a transcription factor that is ligand-activated and mediates the expression of genes specific to fat. It also drives adipocyte differentiation and adipogenesis. The late phases of adipogenesis also result in the production of the protein C/EBP, which controls adipocyte differentiation by collaborating with PPAR to promoting differentiation (Farmer, 2005). SREBP-1, which promotes the expression and synthesis of PPAR-ligands, can also activate PPAR-γ. A transcription factor called SREBP-1 regulate the synthesis of fatty acids, cholesterol, and LDL receptors while also activating the genes and proteins involved in lipogenesis in the liver, including Acetyl-CoA carboxylase and (ACC) and FAS (Farmer, 2005). GEE medications prevent adipogenesis and lipid accumulation in HSD-induced adipose tissues, as evidenced by the decreased mRNA expression of PPAR-γ, C/EBP-α, and ChREBP. Our research also revealed that GEE therapy significantly reduced the levels of adipogenesis-linked protein expression in the liver tissue of obese HSD-fed mice, including SREBP-1c, ACC, and FAS.
5 Conclusion
In conclusion, the GEE dose-dependently inhibited the major transcription factors and markedly reduced WATs weight and body weight gain in HSD-induced obese mice. This shows that the GEE may prevent the obesity caused by a high-starch diet. As a result of these findings, GEE may represent a promising herbal treatment for obesity.
Acknowledgement
This study was carried out with the support of the research program for 'Development of regionally specialized crop technology' of Rural Development Administration, Republic Korea (Project No.: RS-2021-RD012527).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-inflammatory and anti-angiogenic activities of Gastrodia elata Blume. Journal of Ethnopharmacology. 2007;110(3):476-482.
- [CrossRef] [Google Scholar]
- Effects of grape juice, red wine and resveratrol on liver parameters of rat submitted high-fat diet. Anais da Academia Brasileira de Ciências. 2020;92(2):e20191230.
- [Google Scholar]
- Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr.. 2004;79(4):537-543.
- [CrossRef] [Google Scholar]
- Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. The Journal of Biological Chemistry. 2004;279(19):20314-20326.
- [CrossRef] [Google Scholar]
- Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87(1):81-86.
- [CrossRef] [Google Scholar]
- Regulation of PPARgamma activity during adipogenesis. Int. J Obes (Lond). 29. Suppl.. 2005;1:S13-S16.
- [CrossRef] [Google Scholar]
- High carbohydrate intake from starchy foods is positively associated with metabolic disorders: a Cohort Study from a Chinese population. Sci. Rep.. 2015;5:16919.
- [CrossRef] [Google Scholar]
- High-starchy carbohydrate diet aggravates nafld by increasing fatty acids influx mediated by Nox2. Food Sci. Hum. Wellness. 2023;12(4):1081-1101.
- [CrossRef] [Google Scholar]
- Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr.. 2006;83(2):461S-465S.
- [CrossRef] [Google Scholar]
- Anti-tumor activity of Gastrodia elata Blume is closely associated with a GTP-Ras-dependent pathway. Oncol. Rep.. 2007;18(4):849-853.
- [Google Scholar]
- Physiological and biochemical basis of clinical liver function tests: a review. Ann. Surg.. 2013;257(1):27-36.
- [CrossRef] [Google Scholar]
- The protective effects of phenolic constituents from Gastrodia elata on the cytotoxicity induced by KCl and glutamate. Archives of Pharmacal Research. 2006;29(11):963-968.
- [CrossRef] [Google Scholar]
- High-refined-carbohydrate and high-fat diets induce comparable hepatic tumorigenesis in male mice. J. Nutr.. 2014;144(5):647-653.
- [CrossRef] [Google Scholar]
- Neuropharmacological potential of Gastrodia elata Blume and its components. Evid. Based Complement. Alternat. Med.. 2015;2015:1-14.
- [CrossRef] [Google Scholar]
- Protective effects of several components of Gastrodia elata on lipid peroxidation in gerbil brain homogenates. Phytotherapy Research. 2007;21(10):960-964.
- [CrossRef] [Google Scholar]
- Carbotoxicity-Noxious Effects of Carbohydrates. Cell. 2018;175(3):605-614.
- [CrossRef] [Google Scholar]
- Effects of Different Types of Fat or Carbohydrate Sources on Hyperlipidemia and Obesity in Laboratory Animals. The FASEB Journal. 2011;25:991.
- [CrossRef] [Google Scholar]
- Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Archives of Pharmacal Research. 2006;29(10):849-858.
- [CrossRef] [Google Scholar]
- A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol.. 2018;9:24.
- [CrossRef] [Google Scholar]
- Highly diversified fungi are associated with the achlorophyllous orchid Gastrodia flavilabella. BMC Genomics. 2015;16(1):185.
- [CrossRef] [Google Scholar]
- Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy & Behavior. 2006;8(2):376-383.
- [CrossRef] [Google Scholar]
- Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr.. 2011;141(6):1062-1069.
- [CrossRef] [Google Scholar]
- Prolonged oral administration of Gastrodia elata extract improves spatial learning and memory of scopolamine-treated rats. Lab. Anim. Res.. 2015;31(2):69-77.
- [CrossRef] [Google Scholar]
- Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2019;68(1):83-93.
- [CrossRef] [Google Scholar]
- Gastrodin Alleviates Vascular Dementia in a 2-VO-Vascular Dementia Rat Model by Altering Amyloid and Tau Levels. Pharmacology. 2020;105(7–8):386-396.
- [CrossRef] [Google Scholar]
- Tang, W., Eisenbrand, G., 1992. Chinese Drugs of Plant Origin, Subtitle: Chemistry, Pharmacology and Use in Traditional and Modern Medicine. Springer-Verlag Berlin etc. https://doi.org/10.1002/pauz.19920210612.
- Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J. Clin. Invest.. 1996;97(11):2553-2561.
- [CrossRef] [Google Scholar]
- AAV8-mediated Sirt1 gene transfer to the liver prevents high carbohydrate diet-induced nonalcoholic fatty liver disease. Mol. Ther. Methods Clin. Dev.. 2014;1:14039.
- [CrossRef] [Google Scholar]
- Gastrodia and Uncaria (tianma gouteng) water extract exerts antioxidative and antiapoptotic effects against cerebral ischemia in vitro and in vivo. Chin. Med.. 2016;11:27.
- [CrossRef] [Google Scholar]
- Chinese herbal formulas for treating hypertension in traditional Chinese medicine: perspective of modern science. Hypertens. Res.. 2013;36(7):570-579.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102913.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







