Translate this page into:
Fungi species causing dieback and wilt diseases in shisham [Dalbergia sissoo (Roxb)] and impact of various fungicides on their management
⁎Corresponding author at: Ayyub Agriculture Research Institute, Faisalabad, Pakistan. ziaghazali3@gmail.com (Hafiz Muhammad Zia Ullah Ghazali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Dalbergia sissoo commonly known as ‘shisham’ is a multipurpose tree commonly used to produce high-quality timber wood. However, it is under extinction threat due to several biotic and abiotic factors. The fungi species causing dieback and wilt diseases are poorly studied and efficacy of different fungicides for their management has been rarely tested. Therefore, infected root and shoot samples of shisham trees were collected from different localities in Bahawalpur through this study.
Methods
Samples were analyzed for pathological observations which revealed that Fusarium, Alternaria, Curvularia, Dreshcelera, Phoma, Aspergillus, Paecilomyces, Haphlographium, Steganosporium, Ostracoderma, Cephalospora, Penicillium, and Briosia spp were the fungi species infesting the collected samples. Four fungicides were tested to manage/suppress the mycelial growth of identified fungi species.
Results
Mycelial growth of Fusarium, Alternaria, Phoma, Lichenocomium, Penicillium and Aspergillus was significantly inhibited by the application of Chlorothalonil and Procymidone fungicides, while Sulphur proved least effective by inhibiting the mycelial growth of Fusarium spp.
Conclusions
The results revealed that various fungi species infest shisham trees and available fungicides can suppress the growth of these fungi species. Therefore, application of fungicides at appropriate time could lower shisham decline in the country. Nevertheless, field studies are required for further recommendations.
Keywords
Dalbergia sissoo
Die-back
Wilt
In-vitro
Fungicides
Salt injury
1 Introduction
Shisham [Dalbergia sissoo (Roxb)] is a deciduous tree in Papillioniaceae family, which has great economic importance due to its fine quality timber wood (Parveen et al., 2009, Naqvi et al., 2019). Although, shisham is widely accepted common name for the species, it is also known as Indian Rosewood (Kumar 2013, Timilsina et al., 2020). Shisham is abundantly grown in India, Pakistan, Nepal, Bhutan, Bangladesh, Malaysia and Afghanistan (Ashraf et al., 2010). Similarly, shisham forests are profusely found in foothills of Himalayan tract either solely or in combination with other species (Devagiri et al., 2007). Shisham plantations is spread over 10,000 ha producing 28000 m3 timber wood annually (Ashraf et al., 2010, Naqvi et al., 2019).
Shisham is a multipurpose tree, well-known for its rapid growth and production (Kumar et al., 2011). Commonly it is used as fuel wood and good quality timber wood, while other uses of the species include nitrogen fixation, shade, and shelter. Various parts of shisham have high medicinal value (Lal and Sanjay 2012).
Unfortunately, shisham is vulnerable to extinction because of various complex diseases, i.e., wilt, dieback caused by several soils borne fungi (Shah et al., 2010). Dieback disease causes extensive damages to shisham throughout South-Asia and trees of all ages are highly susceptible (Ahmad et al., 2015; Rehman et al., 2012b). Dieback started as an epidemic in central Punjab, Pakistan during 1998 (Mukhtar et al., 2012). Shisham is infested by dieback due to several biotic and abiotic factors in several countries around the world (Bajwa and Javaid 2007). However, the severity of symptoms depends on variety and varies greatly among countries (Webb and Hossain 2005, Shukla 2008). Dieback causes massive damage to plantations and individual trees growing along water channels and borders of agricultural lands (Mukhtar et al., 2015; Rehman et al., 2012b; Shakya and Lakhey, 2007).
Various fungi species are responsible for dieback of shisham trees including Fusarium solani (Mustafa et al., 2004), Cercospora sissoo, Phyllactinia dalbergiae, Phellinus dalbergiae, Ganoderma lucidum and Phytophthora cinnamomi (Dayaram et al., 2003, Harsh et al., 2011, Naqvi et al., 2019). The characteristic symptoms include initial dieback of branches, bark splitting, and gummosis from main stems and consequently death of the whole tree. Likewise, wilting, cankers, internal chlorosis and necrosis are also disease symptoms after severe attack (Javaid 2008, Rehman et al., 2008).
Soil-borne fungi are only managed through the application of highly effective fungicides. These fungicides have significant potential to inhibit the mycelial growth of these fungi (Arif et al., 2021). The application of two fungicides (Captan, Carbendazim) has been found effective against F. solani, which was responsible for shisham dieback. The application of Benomyl fungicide significantly inhibited the mycelial growth of F. solani. The Dithane M−45 and Vitavax also suppressed mycelial growth of F. solani (Mukhtar et al., 2015). Keeping in view the importance of this disease, this study was planned to isolate fungi species infesting shisham trees. Evaluating the efficacy of four fungicides against identified fungi species was the second major objective of the study.
2 Materials and methods
2.1 Sample collection
The infested plant samples of shisham were collected from two different areas (i.e., Dera Nawab and Bahawalpur). Root samples were collected from 30 to 40 cm soil depth. The infected samples were taken from vascular bundles (xylem and phloem) portion. Samples were taken into polythene bags (17 × 13 cm), labeled, and brought in Plant Pathology Lab. Regional Agricultural Research Institute, Bahawalpur, Pakistan. The samples were stored in a refrigerator at 4 °C until use. The trees with varying disease infestation level are given in Fig. 1.
Shisham plants with varying disease severity, slight infection (a), moderate infection (b), slightly sever infection (c), severe infection (d) and dead plant (e).
2.2 Cutting, blending and incubation of infested root samples
Root samples were washed with running tap water for 2–3 min and left for 12 h. The shade-dried root samples were chopped and rinsed with HgCl2 for surface sterilization/disinfestation. These samples were placed on 9 cm sterilized Petri plates containing moistened blotting paper at the bottom. The samples were incubated at 25 ± 2 °C for fungal growth. The samples were incubated until clear colonies of fungi were developed.
2.3 Isolation of pathogens from different plant parts
The isolates were taken from different plant parts, i.e., root, xylem, and phloem for the isolation of pathogens, and transferred to freshly prepared PDA medium in which 0.25 mL streptomycin per Petri plate was added to prevent the growth of bacterial colonies. The Petri plates were sealed with scotch tape and incubated at 25 ± 2 °C.
2.4 Identification of fungal species
The fungi growing from infected root tissues and plant parts were identified on the basis of colony characteristics and conidial morphology using keys, books and already published literature (Ahmad et al., 2015; Ahmadi and Arzanluo, 2019; Bajwa and Javaid, 2007; Javaid et al., 2010; Rehman et al., 2012b; Barnett and Hunter, 1972). Different pathogens identified during the study are given in Fig. 2.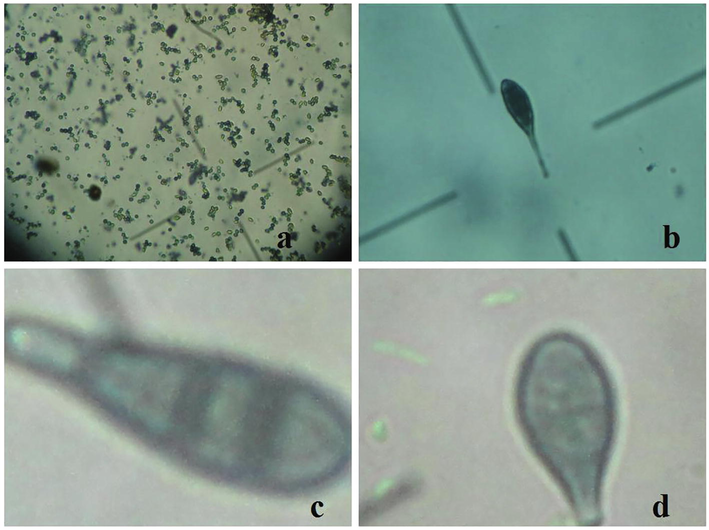
Different pathogens detected during the study, Aspergillus spp. (a), Alternaria alternata (b), Alternaria tenuis (c), and Alternaria sesame (d).
2.5 In-vitro evaluation of fungicides against various isolates of shisham decline
In-vitro efficacy of four fungicides, i.e., Matiram (Cabreotop), Sulphur (Cumulus), Mancozeb (Acrobat) and Chlorothalonil + Procymidone (Protocol) was evaluated by adding calculated quantity of each into autoclaved PDA medium. The 20mLof poisoned melted PDA medium was poured into each sterilized Petri plate and allowed to solidify. The PDA medium without fungicides was regarded as control. After solidification of medium, 6 mm agar plugs of the fungal colonies were transferred to Petri plates containing PDA under Laminar Flow Chamber. Each treatment had three replications. The experiment was laid out according to Completely Randomized Design (CRD). All the plates were incubated at 25 ± 2 °C for 7–12 days.
2.6 Data collection
The growth inhibition rate by each fungicide was recorded after 3 days of incubation. Percent inhibition rate for each fungal colony was calculated by following the methods of (Bajwa and Javaid 2007, Vogel et al., 2011, Rajput et al., 2012).
2.7 Statistical analysis
The collected data were statistically analyzed by Analysis of Variance technique (ANOVA). Normality in the data was tested prior to ANOVA and data having non-normal distribution were transformed by Arcsine transformation technique to meet the normality assumption of ANOVA. Least significant difference post hoc test was used to observe the differences among means at 5% probability where ANOVA indicated significant differences.
3 Results
Various physical and chemical soil properties were determined for the soil samples collected from Dera Nawab (Table 1) and Bahawalpur (Table 2). The soil properties were determined to check the involvement of abiotic factors in disease development. Several abiotic factors including soil type, pH, fertilizer concentration, organic matter content, irrigation source, temperature and relative humidity are responsible for dieback disease in shisham. Shisham decline or dieback has become a threatening disease in shisham growing areas of the world, particularly in Pakistan. The characteristic symptom of disease includes initial dieback of branches, bark splitting, gummosis and consequently death of the whole tree.
Sr. No.
Depth (cm)
Electrical Conductivity (EC) (dS/m)
pH
O.M
%Phosphorus (ppm)
Saturated Hydraulic Conductivity (Ks) (mm/h)
Potassium (ppm)
Saturation (%)
1.
0–6
5.7
7.7
0.69
8.8
44
174
38
2.
6–12
5.3
7.7
0.45
–
–
–
36
3.
0–6
4.8
7.8
0.62
5
48
190
38
4.
6–12
4.7
7.7
0.45
–
–
–
36
Sr. No.
Depth (cm)
Electrical Conductivity (EC) (dS/m)
pH
O.M
%Phosphorus (ppm)
Saturated Hydraulic Conductivity (Ks) (mm/h)
Potassium (ppm)
Saturation (%)
1.
0–6
1.8
7.5
–
6.7
35
135
38
2.
0–6
1.8
7.7
–
4.5
32
126
34
3.
0–6
1.7
7.5
–
5.3
34
131
38
4.
0–6
1.7
7.6
–
7.6
39
150
40
There was slight variation in the percentage infestation of different plant parts. The variation in fungal species may be due to different climatic conditions, isolation periods and different storage containers. The highest infestation percentage was recorded for phloem, xylem, and roots. Fungi species found in the root portion were soil-borne fungi and responsible dieback, while fungi prevailing in the xylem and phloem were aftereffects of disease attack. The highest fungi percentage was in roots; hence, it is predicted that any of the fungi found in the roots is the major cause shisham decline. Botrytis and Alternaria spp. were recorded in roots and can be considered as the major cause of dieback. The highest percentage of Alternaria in xylem and phloem suggested that it played an important role as saprobic fungus as well as main cause of the disease. Presence of Paecilomyces in same proportion of xylem and roots and slight appearance in phloem confirmed that it acted as a pathogenic fungus in the spread of the disease. Cephalosora was only observed in phloem indicating that it acted as saprobic fungi. Ostracoderma acted as soil-borne fungi rather than saprobic. Slight variations were noted in the distribution of Penicillium, Aspergillus, Phoma, Curvularia, Fusarium sp., Lichenocomium, Haphlographium, Briosia, and Steganosporium in all parts of the tree. Fungi species that were suspected to be the cause shisham decline were grown on agar medium to observe their colonial growth. Alternaria and Fusarium showed maximum radial growth than others when grown on agar plates indicating that these could be the prominent cause of shisham dieback. However, the colonial growth of Dreshcelera was suppressed in agar medium.
The results indicated that all the fungicides significantly altered mycelial growth of different fungi (Table 3). The lowest diameter of fungal colony was noted for Drechslera on Potato Dextrose Agar (PDA) medium as 0.1.00 mm/ day, while other fungi species had higher diameter, i.e., Haphlographium (1.40 mm/day), Paecilomyces (1.60 mm/day), Curvularia (3.30 mm/day), Aspergillus (4.10 mm/day), Penicillium (4.60 mm/day), Fusarium spp (5.30 mm/day) and Alternaria spp. (5.70 mm/day). It was observed that Alternaria spp had bigger fungal colony as compared to all other fungi (Table 4). The Phoma spp. expressed minimum fungal mycelial growth (1.00 mm/day) after the application of Chlorothalonil + Procymidone as compared to control (10.0 mm/ day), whereas Mancozeb and Metiram totally inhibited the fungal mycelial growth (0 mm/ day). Aspergillus exhibited the highest colony growth on control (28 mm/ day) and lowest colony growth (1.00 mm/day) after adding Sulphur in PDA medium. Mean values in a column sharing similar letters do not differ significantly as determined by the LSD test (P ≤ 0.05).
Source
DF
SS
MS
F
P
Error
2
0.50
0.25
Fungal growth
8
74.30
9.28
3715.33
0.0000
Error
16
0.04
0.0025
Total
26
74.84
Sr. No.
Fungi
Arithmetic Means
1
Alternaria spp.
5.70a
2
Fusarium spp
5.30b
3
Curvularia spp
3.30f
4
Aspergillus spp
4.10e
5
Penicillium spp
4.60c
6
Haphlographium spp
1.40 h
7
Paecilomyces spp
1.60 g
8
Drechslera spp
1.00i
9
Phoma spp
4.40d
LSD 0.1453
The results revealed that all fungi exhibited statistically higher growth on PDA medium. Drechslera fungi expressed lower growth (1 mm/day) as compared to Haphlographium (1.40 mm/day), Paecilomyces (1.60 mm/day), Curvularia (3.30 mm/day), Aspergillus (4.10 mm/day), Penicillium (4.60 mm/day), Fusarium spp. (5.30 mm/ day) and Alternaria spp. (5.70 mm/day) respectively. Metiram inhibited the growth of all fungi, i.e., Phoma (0.00 mm/day), Aspergillus (0.00 mm/day), Alternaria (1.00 mm/day), Penicillium (0.00 mm/day), Fusarium (0.00 mm/day) and Lichenocomium (0.00 mm/day) as compared to control. Similarly, application of Chlorothalonil + Procymidone caused a significant reduction in fungal growth, i.e., Phoma (0.00 mm/day), Aspergillus (1.00 mm/day), Alternaria (0.00 mm/day), Penicillium (0.00 mm/day), Fusarium (1.00 mm/day) and Lichenocomium (0.00 mm/day) as compared to control treatment.
Alternaria showed higher fungal growth of 1 mm/ day on PDA containing Sulphur, Mancozeb and Metiram, while no growth was observed on Chlorothalonil + Procymidone as compared to control (20 mm/ day). The Sulphur and Mancozeb inhibited the fungal growth, i.e., 1 mm/day of Penicillium on PDA and no colony was observed on PDA with Metiram and Chlorothalonil + Procymidone (0.00 mm/ day) as compared to control (20 mm/ day). Likewise, Fusarium colony growth was retarded by 0.00 mm/day on medium containing Sulphur and Metiram whereas Mancozeb and Chlorothalonil + Procymidone exhibited 1 mm/ day as compared to control (30 mm/ day). Three fungicides, i.e., Mancozeb, Metiram and Chlorothalonil + Procymidone retarded up to 0.00 mm/day fungal colony growth, whereas Sulphur inhibited the Lichenocomium growth by 2.00 mm/ day as compared to control 10.0 mm/ day (Table 5). Mean values in a column sharing similar letters do not differ significantly as determined by the LSD test (P ≤ 0.05).
Sr. No.
Commercially available Fungicides
Fungal mycelial growth (mm/ day) on poisoned food medium
Phoma spp
Aspergillus spp
Alternaria spp
Penicillium spp
Fusarium spp
Lichenocomium spp
1
Sulphur
3.00b
1.00b
1.00b
1.00b
0.00c
2.00b
2
Mancozeb
0.00d
0.00c
1.00b
1.00b
1.00b
0.00c
3
Metiram
0.00d
0.00c
1.00b
0.00c
0.00c
0.00c
4
Chlorothalonil + Procymidone
1.00c
0.00c
0.00b
0.00c
1.00b
0.00c
5
Control
10.0a
28.0 a
20.0a
20.0a
30.0a
10.0a
LSD values
0.6904
0.8062
1.0614
0.3606
0.7008
0.4333
4 Discussion
The wilting, cankers, internal chlorosis and necrosis are the major nefarious disease symptoms observed after sever attack of multiple pathogens (Kumar 2013, Boland and Woodward 2021, Lloret and Batllori 2021). An earlier study (Kumar 2013) reported that wilt disease of shisham in Dehradun and Saharanpur district of western Uttar Pradesh was due to Fusarium solani.
Several researchers also isolated these fungi from shisham seeds. The fungi species included Aspergillus, Penicillium, Rhizopus, Alternaria, Fusarium, Chaetomium, Drechslera and Curvularia, F. solani and Pallidoroseum (Kumar 2013, Kumar 2014). Likewise, Appanah et al. (2000) isolated Alternaria, Aspergillus species and Fusarium, while A. niger, A. flavus, A. tenuis, A. alternata, Chaetomium spp., D. australiensis, F. pallidoroseum, F. solani, Fusarium spp., Penicillium spp., Rhizopus and Geotrichum spp., were observed by Khan et al. (2002). The study of (Mustafa et al., 2004) also observed Rhizoctonia solani, F. solani, F. oxysporum, F. monoliformae, A. niger, A. alternate and Helminthosporium oryzae from shisham tree after severe disease attack. The results of Rajput et al. (2010) are also in line with the current research who isolated F. solani, F. monoliformae, F. equiseti, F. oxysporum, F. semitectum, R. solani, A. alternate, Curvularia lunata, A. niger and Penicillium spp. from shisham.
Dieback disease is caused by number of biotic and abiotic factors and commonly known as syndrome due to complexity of pathogens attack (Parveen et al., 2019). The casual pathogen of dieback disease completes it reproduction in vascular bundles and blocks the translocation of water as well as other essential nutrients. The sever attack causes an extensive damage to shisham in South-Asian territories (Ijaz and Haq, 2021; Rehman et al., 2012a,b; Rizvi et al.,). Shisham tree is facing serious disease attack in Pakistan as well as various other countries of the world. Nevertheless, the severity of disease symptoms varies from plant to plant and country to country (Webb and Hossain 2005, Jones, 2021). Dieback caused extensive damages to the planted trees growing along water channels as well as on the edges of agricultural land (Jones, 2021, Kovač et al., 2021, New et al., 2021).
Several studies proved that dieback disease can only be prevented by the application of fungicides as seed treatment and soil modification with crop remnants. This complex disease was managed successfully by application of Bavistin and Captafol with less propagules per unit of soil. It is not feasible to restrict the soil-borne pathogens either with the help of chemicals or crop rotation from the infected soil. For this purpose, the number of factors, i.e., raising of healthy nursery, correct selection of plantation location having light textured soil, good drainage and sufficient humidity should be kept in mind to minimize this disease of shisham (Arif et al., 2021, Shah et al., 2021).
5 Conclusion
It is concluded from this study that several fungal species are responsible for shisham decline in the study area. The dieback and wilt are the major constraints in the successful production of shisham in different growing areas of Pakistan. The involvement of numerous fungi species indicated that this tree has a major extinction threat. There should be concrete efforts to protect this tree from these fungi species. This disease can only be managed by opting multiple approaches such as soil capacity enhancement via nutritional management, proper selection of nursery, proper selection of plantation sites, prevention of root injury, implementation of normal cultivation practices and avoiding the excessive application of fertilizers, insecticides, and fungicides.
Acknowledgement
The authors extend their appreciation to the Researchers Supporting Project number (RSP2022R483), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Frequency of mycoflora associated with shisham (Dalbergia sissoo) decline in district faisalabad, pakistan. FUUAST J. Biol.. 2015;5(2):225-229.
- [Google Scholar]
- Identification of fungal species associated with dieback and canker disease of elm trees in tabriz metropolis. J. Appl. Res. Plant Protect.. 2019;8(3):25-51.
- [Google Scholar]
- Dieback of sissoo. In: Proceedings of International Seminar, Kathmandu, Nepal, 25-28 April 2000. Forestry Research Support Programme for Asia and the Pacific; 2000.
- [Google Scholar]
- The impact of fosetyl-aluminium application timing on karnal bunt suppression and economic returns of bread wheat (Triticum aestivum L.) PLoS One 2021
- [CrossRef] [Google Scholar]
- A molecular study of genetic diversity in shisham (Dalbergia sissoo) plantation of NWFP, Pakistan. Pak. J. Bot.. 2010;42(1):79-88.
- [Google Scholar]
- Integrated disease management to control shisham (Dalbergia sissoo roxb.) decline in Pakistan. Pak. J. Bot.. 2007;39(7):2651-2656.
- [Google Scholar]
- Barnett, H.L., Hunter, B.B., 1972. Illustrated genera of imperfect fungi. Illustrated genera of imperfect fungi.
- Thick bark can protect trees from a severe ambrosia beetle attack. PeerJ. 2021;9(9:e10755)
- [CrossRef] [Google Scholar]
- Shisham mortality in bihar extent and causes. Indian Phytopathol.. 2003;56(4):384-387.
- [Google Scholar]
- Short note: Seed source variation in seedling and nodulation characters in Dalbergia sissoo roxb. Silvae Genetica.. 2007;56(1–6):88-91.
- [Google Scholar]
- Screening resistance of Dalbergia sissoo clones against ganoderma lucidum root rot disease in field conditions. Forest Pathol.. 2011;41(3):221-226.
- [Google Scholar]
- Dalbergia sissoo: Biology, Ecology And Sustainable Agroforestry. CRC Press; 2021.
- Research on shisham (Dalbergia sissoo roxb.) decline in pakistan–a review. Pak. J. Phytopathol.. 2008;20(1):134-142.
- [Google Scholar]
- Mycoflora associated with stored seeds of different varieties of shisham (Dalbergia sissoo roxb.) Pak. J. Phytopathol.. 2010;22(1):9-12.
- [Google Scholar]
- Global plant virus disease pandemics and epidemics. Plants. 2021;10(2):233.
- [CrossRef] [Google Scholar]
- Isolation and identification of different fungi from diseased shisham tree. Integrated plant disease management. In: Proceedings of 3rd National Conference of Plant Pathology, NARC, Islamabad, 1-3 Oct. 2001. Pakistan Phytopathology Society; 2002.
- [Google Scholar]
- Botryosphaeria dothidea and Neofusicoccum yunnanense causing canker and die-back of sequoiadendron giganteum in Croatia. Forests.. 2021;12(6):695.
- [CrossRef] [Google Scholar]
- Assessment of genetic diversity in different clones of Dalbergia sissoo roxb. By rapd markers. Afr. J. Biotechnol.. 2011;10(35):6686-6694.
- [Google Scholar]
- Inhibition of fusarium causing death before life in sissoo (Dalbergia sissoo roxb.) by a botanical-putranjiva. Int. J. Pharm. Biol. Sci.. 2013;8(6):15-22.
- [Google Scholar]
- Search of a natural remedy for control of fusarial wilting of sisham (Dalbergia sissoo roxb) Int. J. Eng. Res. Sci. Technol.. 2014;3:34-44.
- [Google Scholar]
- Ethnomedicinal uses of Dalbergia sissoo roxb in Jharkhand. Internat. J. Ayurved. Herbal Med.. 2012;2(1):198-201.
- [Google Scholar]
- Climate-Induced Global Forest Shifts Due To Heatwave-Drought. Ecosystem Collapse And Climate Change. Springer; 2021. p. :155-186.
- Isolation of mesophyll protoplasts from leaves of Dalbergia sissoo roxb. J. Appl. Sci. Environ. Manage.. 2012;16(1):11-15.
- [Google Scholar]
- Major constraints on shisham (Dalbergia sissoo) plantations and pathological debate on dieback disease in punjab, Pakistan. J. For. Res.. 2015;26(2):267-271.
- [Google Scholar]
- Fungi associated with shisham (Dalbergia sissoo roxb.) seed and their control. Pak. J. Phytopathol.. 2004;16(2):73-75.
- [Google Scholar]
- Factors leading towards Dalbergia sissoo decline (syndrome) in indian sub-continent: A critical review and future research agenda. Pak. J. Agric. Res.. 2019;32(2):302-316.
- [Google Scholar]
- Roles of roadside vegetation in insect conservation in Australia. Austral. Entomol.. 2021;60(1):128-137.
- [Google Scholar]
- Dieback disease of Dalbergia sissoo trees of some major areas of district swabi. Pure Appl. Bi. (PAB). 2019;8(2):1157-1162.
- [Google Scholar]
- Physiological studies on Lasiodiplodia theobromae and Fusarium solani, the cause of shesham decline. Mycopath.. 2009;7(1):35-38.
- [Google Scholar]
- Isolation of fungi associated with shisham trees and their effect on seed germination and seedling mortality. Pak. J. Bot.. 2010;42(1):369-374.
- [Google Scholar]
- In vitro evaluation of various fungicides against fusarium solani isolated from Dalbergia sissoo dieback. Afr. J. Microbiol. Res.. 2012;6(27):5691-5699.
- [Google Scholar]
- Fungi associated with bark, twigs and roots of declined shisham (Dalbergia sissoo roxb.) trees in Punjab, Pakistan. Pak. J. Phytopathol.. 2012;24(2):152-158.
- [Google Scholar]
- In vitro regeneration of Dalbergia sissoo roxb. And the potential for genetic transformation. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2012:40-42.
- [Google Scholar]
- Incidence of shisham decline in punjab and its correlation with different soil factors. Pak. J. Phytopathol.. 2008;20(2):222-228.
- [Google Scholar]
- Rizvi, R., Gupta, V., Ajit, S., Datta, A., Early selection of dalbergia sissoo progenies suitable for agroforestry.
- Shisham (Dalbergia sissoo) decline by dieback disease, root pathogens and their management: A review. J. Agric. Nat. Res. 2021;4(2):255-272.
- [Google Scholar]
- Medicinal importance and association of pathological constraints with Ddalbergia sissoo. Pak J Phytopathol.. 2010;22(2):135-138.
- [Google Scholar]
- Confirmation of Fusarium solani as the causal agent of die-back of Dalbergia sissoo in Nepal. Plant. Pathol.. 2007;56(6):1041.
- [Google Scholar]
- Resistance of Dalbergia sissoo to Fusarium solani f Sp. Dalbergiae. Forest Pathol.. 2008;38(6):410-418.
- [Google Scholar]
- Sissoo, its pathogenic constraints and their management in Nepal: A review. Grassroots J. Nat. Resour.. 2020;3(4):1-17.
- [Google Scholar]
- Detection of virus particles and double-stranded rna in dieback affected Dalbergia sissoo roxb. from Bangladesh. Bangl. J. Bot.. 2011;40(1):57-65.
- [Google Scholar]
- Dalbergia sissoo mortality in bangladesh plantations: Correlations with environmental and management parameters. Forest Ecol. Manage.. 2005;206(1–3):61-69.
- [Google Scholar]







