Translate this page into:
Fusarium sudanense, endophytic fungus causing typical symptoms of seedling blight and seed rot on wheat
⁎Corresponding author. silvinalar@gmail.com (Silvina Larran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
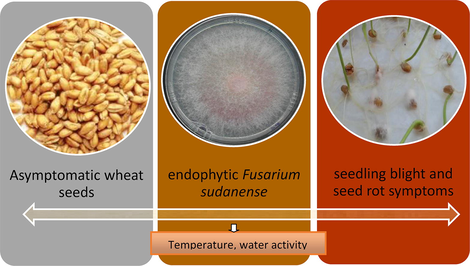
An endophytic fungus isolated from healthy wheat seeds cultivar Klein Yarará in Buenos Aires Province, Argentina, was initially identified as belonging to the Fusarium fujikuroi species complex based on morphological and cultural characteristics. The fungus role in pathogenicity was investigated by artificial inoculation of wheat seeds. Symptoms evaluated 7 and 14 days after inoculation showed that the fungus was pathogenic on seed and seedlings causing symptoms as seed decay, seedling blight and seed rot. The fungus was re-isolated to fulfill Koch’s Postulates and was identified as F. sudanense (strain LBEA 3100), a new species recorded in Argentina. The fungal identity was corroborated using molecular techniques by sequencing the ITS region, D1/D2 domains of the LSU gene and TEF-1 alpha region and by comparison with international databases. Ecophysiological studies of F. sudanense (LBEA 3100) performed at different water activities and temperatures showed faster growth rate at the highest water activity and 25 °C. This is the first report of F. sudanense, isolated from healthy wheat seeds, causing typical symptoms of seedling blight and seed rot on wheat.
Keywords
Fusarium sudanense
Triticum aestivum
Endophytic fungi
Wheat diseases
Healthy wheat seeds
1 Introduction
Wheat (Triticum aestivum L.) is one of the most important cereal grains grown worldwide. In Argentina is the winter cereal crop most widely grown, with a production of 14.95 million tons (USDA, 2017). Like other plants, wheat is a complex community in close association with diverse microorganisms that colonize internal or external tissues (Comby et al., 2016; Porras-Alfaro and Bayman, 2011). Several authors have demonstrated that wheat plants harbor diverse communities of endophytes, mainly fungal species (Larran et al., 2007). Endophytes are important components of plant microbiomes which have been defined as microorganisms that live within plant tissues at least part of their life cycle without causing symptoms of disease (Pimentel et al., 2011). This broad definition not only includes mutualistic and commensalistic symbionts but also includes latent saprotrophs and potential fungal pathogens which have a latent infection period during all or part of their life without showing symptoms (Porras-Alfaro and Bayman, 2011). This latent infection period persists until signs or symptoms can appear by environmental or nutritional conditions or by the stage of maturity of the host or the pathogen (Agrios, 2005).
Moreover, the latent period can end when the plants are under stress. In other words, a change in the environmental conditions or in the host may switch to pathogenic an endophyte that was previously asymptomatic. Latent periods have been studied in different host-pathogen association as on soybean plants and seeds (Sinclair, 1991).
During the last years many researchers have reported the presence of seed endophytes in different plants including gramineous, however the role of seed-associated endophytes for plant growth and health is still underestimated (Ruiz et al., 2011). Although for many years, studies of endophytes have focused on diversity and dynamic of the communities, researches on endophyte-host interaction have had a great increase in the last years (Arnold, 2007; Porras-Alfaro and Bayman, 2011).
Several endophytic species belonging to Fusarium genus have been isolated from leaves, stems and grains of wheat as F. culmorum (Smith) Sacc., F. graminearum Schwabe, F. redolens Wollenw, F. tricinctum (Corda) Sacc. and F. oxysporum Schltdl. (Comby et al., 2016; Larran et al., 2007). In addition, wheat crops are affected by different fungal species of this genus associated with Fusarium crown rot (FCR) that cause symptoms as grain yield loss, stand reduction and rotting of seeds, seedlings, roots, crown seedling blight and rotting of root, crown and stems. FCR is primarily caused by F. culmorum, F. pseudograminearum and F. graminearum, some of them previously identified as endophytes as was mentioned above. Interestingly, it has been reported that all species of this complex can cause Fusarium head blight (FHB), a major cereal disease worldwide (Moya-Elizondo, 2013). In addition, different species among this genus are known to produce diverse secondary metabolites some of which can unfavorably affect human and animal health (mycotoxins) (Leslie and Summerell, 2006). In this sense, the genus Fusarium and its mycotoxicology was widely documented (Desjardins, 2006). Recently new secondary metabolites were detected on a novel species of Fusarium affecting Agapanthus sp. (Edwards et al., 2016). Interestingly, reports on F. sudanense sp. nov. toxins’s production are unknown.
It is known that depending on the environmental conditions the infected plants with Fusarium spp. may either show disease symptoms or remain symptomless (Wilke et al., 2007). Moreover, environmental conditions as temperature and water availability affect the ability of Fusarium species to germinate, to colonize substrates and ecological niches and to produce mycotoxins (Lacey and Magan, 1991).
During routine surveys of wheat seeds at the Centro de Investigaciones de Fitopatología, Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, Buenos Aires Province, an endophytic fungus belonging to the Fusarium fujikuroi species complex (FFSC) was isolated from asymptomatic wheat seeds in Buenos Aires Province, Argentina during the spring of 2016. Considering the fungus could be a new wheat pathogen with an asymptomatic latent infection period, and because the recent introduction of new species in the FFSC (Moussa et al., 2017) the aims of this study were: (1) to evaluate the potential pathogenicity of the endophytic Fusarium on wheat; (2) to verify the fungal identity by molecular techniques and (3) to study the fungal environmental growth conditions in vitro test.
2 Materials and methods
2.1 Isolation and morphological identification of F. sudanense
The fungal strain was isolated from asymptomatic wheat seeds of the cultivar Klein Yarará at the Laboratory of the CIDEFI (34° 52́ S and 57° 58́ W) using the following technique: seeds were surface-sterilized by sequential immersion in 96% ethanol for 1 min, sodium hypochlorite (2% available chlorine v/v) for 3 min, 96% ethanol again for 30 s and finally rinsed twice in sterile distilled water. Then, seeds were kept in Petri dishes containing 2% potato dextrose agar (PDA) and incubated at 24 ± 2 °C in darkness during 7 days. In order to study the morphological characteristics of the fungus, the description of the colonies and grow morphology were inoculated on 2% PDA, Malt Extract Agar (MEA) and Oatmeal agar (OA) growing in darkness at 26 and 37 °C. The morphological characteristics examined included colony colour and those of the microconidia, macroconidia, and conidiophores as size and shape. Measurements of microconidia and macroconidia were made using 7-dand 14-d-old cultures. Microscope slides were prepared by mounting structures in 85% (v/v) lactic acid and 50 measurements were recorded for each characteristic. Taxonomical keys were used for the fungal identification (Leslie and Summerell, 2006).
2.2 Pathogenicity test of F. sudanense
The fungus was kept on 2% PDA and incubated at 24 ± 2 °C for its multiplication. Spore suspension of the endophyte was prepared by flooding the 6-day-old cultures with sterile distilled water and then rubbing the cultures surfaces with a sterile glass rod. The suspension was vortexed and filtrated through two layers of cheesecloth. The conidial concentration used was 6 × 106 conidia/ml adjusted with hemocytometer. Wheat seeds of the cultivar Klein Yarará were surface sterilized with a solution of sodium hypochlorite (5% v/v) for 1 min and then rinsed twice in sterile distilled water. Seeds were kept on filter paper under laminar flow chamber until drying. Then, seeds were immersed in the fungus suspension during 20 min under shaker agitation. Control seeds were immersed in sterile distilled water only under shaker agitation and dried under laminar flow chamber. Inoculated and control seeds were kept into sterile plastic boxes (blotter test) in a temperature-controlled growth chamber at 20 ± 2 °C following rules established by International Seed Test Association (ISTA, 2016). Evaluation was performed by estimating: a) the percentage of germinated seeds at 7 days after inoculation. Germinated seeds were considered when the hypocotyls were bigger than 1.0 cm; b) abnormal seeds and seedlings at 14 days after inoculation: Number of seeds and seedlings with symptoms as seed rot, seedlings with root necrosis, dead seedlings were evaluated and compared with the control. The fungus was re-isolated from inoculated tissues with symptoms attempting to fulfill Koch’s Postulates.
Each treatment (inoculated seeds and control) was performed with eight replications, and each replication, each of them consisting in one plastic box containing 25 seeds (total 200 seeds). Data were analyzed by analysis of variance (ANOVA) for a randomized experiment. Data were arcsin √x transformed to normalize the residual variance. Differences between means were compared by LSD test (P ≤ 0.05).
2.3 DNA extraction, PCR and sequencing
The fungus identification was carried out at the Colección Española de Cultivos Tipo (CECT), Universidad de Valencia, Spain. Strain was grown in 9 cm diameter Petri dishes containing PDA and incubated at 25 °C for 5–7 days. Genomic DNA was extracted from the mycelial lyophilized, using the Yeast DNA Preparation Kit (Jena Bioscience) according to the standard protocol. PCR amplification and cycle sequencing primers used were its1/its4 (White et al., 1990) for Internal transcribed spacer (ITS) region, nl1/nl4 (Kurtzman and Robnett, 1998) for the D1/D2 domain of the Large subunit ribosomal DNA gene (LSU rRNA) and EF1728F and EF1-986R (Carbone and Kohn, 1999) for transcriptional enhancer factor-1 alpha region (TEF-1). Amplified products were examined by agarose gel electrophoresis 1% with Safeview Nucleic Stain (NBS Biologicals) and purified by Gene Clean II Purification Kit (Bio 101, La Jolla, CA, USA).
Sequencing was carried out by ABI PRISM BigDye Terminator V3.1 cycle sequencing ready reactants and ABI PRISM 3730 sequencer (Applied Biosystems) at the Central Service of Support to Experimental Research (SCSIE) of the University of Valencia. Sequencing primers were the same ones used in the amplification reaction. The analysis of the sequences was performed using the BLAST similarity search (performed on January 15, 2017), Fusarium ID database (http://isolate.fusariumdb.org) (Geiser et al., 2004) and Fusarium MLST database (http://www.cbs.knaw.nl/fusarium) (O’Donnell et al., 1998).
Sequencies of Fusarium fujikuroi species complex (FFSC) were searched at the GenBank. Sequencies were aligned by manual adjustments with MEGA v6.06 and BioEdit v7.0.5.3. The analysis included 8 sequencies for TEF 1 (7 types strains and our tested strain, accession number MF581918). The best-fit model of evolution, determined by MEGA v6.06, was used to infer the appropriate substitution model that would best fit the model of DNA evolution for each sequence data set. Maximum likelihood (ML) analyse was used to estimate phylogenetic relationships. The phylogenetic tree was generated based on sequencing of the TEF-1 alpha region.
2.4 Ecophysiological study of F. sudanense
The assay was undertaken to evaluate the effect of temperature and water activity (ɑw) on the fungus growth. The fungus was kept on 2% PDA and incubated at 25 °C in darkness during 7 days for its multiplication. Mycelial discs (8-mm-diameter) were cut out from the margins of the colonies and placed on 90-mm Petri plates containing PDA (pH 5.5) adjusted at following different ɑw: 0.90, 0.95, 0.98 and 0.995 by adding different amounts of glycerol according the technique of Sempere and Santamarina (2006). Plates with the same water activity were placed in water impermeable plastic boxes together with two 100 ml beakers containing a glycerol water solution with an equilibrium relative humidity value identical to the ɑw of the plates. Plates were incubated at 25 °C and 15 °C in the dark. Treatments were performed combining four water activities (0.90, 0.95, 0.98 and 0.995) and two temperatures (15 °C and 25 °C). Each combination at different temperatures and water activities was replicated four times. Petri plates were examined daily during 6 days and the diameter of colonies growth was measured always in the same direction. Then, growth rates were calculated as daily differences of fungus growth radius (mm/day). An ANOVA for repeated measurements by means of Genstat software (2009) was used to evaluate the influence of the factors (water activity, temperature and time) on the fungus growth. Means were compared by LSD (P ≤ 0.05).
3 Results and discussion
3.1 Fungal identification by morphological and cultural features
The fungal colonies incubated on 2% PDA, OA and MEA were floccose, felted and initially white and pale pink but later becoming pinkish violet from both front and reverse. After 4 weeks reverse of the colonies became dark violet. Microconidia one celled, oval, 0-septate, mostly clavate, elliptical or oval, small to medium in length and wide (9.6–15.6 × 3.6–4.8 µm) were formed from monophialides on globose or subglobose false head. These false heads were formed from phialidic conidiophores with monophialides and polyphialides. Microconidia were also formed in short chains. Macroconidia hyaline, falcate to almost straight, 3 to 5-septate, short to medium in length (24–55.5–2.5–4.5 µm) with a short tapered apical cell and foot shaped base were observed. Intercalary chlamydospores were singles or in chains (Fig. 1). Based on these morphological and cultural characteristics the fungus was included in the Fusarium fujikuroi species complex (FFSC).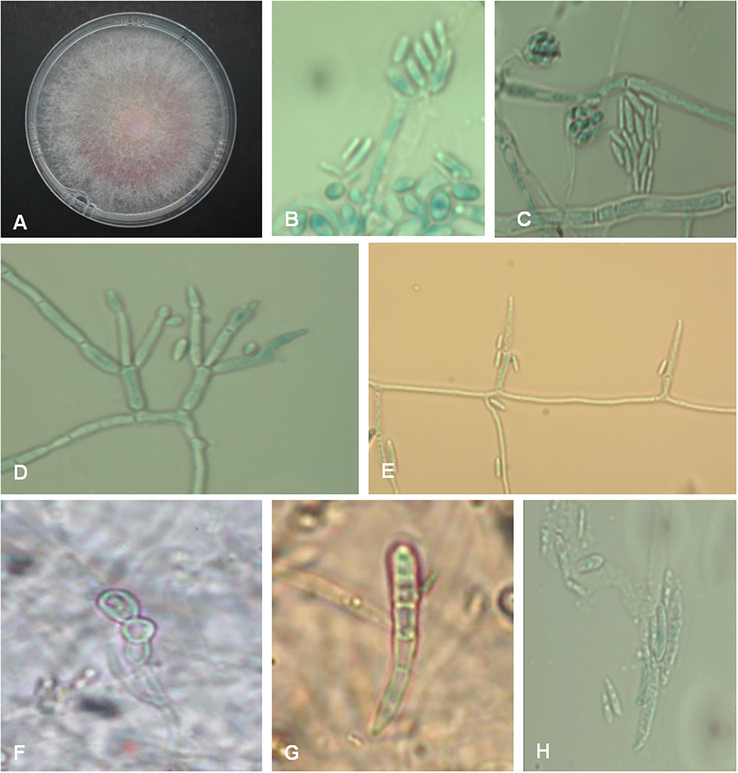
Fusarium sudanense isolated from healthy wheat seeds. A: colony on 2% PDA at 24 ± 2 °C after 4 weeks; B, C: conidial false heads; D, E: poliphialides and monophialides, respectively; F: intercalary chlamydospores; G, H: macroconidia (×40).
3.2 Molecular identification of F. sudanense
The molecular identification of the fungus based on phenotypic and molecular data by PCR amplification and subsequent sequencing of the reference genes for taxonomic purposes was carried out. ITS (including 5.8S rDNA) region, as the standard barcode for fungi (Schoch et al., 2012), the D1 and D2 hypervariable domains of the large-subunit 28S rDNA (LSU) (Kurtzman and Robnett, 1998; Fell et al., 2000) and translational elongation factor 1 alpha (TEF-1) (O’Donnell et al., 1998), were essential barcode for identifying Fusarium species. The Blast similarity for ITS region and the D1/D2 domains of the LSU gene against curated public database as the RefSeq Target Loci (RTL) at NCBI GenBank (http://www.ncbi.nlm.nih.gov/refseq/) (O’Leary et al., 2016) or Fusarium ID database (http://isolate.fusariumdb.org) (Geiser et al., 2004) were not sufficient for species identification. According to these, the endophytic strain LBEA 3100 was included in the Fusarium fujikuroi species complex (FFSC). The BLAST analysis of the TEF-1 region of the strain LBEA 3100 against Fusarium MLST database (http://www.cbs.knaw.nl/fusarium) (O’Donnell et al., 1998) allowed the identification of the isolate at species level as Fusarium sudanense S.A. Ahmed, Al-Hatmi and de Hoog with the highest percentage of similarity of 98.239% (279/284pb) with the sequence AF160309 (type strain CBS 454.97; NRRL 26793), previously identified morphologically as F. nygamai (Moussa et al., 2017).
The strain of F. sudanense, was deposited at the Colección Española de Cultivos Tipo (CECT), Valencia. Spain as LBEA 3100 (CECT 20938). The nucleotide sequences of the ITS, TEF1 and LSU regions determined in this study were deposited at the GenBank database under accession numbers MF540540, MF581918 and MH411162, respectively. The phylogenetic tree was created from TEF-1 sequence (Fig. 2).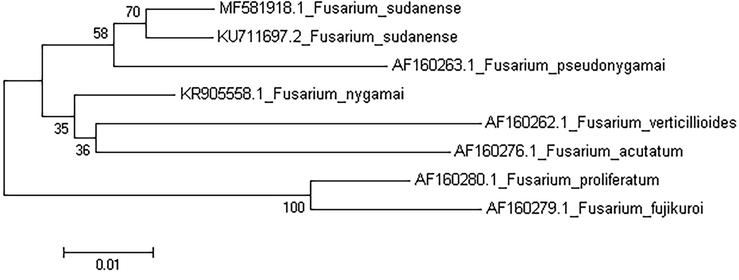
Phylogenetic tree from TEF1 sequence of Fusarium sudanense (LBEA 3100).
3.3 Pathogenicity test of F. sudanense
The results of the pathogenicity test showed that the fungus isolated from healthy wheat grains (LBEA 3100) caused pre and post-emergence symptoms of seedling blight such as seed rots, weak seedlings, poor root development, root necrosis and seedling death after 14 days of inoculation (Table 1 and Fig. 3).
Germinated seeds (%) at 7 days after inoculation
Symptomatic seeds and seedlings at 14 days after inoculation
Seeds rot
Weak seedlings
Seedlings with root necrosis
Dead seedlings
Inoculated seeds
81a*
42.5a
8a
3a
5a
Control seeds
90.5a
0b
0b
0b
0b

Symptoms caused by Fusarium sudanense (LBEA 3100) after 14 days of inoculation: a: radicular necrosis (rn) and seedling showed poor root development (prd); b: weak seedlings (ws); c-f: seeds rot and seddlings with white mycelium on lower stems after 14 days of inoculation.
As it is shown in Fig. 3, abundant white mycelium developed on the surface of the seeds and at the base of the seedlings after 14 days of inoculation. Weak seedlings showed lower height, weak coleoptiles and lower root density when compared with control seedlings. Some infected seedlings also showed a reddish brown discoloration on the lower stem after 7 days of inoculation.
The results of the ANOVA showed significant differences between traits for seeds rot (P = 0.020), weak seedlings (P = 0.028) and dead seedlings (P = 0.02). However, there were not significant differences between traits for germination (P = 0.486).
The fungus was re-isolated from symptoms tissues fulfilling Koch’s Postulates. Symptoms on wheat seeds and seedlings registered in this assay indicate the damage caused for the fungus under in vitro test. Thus, it could be hypothesized a reduction in wheat plant establishment, number of heads per square meter, and grain yield under field conditions that need to be investigated.
Endophytes are ubiquitous in nature and have complex interactions with their hosts, which involve mutualism, antagonism and rarely parasitism (Nair and Padmavathy, 2014). In this sense, among the endophytic fungi isolated from a determined host, well known pathogens are commonly recovered (Larran et al., 2007). In addition, according Porras-Alfaro and Bayman (2011) some of these endophytes may have a dual role, one with a pathogenic lifestyle on certain hosts and as endophytic one on others. Different species of the genus Fusarium are known phytopathogens and are also isolated as endophytes from different hosts (Larran et al., 2007).
Interestingly, two new species in the FFSC were recently introduced (Moussa et al., 2017). One of them is represented by strain CBS 454.97 (in the collection of Centraalbureau voor Schimmelcultures, CBS, Fungal Biodiversity Centre) originated from Striga hermonthica, an important weed of sorghum crop. This strain (CBS 454.97) identified preliminarily as F. nygamai after studies based on phenotypic, physiological and molecular data (Moussa et al., 2017) was reclassified as F. sudanense sp. nov. due to be clearly distinct from all known taxa in the FFSC. Moreover, F. nygamai has been reported causing root rot on sorghum and associated with cotton, maize, millet and rice (Balmas et al., 2000; Leslie and Summerell, 2006).
3.4 Ecophysiological study of F. sudanense
The results obtained in the present study have demonstrated that the growth of F. sudanense (LBEA 3100) was highly affected by temperature and water availability in the medium (Figs. 4 and 5). The results of the statistical analysis of the fungus growth rates (mm/day) showed significant differences for temperature, water activity and time. Also, double and triple interactions were significant (Table 2).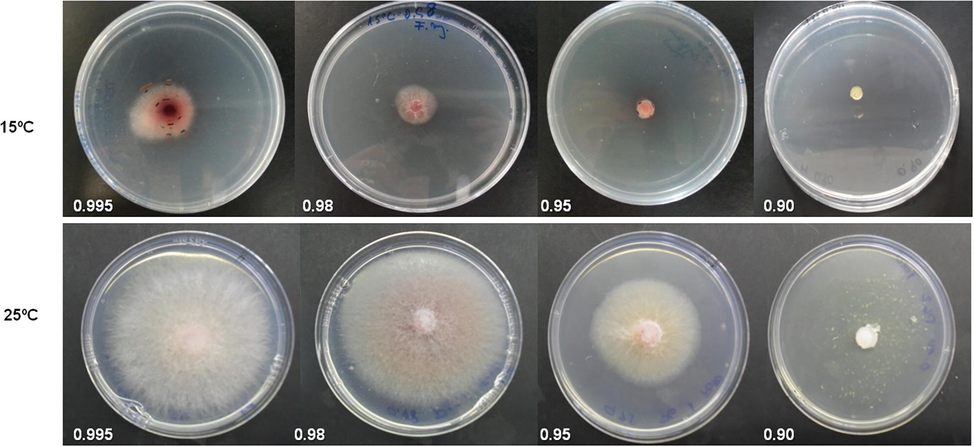
Growth of Fusarium sudanense (LBEA 3100) at different water activities (0.995, 0.98, 0.95, 0.90) and two temperatures (15 and 25°).
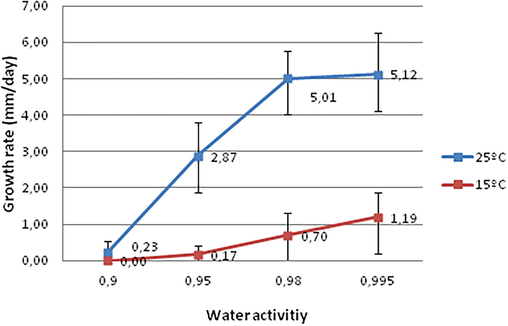
Growth rates (mm/day) of Fusarium sudanense (LBEA 3100) at different water activities and temperatures. Means followed by different letters within the same temperature indicated significant differences, LSD P ≤ 0.05.
Source of variation
DF
Means square
P value*
Replication
3
0.07
Temperature
1
328.23
P ≤ 0.001
Water activity
3
109.16
P ≤ 0.001
Temperature × water activity
3
35.83
P ≤ 0.001
Error
21
0.07
Evaluation times
5
31.51
Evaluation times × temperature
5
15.83
P ≤ 0.001
Evaluation times × water activity
15
5.00
P ≤ 0.001
Evaluation times × temperature × water activity
15
2.23
P ≤ 0.001
Error
120
0.21
It is known that abiotic factors such as temperature, humidity and other environmental factors have a great importance for the incidence and severity of certain Fusarium species (Hudec and Muchová, 2010). As it has been mentioned by Lacey and Magan (1991) water activity determines fungal spores germination and temperature affects both mycelial growth and conidial germination. Therefore, the activity water is one of the essential factors that determine the diseases development. F. sudanense LBEA 3100 grew at all water activities and temperatures except at 0.90 αw and 15 °C. The growth rates of the fungus increased with the water availability and temperature. As it is shown in Fig. 5 the growth rates of the fungus were faster at the highest water activities (0.995 and 0.98 αw) and at the highest temperature tested, with a maximum growth rate at 0.995 αw and 25 °C (average 5.12 mm/day) while the minimum growth rate was at 15 °C and 0.95 ɑw and without growth at 0.90 ɑw (average 0.17 mm/day). Also, similar results were reported by Sempere Ferre et al. (2007) for F. sambucinum which grew most rapidly at 0.995 aw and 25 °C. In addition, the growth rates tended to decrease with the evaluation time, with a few exceptions as those at 0.90 ɑw where at some evaluation times there was not any fungal growth, causing the triple interaction (not shown, only double interactions are indicated in the figures using the times average).
In agreement with Lacey and Magan (1991) for the species in the Fusarium genera our results indicate that the fungus studied here could be also considered hydrophilic because its optimal growth at the highest water availability. Furthermore, the fungus colonies showed variations of coloration between a soft orange to pink accordingly to the availability of water in the culture media (Fig. 4). Our results agree with those recorded by several authors who founded maximum growth rates of different species of Fusarium at 25 °C such as F. culmorum, F. graminearum, F. avenaceum and F. poae (Hudec and Muchová, 2010). Interestingly, these authors reported the highest pathogenicity of F. culmorum and F. graminearum to wheat seedlings roots at 25 °C on in vitro assay. In addition, Lacey and Magan (1991) reported that most Fusarium graminearum infections occurred in the field when the temperatures were between 20–30 °C and only a few cases when temperatures were less than 15 °C.
Many Fusarium spp. have a global distribution and are economically important and as infective agents of plants, animals and humans (Leslie and Summerell, 2006). It is known that species in the FFSC can cause a wide spectrum of often devastating diseases on diverse agricultural crops with typical symptoms including seedling blight, root and crown rot, stunting and the typical symptoms of etiolation and abnormal elongation. In this sense, between FFSC it is known that F. fujikuroi has been recovered from crops other than rice, including maize, wheat and recently from soybean (Wiemann et al., 2013). In particular, F. fujokuroi was report as soybean seedborne with potential to cause pre and postemergent damping off on soybean on the United State (Pedrozo et al., 2015).
F. sudanense LBEA 3100, isolated from asymptomatic wheat seeds caused symptoms on wheat seeds and seedlings after inoculation. So we could suggest that this fungus could be a wheat pathogen that colonizes host tissues without symptoms and similarly to others pathogenic Fusarium species could have a latent period. However, the latent period could be broken by certain environmental or nutritional conditions or by the advance in the plant stage as was defined for different fungi by Agrios (2005).
In this sense, symptomless infection was studied in different hosts such as Fusarium verticillioides (Sacc.) Nirenberg on maize. Bacon and Hinton (1996) have demonstrated that this fungus causes systemic infections of grains that may or may not show disease symptoms. If environmental conditions are favorable it can cause rot in roots, stems and spikes.
According to the results obtained in this work we conclude that the growth of F. sudanense, strain LBEA 3100 was affected by temperature and water activity and showed optimal growth at 25 °C and at the highest values of water activity tested. To our knowledge this is the first report of F. sudanense, isolated from healthy wheat seeds, causing typical symptoms of seedling blight and seed rot on wheat.
The ecophysiological study of this fungus carried out in this work provides an important contribution to the knowledge of its environmental requirements and therefore the conditions under this fungus could act as pathogen. The early detection of this potential pathogen in seeds would contribute to the implementation of effective seed management measures.
Acknowledgements
This work was supported by Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, Buenos Aires Province, Argentina (grant number 11A 296) and by Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires Province, Argentina (grant number (PIP) 819/14).
References
- Agrios, G.N., 2005. Plant pathology. 5th ed. Elsevier, Academic Press, London.
- Understanding the diversity of foliar endophytic fungi: progress challenges, and frontiers. Fungal Biol. Rev.. 2007;21:51-66.
- [Google Scholar]
- Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Botany. 1996;74:1195-1202.
- [Google Scholar]
- Fusarium nygamai associated with Fusarium foot rot of rice in Sardina. Plant Dis. Notes. 2000;84:807.
- [Google Scholar]
- A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553-556.
- [Google Scholar]
- Spatial and temporal variation of cultivable communities of co-occurring endophytes and pathogens in wheat. Front. Microbiol.. 2016;7:403.
- [Google Scholar]
- Fusarium mycotoxins. Chemistry, Genetics and Biology. Minnesota, USA: APS Press, Sta Paul; 2006. p. :260.
- Fusarium agapanthi sp. nov, a novel bikaverin and fusarubin-producing leaf and stem spot pathogen of Agapanthus praecox (African lily) from Australia and Italy. Mycologia. 2016;108:981-992.
- [Google Scholar]
- Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evol. Microbiol.. 2000;50:1351-1371.
- [Google Scholar]
- FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Euro. J. Plant Pathol.. 2004;110:473-479.
- [Google Scholar]
- Influence of temperature and species origin on Fusarium spp. and Microdochium nivale. Pathogenicity to wheat seedlings. Plant Protect. Sci.. 2010;2:59-65.
- [Google Scholar]
- International rules for seeds treating. International Seed Test Association; 2016.
- Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. An van Leeuwenhoek. 1998;73:331-371.
- [Google Scholar]
- Fungi in cereal grain: their occurrence and water and temperature relationship. In: Chelkowsky J., ed. Cereal grain: Mycotoxins, fungi and quality in drying and storage. Amsterdam: Elsevier; 1991. p. :77-118.
- [Google Scholar]
- The endophytic fungi from wheat (Triticium aestivum L.) World J. Microbiol. Biotechnol.. 2007;23:565-572.
- [Google Scholar]
- The Fusarium Laboratory Manual. USA: Blackwell Publishing; 2006. p. :388.
- Fusarium crown rot disease: biology, interactions, management and function as a possible sensor of global climate change. Cien. Invest. Agr.. 2013;40:235-252.
- [Google Scholar]
- Two new species of the Fusarium fujikuroi species complex isolated from the natural environment. An van Leeuwenhoek. 2017;110:819-832.
- [CrossRef] [Google Scholar]
- Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014 Article ID 250693
- [CrossRef] [Google Scholar]
- Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia. 1998;90:465-493.
- [Google Scholar]
- Nucleic Acids Res.. 2016;4:D733-D745.
- First report of seedborne Fusarium fujikuroiand its potential to cause pre- and post-emergent damping-off on Soybean (Glycine max) in the United States. Plant Disease Note. 2015;12:1865.
- [Google Scholar]
- The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol. Res. Int.. 2011;2011:576286
- [Google Scholar]
- Hidden fungi, emergent properties: endophytes and microbiomes. Ann. Rev. Phytopathol.. 2011;40:291-315.
- [Google Scholar]
- Characterization and screening of plant probiotic traits of bacteria isolated from rice seeds cultivated in Argentina. J. Microbiol.. 2011;49:902-912.
- [Google Scholar]
- Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. PNAS. 2012;109:6241-6246.
- [Google Scholar]
- Ecofisiología de Drechslera oryzae Subram. & Jain en condiciones in vitro. Phytoma. 2006;178:49-51.
- [Google Scholar]
- Interacciones competitivas entre Fusarium sambucinum Fuckel y Phoma glomerata (Corda) Wollenweber & Hochapfel en condiciones in vitro. Rev. Iberoam. Micol.. 2007;24:29-33.
- [Google Scholar]
- Latent infection of soybean plants and seeds by fungi. Plant Dis.. 1991;75:220-224.
- [Google Scholar]
- USDA. United States Department of Agriculture. Foreign Agricultural Service https://apps.fas.usda.gov/psdonline/circulars/grain-wheat.pdf accessed 01.11.2017
- Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., eds. PCR protocols. A guide to methods and applications: Academic Press, San Diego, Cali; 1990. p. :315-322.
- [Google Scholar]
- Seed transmission of Fusarium verticillioides in maize plants grown under three different temperature regimes. Plant Dis.. 2007;91:1109-1115.
- [Google Scholar]
- PLos Pathog. 2013;9:e1003475
- [CrossRef]







