Ganoderma lucidum mitigates gamma radiation-induced oxidative stress in Swiss albino mice
⁎Corresponding authors. shilpajshetty@nitte.edu.in (Shilpa S Shetty), kumarin@nitte.edu.in (Suchetha Kumari N)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
Radiotherapy is well established in cancer therapeutics. Though radiation targets tumor cells, they damage normal tissues by bystander effect. The present study aimed to evaluate the radioprotective efficacy of Ganoderma lucidum (GL) mycelium powder on gamma radiated swiss albino mice.
Materials and method
Qualitative phytochemical tests and in-vitro antioxidant activity of GL were carried out using standard protocols. Institutional animal ethical committee clearance was obtained. The pre and post-treated mice were irradiated using Gamma chamber 2000 (CARRT), Mangalore University. Biochemical estimations (TAC, SOD, NO, GPx, MDA, reduced glutathione), hematological markers (RBC, WBC, Hb, Platelet), lipid profile, inflammatory markers (Il-2, TNF-alpha, IF-gamma) and genotoxic markers (micronucleus and comet assay) were performed using standard protocols and commercially available kits.
Conclusion
The pre and post-treatment groups showed statistically significant differences (p < 0.05) compared to radiation control in hematological parameters, lipid profile, micronucleus, and comet formation. In pre-treatment and post-treatment groups, Ganoderma lucidium mycelium extracts mitigated radiation-induced hematological, oxidative stress, hyperlipidemia, and genotoxic changes.
Keywords
Radioprotection
Antioxidants
Ganoderma lucidium
Hematology
Lipid
1 Introduction
Radiation, a conventional weapon in diagnosing and cancer therapy, destroys cells in the “target tissue”. Radiation forms free radicals, unstable molecules that easily react with DNA, proteins, and lipids by altering their chemical structure. When a molecule is attacked by a free radical, it becomes a free radical itself, resulting in a chain reaction that destroys the cell (Martemucci et al., 2022). Radiation's biological efficacy is determined by linear energy transfer, total dosage, fractionation rate, and the radiosensitivity of the cells or tissues targeted (Zhao et al., 2017). Radiation treatment reduces the dosage administered to normal cells or tissues while optimizing the dose–effect on malignant cells (Zhao et al., 2017; Baskar et al., 2014).
The antioxidant system that scavenges free radicals and reactive oxygen species (ROS) generated by ionizing radiation is at the top of the defensive systems established during evolution in living organisms (Sharapov et al., 2019). Superoxide dismutases (SODs), glutathione peroxidases (GPxs), and other enzymatic and non-enzymatic antioxidants are part of the antioxidant protection system **[5–7]. When the body is exposed to radiation, it produces many free radicals and reactive oxygen species (ROS) (Sharapov et al., 2019; Kuruba and Gollapalli, 2018). In this situation, the intrinsic antioxidative enzymes cannot wholly suppress the free radicals, and ROS produced, therefore, requires the administration of an antioxidative product that facilitates scavenging and prevents radiation-induced cellular damage (Sharapov et al., 2019). With this usefulness in military, industrial, and therapeutic applications, radio-protective chemicals have been the subject of extensive research for many years. In cancer therapy, the development of safe and effective radioprotective chemicals for human use is especially important (Kuruba and Gollapalli, 2018). Although radiotherapy is a common and successful cancer treatment, normal tissues, particularly those close to tumours, are radiosensitive, limiting its therapeutic effectiveness (Obrador et al., 2020). As a result, protecting normal tissues from radiation-induced cellular harm is critical in radiotherapy.
The low toxicity and ease of administration, dietary components, particularly mushrooms, can be effective medicinal agents. Ganoderma lucidium (GL) or reishi mushroom, also referred to as “Herb of Spiritual Potency”, “Ten Thousand Year Mushroom,” and “King of the Herbs” (Yang et al., 2019). It belongs to the species of red mushrooms and is the most revered Chinese traditional medicine herb with over 200 years of documented history for several disease treatments like body aches, liver diseases, cancer, and psychiatric and neurological disorders (Bijalwan et al., 2021). Ganoderma contains a whopping 400 nutrients! Some of which include polysaccharides, antioxidants, adenosine, protein, potassium, calcium, and Vitamin A, C, E, D, and B complex vitamins. G. lucidum has been explored scientifically to determine its content of bioactive components that affect human physiology, and it has been used in the pharmacology, nutraceuticals, and cosmetics sectors for powerful components (Wachtel-Galor et al., 2011). Indian varieties contain Ganoderic acids, which is the most abundant triterpenoids that showed pharmacological activity (El Sheikha, 2022).
Components with lipid-lowering and antihypertensive effects have been found in G. lucidum herbal materials, and compounds with unique mechanisms of action are extracted (El Sheikha, 2022). Evidence is developing that this mushroom species has the potential to be a useful antiviral treatment for treating a variety of viral infections, including dengue virus, enterovirus 71, and the recently discovered coronavirus sickness (COVID-19) and also anti-tumor **(El Sheikha, 2022a; El Sheikha, 2022b; Ansari et al., 2022; Zhang et al., 2019). Majority of Reishi research has focused on the effects of specific components as radioprotectors rather than the whole mushroom as a dietary supplement or medicinal plant (Chan et al., 2021; Gonzalez et al., 2020).The present study aims to assess the radioprotective efficacy of Ganoderma lucidum aqueous extract (AGL) on mice exposed to Gamma radiation.
2 Materials and methods
2.1 Collection of Ganoderma lucidum (GL) mycelium powder
Ganoderma lucidum (GL) mycelium powder was procured from DRDO-DIBER, Haldwani.
2.2 Preparation of Ganoderma lucidum (GL) mycelium extract
Aqueous extract: Aqueous extract was prepared by boiling 5 g of GL in 200 ml distilled water for 1 h. Then the cooked mixture was stored overnight at room temperature and filtered through Whatman filter paper. The filtrate was dried by evaporation with a heating mantle, extract condensed and stored at 40C for further analysis.
Phytochemical analysis: The preliminary screening of phytochemicals and antioxidants was performed following standard procedures (Raman, 2006; Harborne, 2005).
2.3 In vitro antioxidant activity
The In vitro antioxidant potential of aqueous extract Ganoderma lucidum (AGL) was assessed using DPPH Radical Scavenging, ABTS radical scavenging, Ferric Reducing Antioxidant Power (FRAP), and Total Antioxidant Capacity assays (Raman, 2006; Harborne, 2005; Benzie and Strain, 1996; Prieto et al., 1999).
2.4 Animal study
Male Swiss albino mice (Mus musculus), 6–8 weeks old with 25 ± 2 gm body weight were used for the study following approval from the Institutional animal ethical committee (Ref. KSHEMA/IAEC/12/2019) and were housed under standard environmental conditions (temperature 22 ± 2 °C; humidity 55 ± 5%) with a 12 h light/and dark cycle, fed with standard laboratory pellets and water ad libitum.
2.5 Irradiation of mice
The mice were irradiated using Gamma chamber 2000, (CARRT), Mangalore University (maximum dose rate of 7.556 kGy/h approximately at the center of the sample chamber). The mice were placed in a well-ventilated 3 cm × 6 cm Perspex box for irradiation.
2.6 Experimental design
The study included of 9 groups with 6 mice in each group. The mice were treated with Ganoderma lucidum aqueous extract (400 mg/kg BW and 800 mg/kg BW) and gallic acid (100 mg/kg body weight) for the respective group (Fig. 1).
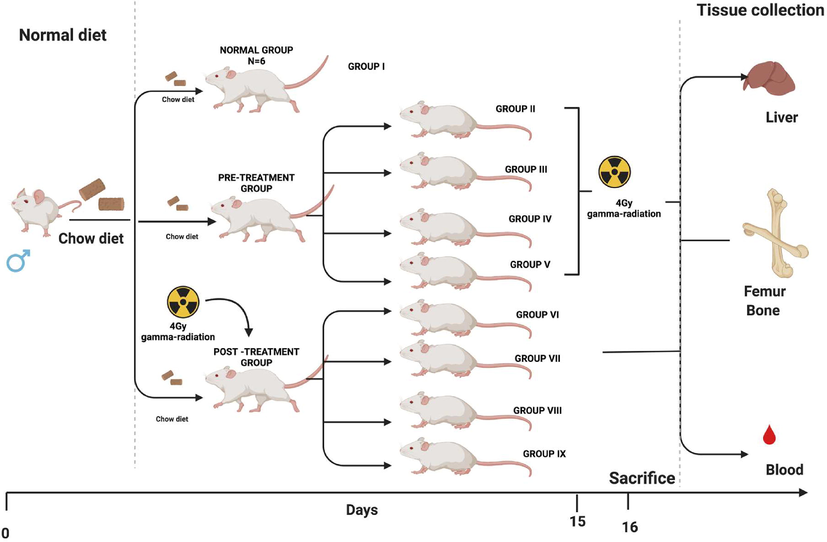
- Representation of experimental design (created using Biorender).
Group I: Normal Control (NC): Mice received standard mice feed and water ad libitum daily.
A. Pre-treatment group
Group II: Radiation Control (Pre RC): Mice were maintained for 15 days before 4 Gy gamma-radiation exposure.
Group III: Mice were treated with Gallic acid (GA-100 mg/kg body weight) orally for 15 days before 4 Gy gamma-radiation exposure.
Group IV: Mice were treated with Ganoderma lucidum aqueous extract (GLA 1–400 mg/kg BW) orally for 15 days prior to 4 Gy gamma-radiation exposure.
Group V: Mice were treated with Ganoderma lucidum aqueous extract (GLA 2–800 mg/kg BW) orally for 15 days prior to 4 Gy gamma-radiation exposure.
B. Post-treatment group
Group VI: Radiation control (Post RC): Mice were maintained for 15 days post to 4 Gy gamma-radiation exposure.
Group VII: Mice were treated with Gallic acid (GA-100 mg/kg body weight) orally for 15 days post to 4 Gy gamma-radiation exposure.
Group VIII: Mice were treated with Ganoderma lucidum aqueous extract (GLA 1–400 mg/kg BW) orally for 15 days post to 4 Gy gamma-radiation exposure.
Group IX: Mice were treated with Ganoderma lucidum aqueous extract (GLA 1–800 mg/kg BW) orally for 15 days post to 4 Gy gamma-radiation exposure.
On day 16 of the experiment, the mice were sacrificed under mild ether anesthesia, whole blood via cardiac puncture, liver tissue, and bone marrow were collected for further analysis.
2.7 Tissue homogenate preparation
The excised liver tissues were rinsed in ice-cold saline and homogenized in phosphate buffer saline (PBS) (pH 7.4) in a homogenizer at 1000 rpm for 3 min. Then the homogenate was centrifuged at 3000 rpm for 10 min at 4 °C, and the supernatant was collected and stored for biochemical estimations.
2.8 Hematological, biochemical and in vivo antioxidant studies
The hematological parameters were recorded using Erma veterinary blood cell counter (PCE-210 VET). Serum was separated, and lipid profile status was analyzed using semi-auto analyzer star 21 plus, with commercially available kit (Agappe Diagnostics Ltd.). The total antioxidant capacity (TAC) (Prieto et al., 1999), Superoxide Dismutase (SOD) (Beauchamp and Fridovich, 1971) Nitric oxide (NO) (Bredt and Snyder, 1994), Glutathione Peroxidase (GPx) (Rotruck et al., 1973), Lipid Peroxidation (Buege and Aust, 1978), Reduced Glutathione (Sharma et al., 2009) were estimated using standard protocols.
2.9 Estimation of inflammatory and genotoxic markers
The levels of inflammatory markers like IL-2, TNFα and Interferon γ were estimated using Commercially available ELISA kits. The micronucleus assay in erythrocytes of mouse bone marrow was proposed as a screening test by Schmid (1975). Comet images were scored with comet score software. Duplicates were maintained for each sample and 50 cells per slide were examined (Singh et al., 1988).
2.10 Statistical analysis
Statistical analysis was performed using SPSS statistical software. The data is expressed as mean ± SE. One-way analysis of variance (ANOVA) followed by Tukey's test was used to study the differences between experimental groups. P ≤ 0.05 was considered as statistically significant.
3 Results
The results of the qualitative phytochemical screening are shown in Table 1.
| Chemical Tests | Aqueous Extract of Ganoderma lucidum mycelium (AGL) |
|---|---|
| 1.Test For Triterpenoids & Steroids | |
| Liebermann burchard test | + |
| 2.Test For Glycosides | |
| Kellernkilliani test | + |
| Bromine water | – |
| 3.Test For Saponins | |
| Foam test | – |
| Froth test | – |
| 4.Test For Alkaloids | |
| Hager ‘s test | + |
| 5.Test For Flavonoids | |
| Ferric chloride test | + |
| Alkaline reagent test | – |
| Lead acetate solution test | + |
| 6.Test For Tannins | |
| Gelatin test | – |
| 7.Test For Proteins | |
| Biuret test | – |
| Xanthoproteic test | – |
| 8.Test For Free Amino Acids | |
| Ninhydrin test | – |
| 9.Test For Carbohydrates | |
| Benedict’s test | + |
| 10. Test For Vitamin C | |
| DNPH test | + |
| 11.Test For Sterols | |
| Salkowski test | + |
| 12.Test For Resins | – |
3.1 In-vitro antioxidant activity of Ganoderma lucidum mycelium powder
The free radical scavenging potential of Ganoderma (GL) aqueous extract was evaluated using Ellagic acid (EA) as standard. A dose-dependent increase was observed for DPPH radical scavenging effect (Fig. 2A), ABTS Radical Scavenging activity (Fig. 2B), protein (Fig. 2D) and total antioxidant activity (Fig. 2E) and a decrease was observed in FRAP assay (Fig. 2C).
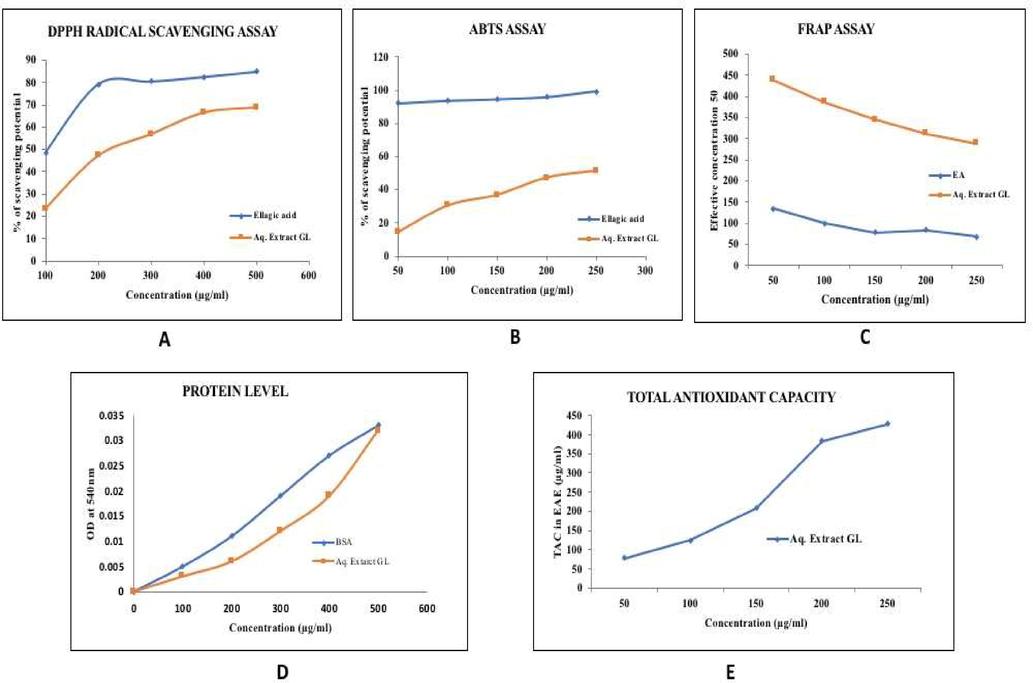
- (A–D): In-vitro antioxidant activity of Ganoderma lucidum mycelium powder.
3.2 Hematological changes
The RBC count showed significant (p < 0.01) decrease in the radiation control group compared to the normal control group. In the pre-and post-administration of Ganoderma lucidum aqueous extract RBC levels were similar in both the groups (Fig. 3A&B). White blood cell (WBC) and hemoglobin count of the radiation control group was significantly reduced (p < 0.001) when compared to the normal control group. In contrast, near-normal levels were seen in the group pre and post-treated with Ganoderma lucidum aqueous extract (Fig. 3A&B). There is a decrease in platelet levels in the radiation control group when compared to the normal control group. The platelet level was higher in the Ganoderma lucidum aqueous extract and gallic acid group (p < 0.05) when compared to the radiation control group (Fig. 3A&B).
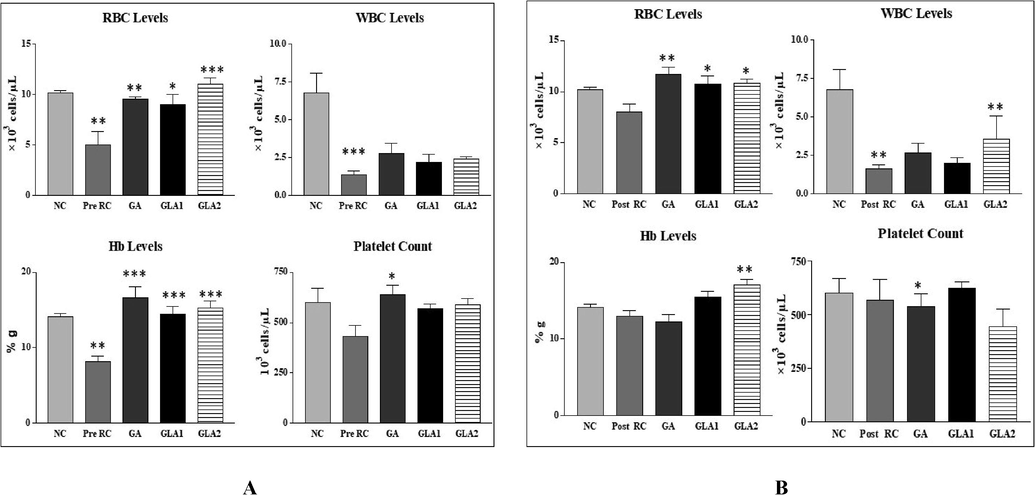
- (A&B): Changes in RBC levels of mice after exposure to 4 Gy gamma radiation with Ganoderma lucidum aqueous extract pre (A) and post treatment groups (B). NC- Normal control, RC- Radiation control, GA- Gallic acid (100 mg/kg body weight), GLA 1-Ganoderma lucidum aqueous extract (400 mg/kg BW) and GLA 2-Ganoderma lucidum aqueous extract (800 mg/kg BW). Values are expressed as mean ± SE, n = 6. **p < 0.01 compared to NC group. ***p < 0.001, **p < 0.01, *p < 0.05 compared to RC group.
3.3 Lipid profile status
The total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL-C) and very low-density lipoprotein (VLDL-C) levels were significantly elevated in the radiation control group when compared to the control group, while HDL-C showed significant (p < 0.01) reduction. The pre-and post-administration of Ganoderma lucidum aqueous extract significantly decreased the total cholesterol, triglyceride, LDL-C and VLDL-C level (Fig. 4 A &B), whereas in the HDL-C level showed a significant (p < 0.001) increase in the Ganoderma lucidum aqueous extract administered group when compared to the radiation control group (Fig. 4A & B).
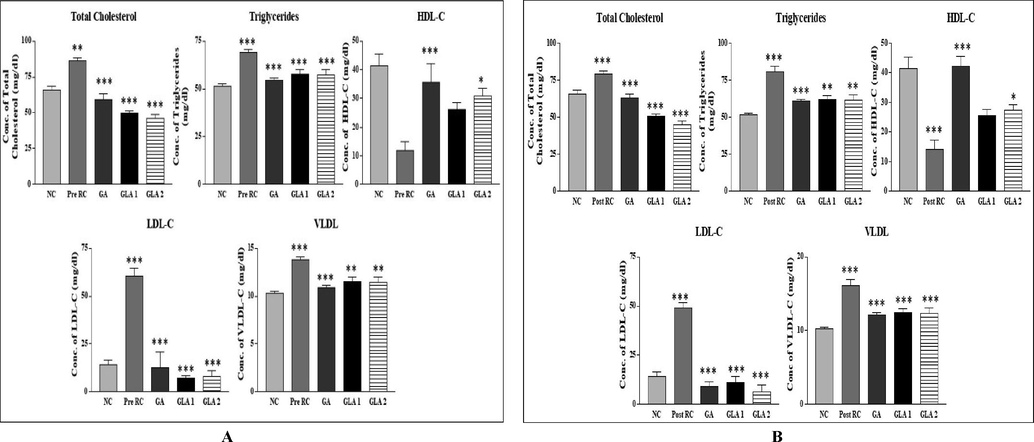
- (A&B): Changes in lipid profile in mice liver homogenate after exposure to 4 Gy gamma radiation with Ganoderma lucidum aqueous extract, pre (A) and post treatment groups (B). NC- Normal control, RC-Radiation control, GA- Gallic acid (100 mg/kg body weight), GLA 1- Ganoderma lucidum aqueous extract (400 mg/kg BW) and GLA 2- Ganoderma lucidum aqueous extract (800 mg/kg BW). Values are expressed as mean ± SE, n = 6. **p < 0.01, ***p < 0.001 when compared to the NC group. ***p < 0.001 when compared to the RC group.
3.4 In vivo antioxidant activity
The total antioxidant capacity has significantly (p < 0.001) reduced in the radiation control group as compared to the normal control group. Whereas the Ganoderma lucidum pre and post-treatment group has shown a significant (p < 0.01, p < 0.001) increase in total antioxidant capacity as compared to the radiation control group (Fig. 5A & B). A significantly increased activity was observed in the radiation control group as compared to the normal control group. Whereas in the Ganoderma lucidum extract pre and post-treatment group, the nitric oxide level decreased significantly (p < 0.001) when compared to the radiation control group (Fig. 5A & B). The SOD activity of the radiation control group was significantly (p < 0.001) decreased when compared to the normal control group. The Ganoderma lucidum pre and post-treatment group has a significant (p < 0.001) increase in SOD activity compared to the radiation control group. Similarly, GPx activity was significantly (p < 0.01, p < 0.001) decreased in the radiation control group compared to the normal control group. Whereas GPx activity was significantly (p < 0.05) increased in the Ganoderma lucidum extract pre and post-treatment group at the dose of 400 mg/kg body weight compared to the radiation control group (Fig. 5A & B). Radiation increased lipid peroxidation, whereas post-administration of Ganoderma lucidum extract decreased lipid peroxidation when compared to the radiation control group (p < 0.001) (Fig. 5A&B). The reduced GSH level was significantly (p < 0.001) decreased in the radiation control group compared to the normal control group. The pre and post-administration of Ganoderma lucidum extract were significantly (p < 0.001) increased compared to the radiation control group (Fig. 5A &B).
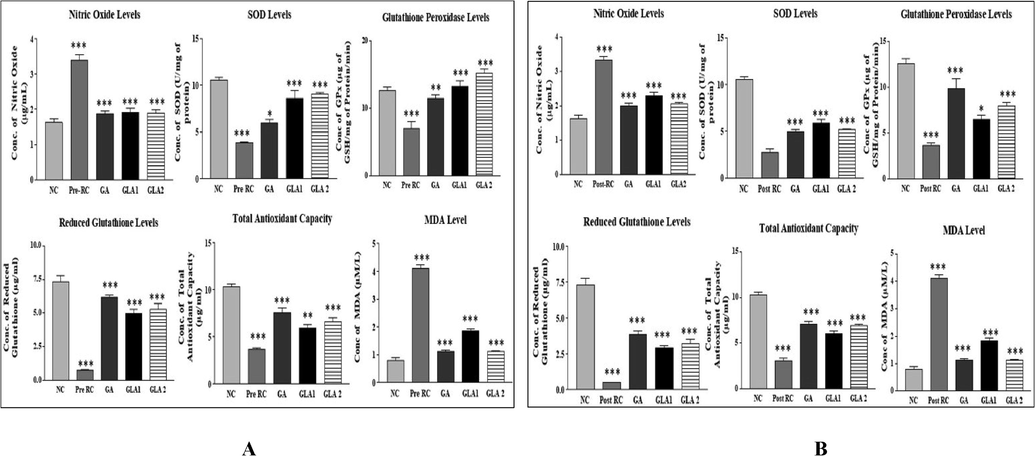
- (A&B): Antioxidant levels in mice liver homogenate after exposure to 4 Gy gamma radiation with Ganoderma lucidum aqueous extract pre (A) and post-treatment (B) groups. RC-Radiation control, GA- Gallic acid (100 mg/kg body weight) and GLA 1: Ganoderma lucidum aqueous extract (400 mg/kg body weight), GLA 2: Ganoderma lucidum aqueous extract (800 mg/kg body weight).Values are expressed as mean ± SD, n = 6. ***p < 0.001 compared to NC group, **p < 0.01, ***p < 0.001compared to RC group.
3.5 Inflammatory markers
The levels of inflammatory markers like IL-2 and TNF-Alpha were significantly (p < 0.001) increased in the pre and post-treatment group, whereas the levels of interferon γ were reduced in the pre-treatment group compared to the normal control group. The gallic acid and Ganoderma lucidum aqueous extract treated groups had significantly (p < 0.001, p < 0.01) decreased levels of IL-2, TNF-Alpha, and Interferon γ when compared to the radiation control group (Fig. 6A & B).
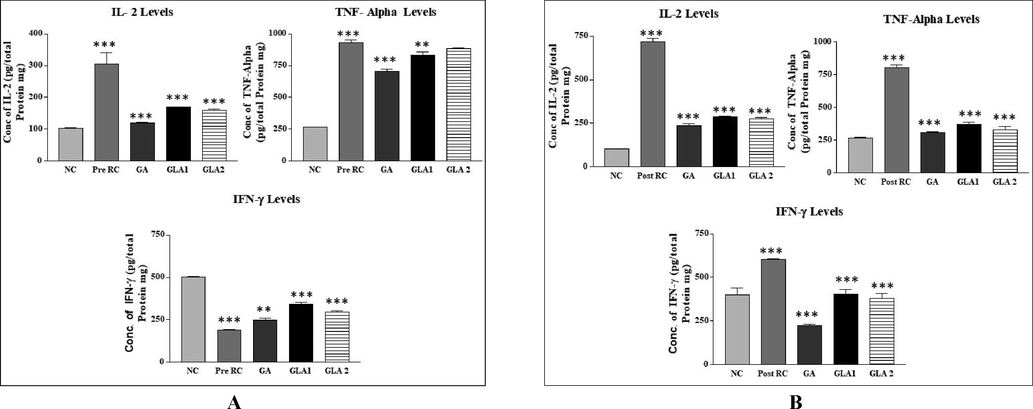
- (A&B): Inflammatory markers in mice liver homogenate after exposure to 4 Gy gamma radiation with Ganoderma lucidum aqueous extract pre (A) and post-treatment (B) groups. RC-Radiation control, GA- Gallic acid (100 mg/kg body weight) and GLA 1: Ganoderma lucidum aqueous extract (400 mg/kg body weight), GLA 2: Ganoderma lucidum aqueous extract (800 mg/kg body weight).Values are expressed as mean ± SD, n = 6. ***p < 0.001 compared to NC group, **p < 0.01, ***p < 0.001compared to RC group.
3.6 Genotoxicity markers
3.6.1 Micronucleus assay and comet assay
Table 2 summarizes the micronucleus assay with Ganoderma lucidum aqueous extract, gallic acid treatment before irradiation. A significant (p < 0.05, p < 0.01) increase in MnPCE and MnNCE was seen in the radiation control compared to the normal control. A significant reduction in the number of MnPCE and MnNCE was found in gallic acid and Ganoderma lucidum aqueous extract pre-treatment prior radiation. The alteration in the levels of comet attributes is shown Fig. 7 (A-C). Gamma irradiated groups showed a dose-dependent increase in all comet parameters (tail length, tail moment, olive moment and % DNA in tail) compared to the normal control groups. Whereas pre and post-treatment of Ganoderma lucidum aqueous extract showed a significant decrease in the levels of comet attributes (Fig. 8, Fig. 9(A–C)).
| Treatment | MnPCE/100PCE cells (mean ± SE) |
MnNCE/100NCE cells (mean ± SE) |
|---|---|---|
| NC | 2.25 ± 0.05 | 0.5 ± 0.1 |
| RC | 56 ± 12.6** | 4.55 ± 0.05** |
| GA | 15 ± 4.3* | 1.6 ± 0.6* |
| GLA 1 | 5.95 ± 0.05* | 2.6 ± 0.3 |
| GLA 2 | 4.05 ± 0.07** | 2.4 ± 0.565* |
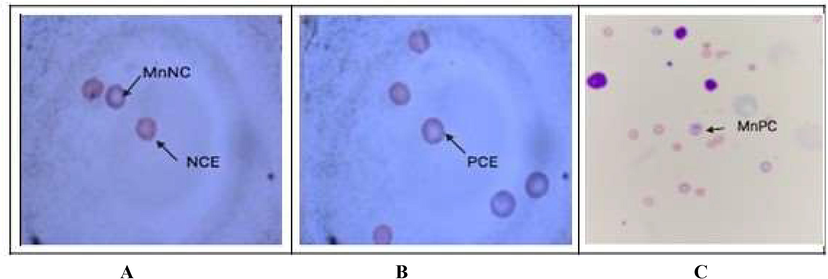
- (A–C): Image showing Giemsa-May Grunwald stained mouse bone marrow with pink coloured normochromic erythrocytes (NCE) and purple colored polychromatic erythrocytes (PCE) and micronucleated NCE or PCE appearing as a dot towards the membrane.
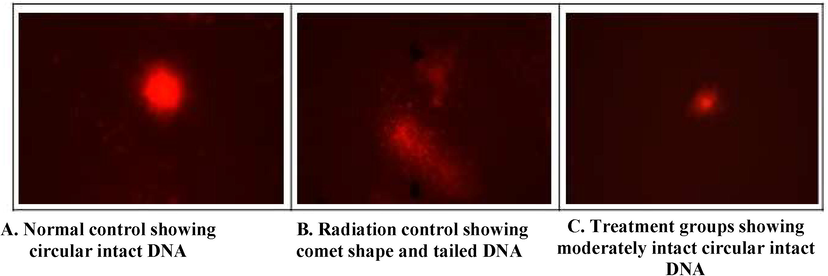
- Representation of Comet assay (A,B,C).
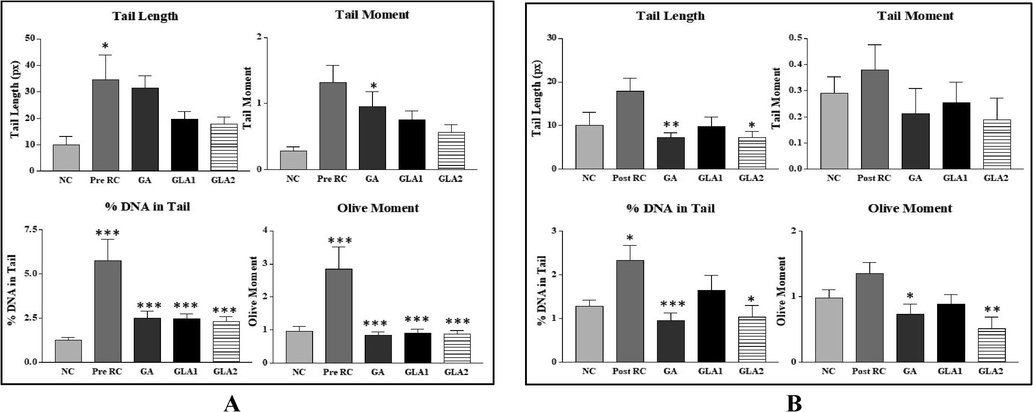
- (A & B): Effect of Ganoderma lucidum aqueous extract and Gallic acid pre-treatment and post-treatment on radiation-induced DNA damage in Swiss albino mice. NC- Normal control, RC-Radiation control, GA- Gallic acid (100 mg/kg body weight), GLA 1- Ganoderma lucidum aqueous extract (400 mg/kg BW) and GLA 2- Ganoderma lucidum aqueous extract (800 mg/kg BW). Values are expressed as mean ± SE, n = 6. *p < 0.05 compared to NC group. **p < 0.001 compared to RC group, *p < 0.05 compared to RC group (A- Pre-treatment Group; B-Post-treatment Group).
4 Discussion
Gamma radiation is a commonly utilized therapeutic source in treating several cancers (Obrador et al., 2020; Kim et al., 2015; Jalal et al., 2014). Apoptosis, necrosis, autophagy, mitotic catastrophe, and senescence are all modes of cancer cell death mediated by gamma irradiation (Selim et al., 2009). On the contrary, it affects both tumor and normal cells (Kiang et al., 2012). According to significant research on sublethal dosages, the sublethal dose studies Gamma radiation affects the cell membrane (Sylvester et al., 2018), reduces overall antioxidant capacity, and increases lipid peroxidation (Kuruba and Gollapalli, 2018; Mansour et al., 2014). The radiolysis of water generates free radicals within the cell, which causes these consequences.
Hemoglobin, red blood cell count, WBC count, and platelets are the hematological parameters generally influenced by radiation (El-Shanshoury et al., 2016). Hematological indices such as hemoglobin, RBC, WBC, and platelet counts were lower in the radiation control groups than the normal control groups in the current study. According to our data, the irradiation of mice resulted in large increases in total cholesterol, LDL-C, VLDL-C, and triglyceride levels, but a reduction in HDL-C levels. This is in line with previous studies that showed hyperlipidemia due to whole-body irradiation (El-khafif et al., 2003; Eckarstein et al., 2002; Diab et al., 2012). Irradiation-induced hyperlipidemia is linked to alterations in hepatic lipid metabolism and might be caused by the radiation's indirect influence on the production of several inflammatory mediators (O'Connell et al., 2015). Excessive levels of free radicals and reactive oxygen species (ROS) are produced in the body as a result of radiation exposure, as previously stated. According to our findings, in mice exposed to radiation, Ganoderma lucidium extract boosted antioxidant activity and decreased inflammation. Micronucleus (MN) production is caused by cytogenetic damage, measured in the micronucleus assay by scoring differently colored cells. It's a cytogenetic approach for determining the cytotoxicity of chemical compounds or irradiation in various systems. When a harmful chemical is present in a system that affects bone marrow proliferation, the amount of PCE, or immature erythrocytes, increases compared to NCE, or mature erythrocytes (Xu et al., 2014). In conditions that cause anemia, reduced splenic function, or myelodysplastic syndrome and myeloproliferative disorders, the amount of nucleated RBC (nRBC), micronucleated RBC (also termed Howell–Jolly bodies) (or both) may rise. This study found increased MN formation in the radiation control and normalized levels in the Ganoderma lucidum aqueous extract and gallic acid treatments before irradiation, suggesting that Ganoderma lucidum aqueous extract may have an anti-clastogenic activity in vivo.
Ionizing radiation can cause DNA damage either directly by breaking strands or indirectly by creating reactive oxygen species as superoxide anions and hydrogen peroxide (Barker et al., 2005). The cell's function is harmed or dies due to the indirect impact of radiation on DNA molecules. Ionizing radiation exposure can generate intra-strand and inter-strand DNA–DNA cross-links, DNA single-strand breaks, DNA double-strand breaks, and DNA–protein cross-links, as well as intra-strand and inter-strand DNA–DNA cross-links (El-Khafif et al., 2003; Eckarstein et al., 2002; Diab et al., 2012; Nair and Menon, 2013).
Damage to genetic material was discovered in the irradiated group, as evidenced by increases in the olive moment, tail length, tail moment, and percent DNA in tail. Furthermore, all comet characteristics were reduced in the treatment groups, showing a protective effect against genetic material damage. Ganoderma is thought to supply H+, which inhibits the activity of free radicals by neutralizing oxygen radicals and protecting cells from DNA damage (El-khafif et al., 2003; Eckarstein et al., 2002; Diab et al., 2012; Nair and Menon, 2013).
5 Conclusion
The findings show that Ganoderma lucidium mycelium extracts are protective in preventing radiation-induced hematological, hyperlipidemia, and genotoxic alterations in both pre-and post-treatment groups. More research on the isolation and characterization of active components with known biological activities and bioavailability is required. Further molecular analysis is needed to confirm the findings of this study. Identifying key elements will help elucidate the radioprotective factor and mechanisms behind its efficacy directing towards using Ganoderma lucidium to treat malignant cells as a radioprotector.
Declarations
Ethics and consent to participate: The Institutional animal ethical committee approved the experimental protocol (Ref. KSHEMA/IAEC/12/2019). All the animal experiments were performed according to the institutional ethical committee for care and handling of experimental animals.
Consent to publish: NA.
Competing interests: The authors declare that they have no competing interests.
Funding
Defence Institute of Bio-Energy Research (DIBER) financial support (DIBER/T/TC/Proj sanc/897).
Acknowledgement
The authors wish to express their sincere appreciation to the Centre for Application of Radioisotopes and Radiation Technology (CARRT), Mangalore University, Mangalore, for providing the irradiation facility. The authors would like to extend earnest gratitude to the Defence Institute of Bio-Energy Research (DIBER) for providing financial support (DIBER/T/TC/Proj sanc/897).
Author’s contributions
MB, RS, SKB, SSS, SKN- contributed in acquiring the funds, substantial contribution to the concept or design of the study.
MB, RS- provided the tested material (Ganoderma lucidium powder).
SKN, SSS, RPG, S- the acquisition, analysis, or interpretation of data for the article.
Conflict of interest
The authors declare no conflict of interest
References
- Pharmacokinetic, metabolomic, and stability assessment of ganoderic acid H based triterpenoid enriched fraction of Ganoderma lucidum P. Karst. . Metabolites. 2022;12(2):97.
- [Google Scholar]
- DNA–protein crosslinks: their induction, repair, and biological consequences. Mutat. Res./Rev. Mutat. Res.. 2005;589(2):111-135.
- [Google Scholar]
- Biological response of cancer cells to radiation treatment. Front. Mol. Biosci.. 2014;24(1):1-9.
- [Google Scholar]
- Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem.. 1971;44(1):276-287.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239(1):70-76.
- [Google Scholar]
- Growth performance of Ganoderma lucidum using billet method in Garhwal Himalaya, India. Saudi J. Biol. Sci.. 2021;28(5):2709-2717.
- [Google Scholar]
- Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem.. 1994;63(1):175-195.
- [Google Scholar]
- The beneficial effects of Ganoderma lucidum on cardiovascular and metabolic disease risk. Pharm. Biol.. 2021;59(1):1161-1171.
- [Google Scholar]
- Assessment of genotoxicity and histopathological changes induced by polyethylene glycol (PEG6000) in male mice. J. Cytol. Histol.. 2012;3(5):1000153.
- [Google Scholar]
- High density lipoproteins and atherosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vase Biol.. 2002;21:13-27.
- [Google Scholar]
- Nutritional Profile and Health Benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as Functional Foods: Current Scenario and Future Perspectives. Foods. 2022;11(7):1030.
- [Google Scholar]
- Effect of taurine treatment on some biochemical parameters in gamma irradiated mice. Environ. Sci.. 2003;6(2):939-1401.
- [Google Scholar]
- Evaluation of low dose ionizing radiation effect on some blood components in animal model. J. Radiat. Res. Appl. Sci.. 2016;9(3):282-293.
- [Google Scholar]
- Use of Ganoderma lucidum (Ganodermataceae, Basidiomycota) as radioprotector. Nutrients. 2020;12(4):1143.
- [Google Scholar]
- Phytochemical Methods. Springer (India) Pvt.; 2005.
- Radiation induced bystander effect and DNA damage. J. Cancer Res. Ther.. 2014;10(4):819.
- [Google Scholar]
- Lipid peroxidation after ionizing irradiation leads to apoptosis and autophagy. Lipid Peroxid. 2012:261-278.
- [Google Scholar]
- Therapeutic implications for overcoming radiation resistance in cancer therapy. Int. J. Mol. Sci.. 2015;16(11):26880-26913.
- [Google Scholar]
- Natural radioprotectors and their impact on cancer drug discovery. Radiat. Oncol. J.. 2018;36(4):265-275.
- [Google Scholar]
- Modulatory effect of moringa oelifera against Gamma- radiation-induced oxidative stress in rats. Biomed. Aging Pathol.. 2014;4:265-272.
- [Google Scholar]
- Free radical properties, source and targets, antioxidant consumption and health. Oxygen. 2022;2(2):48-78.
- [Google Scholar]
- Consumption of antioxidant dietary agents, curcumin and vitamin C, protects cellular DNA from gamma-radiation. Int. J. Radiat. Res.. 2013;11(1):11.
- [Google Scholar]
- Practical murine hematopathology: a comparative review and implications for research. Comp. Med.. 2015;65(2):96-113.
- [Google Scholar]
- Radioprotection and radiomitigation: from the bench to clinical practice. Biomedicines. 2020;8(11):461.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269(2):337-341.
- [Google Scholar]
- Phytochemical Technique. New Indian Publishing Agencies; 2006.
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588-590.
- [Google Scholar]
- Effect of gamma radiation on some biophysical properties of red blood cell membrane. Romanian J. Biophys.. 2009;19(3):171-185.
- [Google Scholar]
- Evaluation of glutathione in oral squamous cell carcinoma. J. Maxillofac. Oral Surg.. 2009;8(3):270-274.
- [Google Scholar]
- A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res.. 1988;175(1):184-191.
- [Google Scholar]
- Radiation-induced cardiovascular disease: mechanisms and importance of linear energy transfer. Front. Cardiovasc. Med.. 2018;5:5.
- [Google Scholar]
- Wachtel-Galor, S., Yuen, J., Buswell, J.A. and Benzie, I.F., 2011. Ganoderma lucidum (Lingzhi or Reishi). Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition.
- Polysaccharide isolated from Parmelia tinctorum ameliorates ionizing irradiation-induced damage in mice. J. Radiat. Res.. 2014;55(4):641-647.
- [Google Scholar]
- Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol.. 2019;3(1)
- [Google Scholar]
- Ganoderma lucidum exerts an anticancer effect on human osteosarcoma cells via suppressing the Wnt/β-catenin signaling pathway. Integr. Cancer Therap.. 2019;18
- [Google Scholar]
- Radiosensitivity and relative biological effectiveness based on a generalized target model. J. Radiat. Res.. 2017;58(1):8-16.
- [Google Scholar]







