Translate this page into:
Function-specific volatiles and volatilization characteristics of Dendrobium officinale
⁎Corresponding author. zhangdangquan@163.com (Dangquan Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The orchid Dendrobium officinale is a medicinal herb that has increasingly been in demand in recent years. It is mainly used for medicinal purposes, and other functional products from it are rarely developed. Here, the volatile organic compound (VOC) composition and functional characteristics of the solid powders and solvent extractives of D. officinale were expounded, and the laws of thermal weight changes of group and surface microstructures were revealed. Many beneficial constituents were detected in the VOCs from powders of the root, stem, and leaf of D. officinale. The roots, stems, and leaves were rich in vitamin E and heptacosane, which can be used as nutritional supplements. The root also contains rich glycerol, can be used as a sweetener and absorbent; the stem is rich in dimethyl phthalate, which can be used to produce mosquito repellent oil; and the leaves contain abundant aldehydes, which can serve as food additives and a component of orange flavoring. However, the root also contains a trace amount of pyrrole (0.09%), which could be used as a negative indicator for the quality evaluation of D. officinale. In addition, many other beneficial VOCs have been detected in extractives of D. officinale, such as phytol, melezitose, hexadecanoic acid, associated esters, and other bioactive components. The total content trend is: ethanol extractive > phenylethanol extractive > petroleum ether extractive, suggesting that the ethanol extractive has the best development prospect. The infrared spectroscopy analysis showed that D. officinale contains aromatic compounds, organic acids, esters, alcohols, aldehydes, and alkanes. It was further confirmed that the alkanes detected in the volatile compounds of D. officinale are natural components of D. officinale, and it was shown that an extraction of organic solvents can cause subtle compound group variation. During the heat loss of D. officinale, there were three distinct stages: 30 °C–130 °C, 130 °C–400 °C, 400 °C–600 °C. The order of quality loss is: second stage > last stage > first stage. The microscopic influence of extraction treatment on D. officinale and the characteristics of microstructural changes showed that the microscopic surface structure characteristics of solid particles of D. officinale, including aggregation degree, shape uniformity, and structure size, are slightly changed.
Keywords
Dendrobium officinale
Volatile organic compounds (VOCs)
Composition characteristics
Organic solvent extract
Microscopic surface structure
1 Introduction
D. officinale is one of the most important traditional Chinese medicines. which has a history of hundreds of years. Because of its antipyretic, immune regulation, eye-benefiting and other functions, it is also used as a folk medicine. (He et al., 2015). In recent years, as a result of overexploitation and great demand, the D. officinale growing in the wild has become endangered (Li et al., 2008).
Dendrobium provides some beneficial functions to the body including heat removal, improvement of eyesight, maintaining stomach tension, increasing body fluid production, and beneficial immunoregulation (Shen et al., 2017; Zhang et al., 2016). Recent studies have found that D. officinale contains polysaccharides, alkaloids, flavonoids, phenanthrene, amino acids, trace elements, and many other nutrients with anti-tumor, antioxidant, hypoglycemic, anti-aging, anti-bacterial, and other functions (Tsai et al., 2010).
Most previously conducted analyses of D. officinale have used only instruments such as GC/MS to detect and analyze without the use of an all-encompassing detection and analysis system. There also have not been analyses conducted on the compositions of volatile organic compounds (VOCs) of D. officinale at different temperatures and that of D. officinale extractives from different organic solvents, as well as the residues after extraction. In this experiment, D. officinale solids were analyzed by thermal desorption gas chromatography (TD-GC/MS) and thermogravimetry (TG), and the VOCs of the D. officinale extractives from ethanol, benzene/ethanol, and petroleum ether were detected by GC/MS. Their residues were analyzed by infrared (IR) spectroscopy and scanning electron microscopy (SEM).
2 Materials and methods
2.1 Materials and reagents
Dendrobium officinale was provided by the Biotechnology Laboratory of Central South University of Forestry and Technology. After being dried at approximately 40 °C, D. officinale was ground into powders with a Disintegrator (China). 200 meshes of powder were screened by screening instrument (USA). All reagents are purchased from Sigma Chemical Company (USA), unless otherwise specified.
2.2 Methods
2.2.1 D. officinale extraction by three solvents
D. officinale samples were extracted by reflux of solvent (ethanol, benzene/ethanol (2:1) and petroleum ether) for 5 h. After 12 h at room temperature, the samples were completely extracted by FOSS Automated Soxhlet Extraction apparatus (Agilent, USA). Place it at 70C for 5 h and then filter it with filter paper soaked in ethanol for 24 h (Wei et al., 2011). The filtered extract was evaporated at 45C in 0.01 MPa, concentrated to 20 ml, transferred to a sealed reagent bottle, and then dried at 50C to extract the residue (Tian et al. 2015). Concentrated extracts and residues are stored in refrigerators at 4C for the following determination (Liu et al., 2017).
2.2.2 Solid volatilization analysis by TD-GC/MS
After the samples had been refrigerated for several hours, they were lyophilized with a freeze-drying apparatus (Yamato DC801). Three different anatomical parts of D. officinale were each placed into sample tubes. Then, the VOCs were released in a split mode (ratio of 1:10) to a dimethyl polysiloxane capillary column (DB-5MS, 30 m × 250 μm × 0.25 μm) coated with a neutral phase (Hewlett-Packard-5 cross-linked 5% phenyl methyl silicone) (Lukic, et al. 2016). GC/MS was carried out with an Agilent GC7890B/MS5977A, which was linked to a mass selective detector (MSD). The carrier gas was helium, the system gas pressure was 20 kPa, and the injection port temperature was 250 °C (Rong et al., 2016).
The GC temperature program initiated at 30 °C and increased at the rate of 10 °C/min until 50 °C,and then increased at the rate of 8 °C/min until 250 °C, followed by a temperature ramp of 5 °C/min to 300 °C, which was then held for 5 min (Okazaki et al., 2016) . The MS program scanned over 30–600 atomic mass units (AMUs) (m/z), with an ionizing voltage of 70 eV and an ionization current of 150 µA for electron ionization (EI)(Gil-Molto et al., 2009). The flow velocity of helium was 1.2 ml/min, the ion source temperature was 230 °C, and the quadrupole temperature was 200 °C (Tolgyessy et al., 2011).
2.2.3 Thermostability of D. officinale by TGA
Thermogravimetric analysis (TGA) was conducted using a thermogravimetric analyzer (TGA Q50 V20.8 Build 34, USA), with a sensitivity of 0.1 μg, using an empty crucible clamp as a reference. The furnace temperature control ranged from 30 °C to 1000 °C. An accurate mass of D. officinale sample (6606.00 μg) was placed in a TG crucible (Jaiswal and Koul, 2013). The TGA temperature program started at 30 °C and increased to 600 °C at 10 °C/min; the carrier gas used was high purity nitrogen with a flow rate of 40 ml/min.
2.2.4 VOC analysis by GC/MS
For the GC/MS analysis of VOCs, 1.0 ml concentrated extractives were aspirated into a 1.5-ml centrifuge tube, mixed with an appropriate amount of anhydrous sodium sulfate at room temperature for 2 h, centrifuged at 1200 rpm for 2 min, and then 700 μL of supernatant was removed and placed into a reagent bottle for detection by GC/MS (Rohrmann et al., 2011). For the GC analysis, a capillary column (30 m × 250 μm × 0.25 μm) was used. The initial temperature of 50°Cwas maintained for 0 min, then increased at 8 °C/min to 200 °C, maintained for 0 min, increased at 5 °C/min to 260 °C, and then maintained for 2 min. The carrier gas used was high purity He (99.99%), the column pressure was 57.4 KPa, the injection volume was 1 μL, no split mode was used, and the vaporization chamber temperature was 280 °C (Jung et al., 2011). Detection with MS was controlled with a program that scanned from 30 to 600AMU (m/z); an ionizing voltage of 70 eV was used with an ionization current of 150µA for electron ionization (EI). The flow velocity of helium was 1.2 ml/min, the ion source temperature was 230 °C, and the quadrupole temperature was 200 °C (van Oort et al., 2017).
2.2.5 D. officinale solid and extracted residue analysis by FTIR
Fourier transform infrared spectroscopy (FTIR) is widely used to identify chemical bonds and functional groups of various compounds (Mi et al., 2019). A sample of D. officinalis and its three extractive residues was dried continuously at 100 °C for 4 h, then stored with desiccant to prevent moisture absorption, thereby reducing any adverse effects on backward. It was placed in a drying pot and dried in a muffle furnace at 150 °C for 5 h. Then it was removed under a heating lamp shade. Then 200 mg potassium bromide is ground to a smooth surface with agate mortar. The sample of 0.5–2 mg is fully mixed with potassium bromide and ground to a smooth surface with mortar. The powder mixture is then put into a tablet press and compressed into tablets (Savchenko et al., 2017). The pressed samples were tested by infrared spectroscopy.
2.2.6 D. officinale solid and extracted residue analysis by SEM
For SEM analysis, a sample of D. officinale and its extracted residue were baked at 70 °C for 12 h. A small amount of the sample was spread onto double-backed cellophane tape attached to a stub before coating with gold–palladium at room temperature 32 °C for 1 min (De Basanta et al., 2009). Scanning electron micrographs of the samples were taken with a JEOL scanning electron microscope (JSM-6380LV) at an acceleration voltage of 15.00 kV and a distance of 100 μm; particles were imaged between 200× and 2000× nominal magnification (Ku et al., 2016).
3 Results and analysis
3.1 Characteristics of volatile constituents in different organs of D. officinale
A total of 57 substances was detected in different organs of D. officinale by TD-GC/MS, of which 35 species were detected in the root, 33 species in the stem, and 31 species in leaves (Fig. 1). The components of the stem and leaf were similar, but the content was different. It was found that 11 types of substances were jointly detected in three parts, and 14 types of substances were jointly detected in two parts. The most common substance in three organs of D. officinale was phthalic acid (hex-3-yl isobutyl ester) (Table 1). Its physiological activity and pharmacological toxicity have not been researched yet, only detected in Chinese bayberry, Myricarubra cv. DingAo Orient Pearl.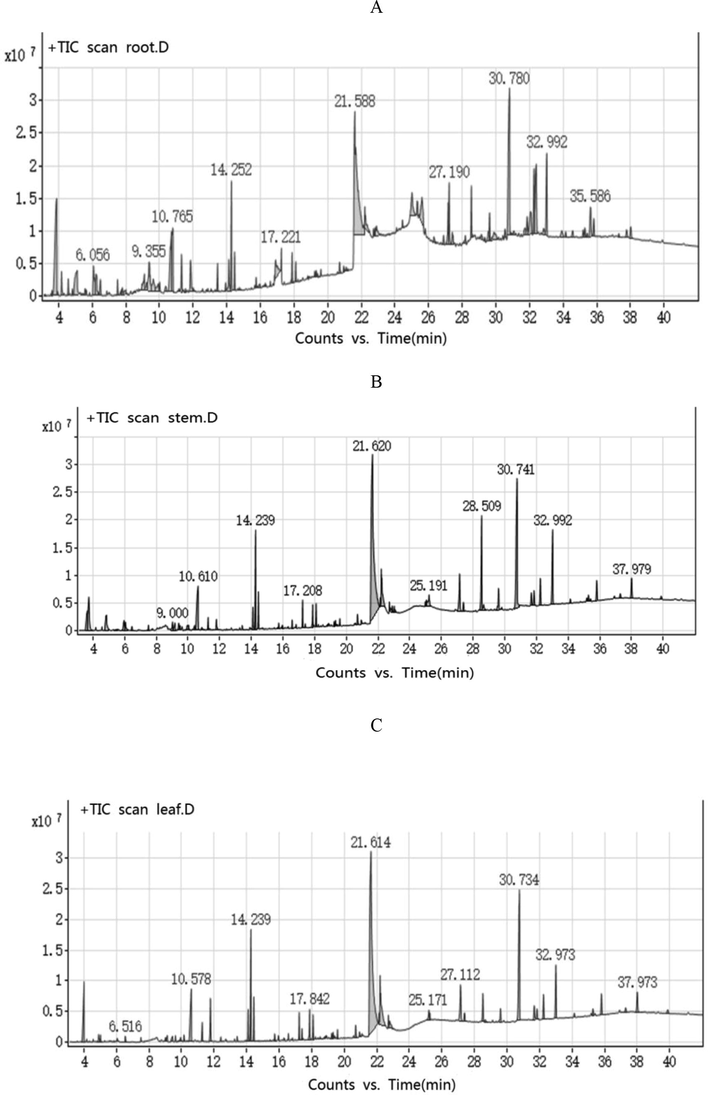
Gas chromatograms of different organs of D. officinale. (A) Root; (B) stem; (C) leaf.
No.
Component names
Relative content/%
Root
Stem
Leaf
1
Pyrrole
0.09
2
Acetic acid
7.50
5.23
4.06
3
Hexanal
0.68
4
Pyrazine, methyl-
0.37
5
1 h-Pyrrole, 2-methyl-
0.12
6
Acetamide
2.57
1.92
0.39
7
2-Furanmethanol
0.38
0.17
2-Propenamide
1.39
9
Butyrolactone
0.55
0.30
10
Pyrazine, 2-ethyl-5-methyl-
0.08
11
4-Pyridinamine, 2,6-dimethyl-
0.23
12
Alpha-l-rhamnopyranose
0.33
13
Glycerin
1.01
14
Maltol
0.27
15
4 h-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-
7.13
4.57
4.81
16
Benzofuran, 2,3-dihydro-
1.40
0.56
17
11-Oxa-dispiro[4.0.4.1]undecan-1-ol
0.18
18
Dodecanoic acid, 3-hydroxy-
0.10
0.22
19
Desulphosinigrin
0.32
20
2-Methoxy-4-vinylphenol
0.65
21
7-Methyl-z-tetradecen-1-ol acetate
0.98
2.70
2.67
22
2-(2-Butoxyethoxy)-ethanol, acetate
2.56
3.77
23
Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester
0.84
1.24
24
Estra-1,3,5(10)-trien-17. beta. -ol
0.74
25
1-Penten-3-one, 1-(2,6,6-trimethyl-1-cyclohexen-1-yl)-
0.09
26
Benzene, 1,2,3-trimethoxy-5-(2-propenyl)-
1.90
0.95
1.00
27
Cedrol
0.40
0.81
0.90
28
Phthalic acid, hex-3-yl isobutyl ester
16.49
31.93
37.0
29
Oleic acid
6.56
3.87
0.97
30
9-Octadecenamide, (z)-
1.63
2.55
2.80
31
Heptacosane
11.50
16.00
13.46
32
Beta-sitosterol
1.86
33
Ethyl iso-allocholate
1.53
0.18
34
Gamma-sitosterol
1.45
1.32
1.45
35
Ethanone, 1-(1 h-pyrrol-2-yl)-
0.50
0.26
36
Furaneol
0.56
0.38
37
[1,3] Diazepan-2,4-dione
0.48
38
2-Pyrrolidinone
0.26
39
2-Hepten-4-one, 2-methyl-
0.42
40
Ethanol, 2-(2-butoxyethoxy)-
0.43
41
2,2,4-Trimethyl-1,3-pentanediol diisobutyrate
1.74
42
Dimethyl phthalate
0.20
43
3-Buten-2-one, 4-(5,5-dimethyl-1-oxaspiro [2.5] oct-4-yl)
0.17
44
2,4-Di-tert-butylphenol
0.27
0.23
45
Tridecanoic acid, 12-methyl-, methyl ester
0.24
0.30
46
1,2-Benzenedicarboxylic acid, butyl cyclohexyl ester
0.23
0.75
47
Phthalic acid, butyl undecyl ester
0.62
0.97
48
Pentacosane
5.03
49
Formic acid, 3,7,11-trimethyl-1,6,10-dodecatrien-3-yl ester
1.39
50
Vitamin E
4.03
1.11
1.23
51
2-Hexenal, (e)-
0.27
52
Nonanal
0.21
53
Dimethyl dl-malate
0.28
54
2′,4′-Dimethoxyacetophenone
0.42
55
Tetradecane, 2,6,10-trimethyl-
0.27
56
9,12-Octadecadienoic acid (z,z)-
1.31
Abundant vitamin E was detected in D. officinale. Vitamin E is a fat-soluble antioxidant, although it has many other biological functions (Peng et al., 2017a). As an antioxidant, vitamin E is a fat-soluble antioxidant, although it has many other biological functions. As an antioxidant, vitamin E acts as a peroxy radical scavenger, reacts with them to form tocopherol groups and cannot produce harmful free radicals in tissues, and then returns through hydrogen donors (such as vitamin C) to return to a reduced state (Traber and Stevens, 2011). Vitamin E has also been found to be a commercial antioxidant and biocompatible modifier for biomaterials and medical devices, such as ultra-high molecular weight polyethylene (UHMWPE) for hip and knee implants and for adrenal glands hemodialysis therapy (Piroddi et al., 2012).
4 h-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- (root 7.13%, stem 4.57%, leaf 4.81%), a pharmaceutical intermediate, can be used in the synthesis of edible essence and flavors (Peng et al., 2017b). Oleic acid (6.56% root, 3.87% stem, 0.97% leaf) is an essential nutrient in animal foods, and its lead salt, manganese salt and cobalt salt can be used as paint desiccant. In addition, it can produce many other products.9,12-Octadecadienoic acid (z, z)- (leaf 1.31%), a nutrient fortifier, can be used as a lipid-lowering drug and for the treatment and prevention of atherosclerosis.
The root, stem, and leaf of D. officinale contained a large amount of heptacosane (C27H56), 11.5%, 16%, 13.46% respectively. Heptacosane is a nutritional supplement that can increase sweetness and improve the aftertaste, and also explains why the stem of D. officinale is sweeter than the root and leaf. In addition, the root was rich in glycerol (1.01%), which can be used as a sweetener and moisture absorber; the stem was rich in dimethyl phthalate (0.20%), which can be synthesized into an insect repellent. The leaf was rich in nonanal (0.21%), which had a strong smell of sweet oranges (Cullere et al., 2011). A dilute ethanol solution of Nonanal contains vanilla aldehyde that confers a fragrance; it is often used as a food additive and also can be used to manufacture the fragrance of roses, orange blossoms, violets, and other fragrances. However, the root of D. officinale also contained traces of pyrrole (0.09%), which is a negative indicator for the quality evaluation of D. officinale (Heeba and Abd-Elghany, 2010).
3.2 Vocs characteristics of D. officinale extractives
17, 16, and 9 VOCs were detected from three D. officinale extractives that were obtained by solvents (benzene/ethanol (2:1), ethanol, petroleum ether), respectively (Fig. 2, Table 2). The substance detected at the 31.2 min peak was heptacosane(C27H56) (Peschel et al., 2007). All three extracts contained a large amount of heptacosane. According to the results of TD-GC/MS and GC/MS, the heptacosane content was indeed high, and it was one of the main components of D. officinale. The combination of the two instruments could be used to verify the results and reduce interference. The largest amount of heptacosane was extracted by petroleum ether. Melezitose is a nonreducing trisaccharide sugar that can be partially hydrolyzed to glucose and turanose, the latter of which is an isomer of sucrose (Peschel et al., 2007). Melezitose is mainly extracted by ethanol. In addition, ethanol can extract linoleic acid (9,12-octadecadienoic acid (Z, Z)-) and palmitic acid (N-hexadecanoic acid) from D. officinale.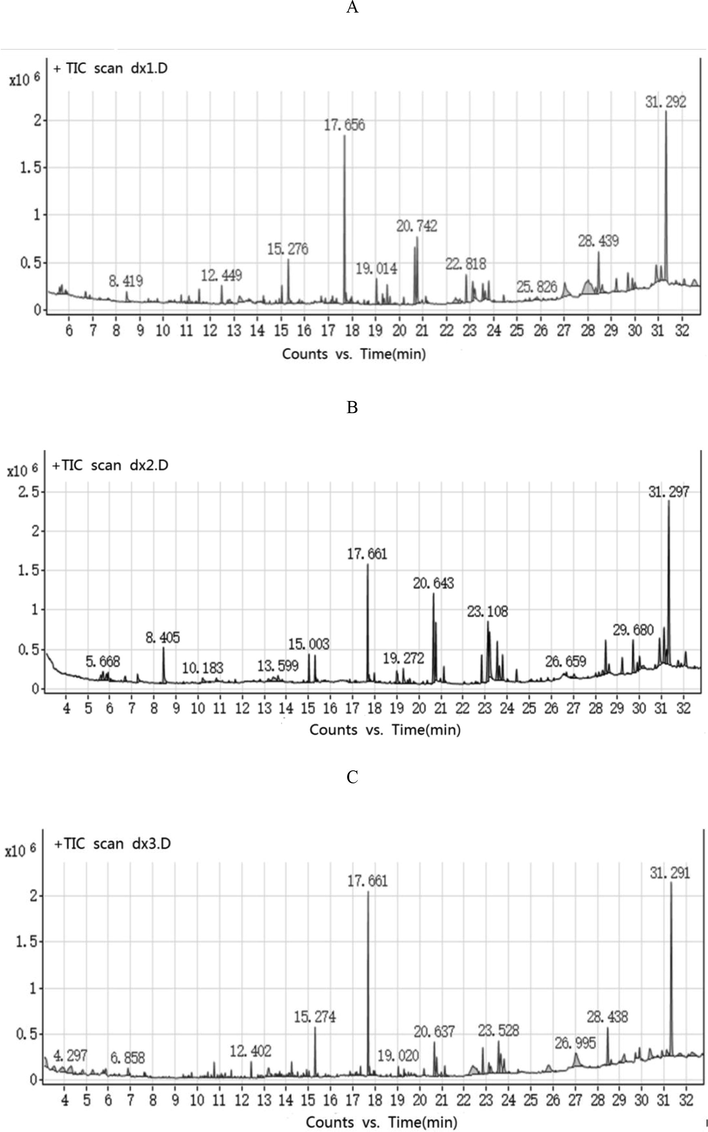
Volatile extraction spectra of D. officinale with different organic solvents. (A) Phenylethanol; (B) ethanol; and (C) petroleum ether.
No.
Component names
Relative content/%
Benzene/ethanol (2:1)
Ethanol
Petroleu-m ether
1
(1,4-Dimethylpent-2-enyl) benzene
1.08
2
Benzenemethanol, 3-hydroxy-5-methoxy-
0.18
3
1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-
2.47
1.26
2.64
4
3-Methyl-2-butenoic acid, tridec-2-ynyl ester
8.50
5.90
11.34
5
Phytol, acetate
1.42
6
Pentadecanoic acid
0.62
1.26
0.82
7
[1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester
0.41
1.36
1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester
1.08
9
1,2–15,16-Diepoxyhexadecane
0.53
10
N-Hexadecanoic acid
3.98
6.36
2.51
11
Phthalic acid, butyl isohexyl ester
4.17
3.42
1.73
12
Hexadecanoic acid, ethyl ester
0.37
0.89
13
Phytol
1.80
1.81
1.80
14
9,12-Octadecadienoic acid (Z, Z)-
0.89
13.44
15
Linoleic acid ethyl ester
1.22
16
Gamma-Sitosterol
7.69
17
Heptacosane
14.06
13.80
14.98
18
4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-
2.84
19
Melezitose
1.97
20
Tetradecanoic acid
0.60
21
17-Octadecynoic acid
1.39
22
Dodecanoic acid, 3-hydroxy-
0.40
0.65
23
Ethyl oleate
1.43
24
Undec-10-ynoic acid, dodecyl ester
0.81
Most of the VOCs in ethanol extracts of D. officinale were alcohols, the VOCs of ethanol and petroleum ether extracts were alkanes and organic acids, and the VOCs extracted by ethanol were esters. In addition, a variety of other beneficial VOCs, such as phytol, melezitose, hexadecanoic acid, associate esters, and other bioactive components, were also detected in the extracts of D. officinale, but their relative content in the three solvents was different. The order of the total content was ethanol extract > phenylethanol extract > petroleum ether extract. Therefore, in the actual processing and utilization, we can choose suitable solvents for extraction and preparation according to the different VOC requirements, in which the development foreground of ethanol extract is best.
3.3 Chemical group changes of D. officinale and residues
In the range of 4000 cm−1 to 400 cm−1, the FTIR absorption peak mainly occurred between 1800 cm−1 and 800 cm−1, and a slight change in the FTIR absorption peak was observed between D. officinale and the extracted residue. (Fig. 3). A strong absorption peak appears near 3300 cm−1(Fig. 3), indicating that there is an –OH bond. Consistent with the results of GC/MS, the VOCs of D. officinale are abundant in ethanol and phenolic compounds (Chen et al., 2011). The peak near 2900 cm−1 corresponds to the C–H bond of the alkane. Heptacosane is a major component of VOCs of D. officinale (Taira et al., 2011).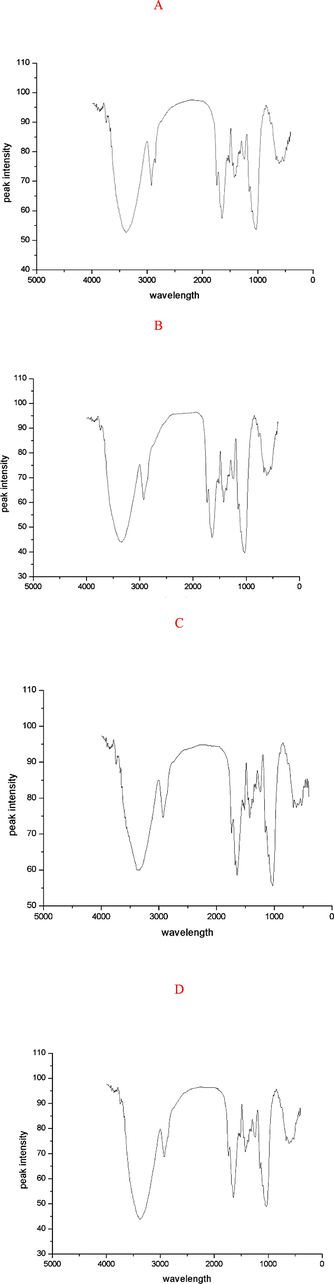
(A) Infrared spectra of D. officinale samples; (B) infrared spectra of the residue of benzyl ethanol extracts from D. officinale; (C) infrared spectra of ethanol extracts from D. officinale; (D) infrared spectra of the residues of petroleum ether extracts from D. officinale.
At a wavelength of 2800 cm−1, a peak can be observed from the infrared spectrogram of D. officinale, while the residue does not have this peak. According to the rule of infrared group peak position, this region corresponds to alkanes. It was probable that the alkane or aldehyde substances were proposed after extracting, causing perssad changed (Filip et al., 2004). The peak near 1700 cm−1 corresponding to the C = O indicates that VOCs of D. officinale may contain aldehydes, ketones, acids, and esters. Consistent with the result of GC/MS, the VOCs of D. officinale are abundant in acids and esters (Wu et al., 2010).
At wavelengths near 1600 cm−1, the absorption peak corresponds to olefins. The C–H bond in the double bond of olefins has a bending vibration of 1500–1000 cm−1, which is insensitive to the structure and less used. The vibration of the outer surface is the most useful in the range of 1000–700 cm−1, the absorption peak is obviously characterized, the intensity is larger, and it is easy to recognize (Aramwit et al., 2010).
3.4 Volatility characteristics of D. officinale
In the differential thermogravimetric analysis (DTG) analysis from 30 °C to 600 °C, D. officinale has three distinct heat loss stages. (Fig. 4). The mass loss in the first stage occurred from an initial temperature of 30 °C to 130 °C, a reduction of 5319.662 μg at a temperature of 130 °C and a loss of 3.92%. The loss of mass is likely attributed to water evaporation but also could be caused by further condensation between molecules. The quality of the second stage was significantly reduced from 240 °C to 400 °C by 2640.089 μg, with a loss of 51.33%. During the TG treatment from 30 °C to 600 °C, a distinct peak was observed in the corresponding DTG curve, which reached its maximum at 319 °C, indicating that the rate of mass loss of D. officinale was the fastest in this temperature range. At 319 °C, the maximum weight loss rate was 0.4538834. During the third stage, minor mass loss occurred starting at 400 °C, and at 600 °C, the pyrolysis of D. officinale was basically complete. The quantity was reduced to 1.331679 mg, with a loss of 20.7%. According to the DTG curve, the graded mass loss rate: the second phase > the third phase > the first phase. In addition, there were three critical temperature turning points at 130 °C, 319 °C and 400 °C during the TG treatment. At three temperature points, the mass of D. officinale varies greatly, which may be caused by significant chemical changes, such as pyrolysis of macromolecules into small molecules.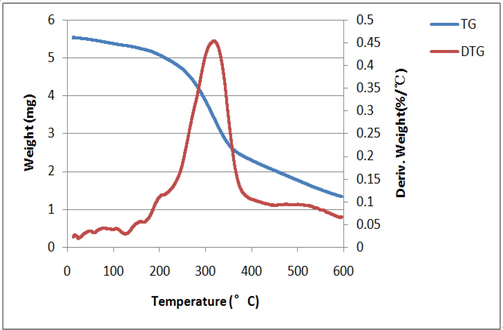
TG Curve and DTG curve.
3.5 Microstructure change of D. officinale solid and extracted residue by SEM
D. officinale and its extraction residues were scanned at multiple sites at magnifications of 200× and 2000× (Grímsson et al., 2011), and the sites that could represent the entire microcosmic morphological characterization were selected and photographed (Fig. 5). At a magnification of 200×, D. officinale powder showed a variety of different micromorphologies, such as spherical, ellipsoidal, massive, etc., and it was obvious that the sizes of the particles were different and that the surfaces were rough.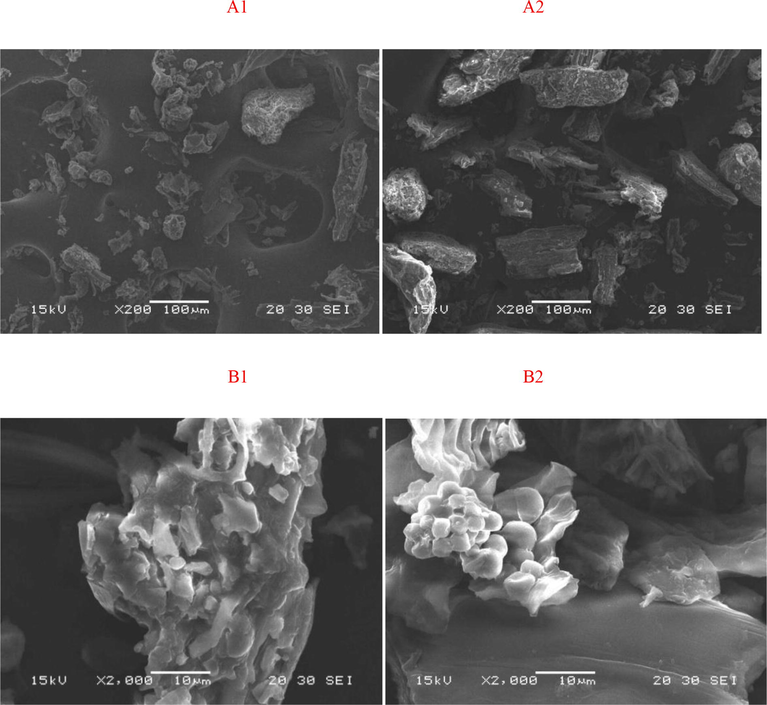
. (A1) SEM image of D. officinale powder at 200× magnification; (A2) SEM image of D. officinale residue at 200× magnification; (B1) SEM image of D. officinale powder at 2000× magnification; (B2) SEM image of D. officinale residue at 2000× magnification.
Extraction of the residues revealed massive particles that were almost the same size, but the surface roughness did not significantly change. With a magnification of 2000×, the surface of D. officinale powder appeared uneven because it was composed of all types of tiny particles that were adhered to one another. The shapes and sizes of the particles were not obvious. Many tiny particles were also adhered to the surface of the extracted residue, and the shapes of the tiny particles were mostly spherical or ellipsoidal with not much difference in the size of the particles (Schemehorn et al., 2004).
The electron microscopy image of the extracted residue is brighter than that of the powder, indicating that the particle surface of the extracted residue is smoother, which may be due to some material being extracted from the powder surface. There may be two reasons why the particle morphology of the extracted residue is more unitary: one is the physical change caused by heating in the process of extraction, and the other is that the morphological change results because a great deal of the material is extracted (Wallace et al., 2011).
4 Discussion and conclusions
In the direct VOCs of the solid powder of roots, stems, and leaves f D. officinale, many types of beneficial components were detected, but there was a great difference in the material category and content from the different parts. For example, the roots, stems, and leaves were rich in vitamin E, with the content of roots reaching 4%, and all contained a large quantity of heptacosane (roots 11.5%, stems 16%, and leaves 13.46%), which could be used as a nutritional supplement or made into a sweetener to improve taste. In addition, the roots were also rich in glycerol (1.01%), which could be used as a sweetener or absorbent; the stems were rich in dimethyl phthalate (0.20%), from which insect repellent oil could be produced; the leaves were rich in aldehyde (0.21%), with a strong smell of sweet orange, which could be used as a food additive and could also be used to prepare the fragrance of roses, orange blossoms, fragrant violets, and other fragrances. The roots of D. officinale also contained trace amounts of pyrrole (0.09%), although excessive amounts will reduce the quality of D. officinale and can be used as a negative indicator for the quality of D. officinale. In actual production, the different parts of D. officinale can be used in a different manner according to the content of the substance, in order to achieve the maximum value (Rohrmann et al., 2011).
Most of the VOCs in ethanol extracts of D. officinale were ethanol, in the petroleum ether extracts were alkanes and organic acids, and in the benzene ethanol extract were esters. All three extracts contained a large quantity of heptacosane. In addition, a variety of other beneficial VOCs, such as phytol, melezitose, hexadecanoic acid, associated esters, and other bioactive components, were also detected in the extracts of D. officinale, but their relative content in the three solvents was different. The order of the total content was: ethanol extract > phenylethanol extract > petroleum ether extract. Therefore, in the actual processing and utilization, we can choose the appropriate solvent for extraction and preparation according to different VOC requirements, and the ethanol extract has the best development prospect.
The results of infrared spectroscopy indicated that the extract structure and extracted residue of D. officinale were consistent with the structure contained in the chemical structure detected by GC/MS. The samples of D. officinale contained aromatic compounds, organic acids, esters, alcohols, aldehydes, and alkanes. It was further verified that the alkanes detected in the volatile compounds of the samples are natural components of D. officinale, which showed that the extraction of organic solvents could cause subtle changes in the compound perssads in D. officinale.
The differential thermogravimetric analysis of D. officinale revealed that there were three obvious stages of thermal loss. The first stage was 30 °C–130 °C, the second stage was 130 °C–400 °C, and the third stage was 400 °C–600 °C. The quality loss order was second stage > third stage > first stage. During the thermogravimetric process, the DTG curve reached the maximum at 319 °C and formed a spike, and there were three critical temperature turning points at130 °C, 319 °C, and 400 °C. At these temperature points, significant changes in mass can cause significant chemical changes, such as pyrolysis of macromolecules into smaller, more volatile molecules.
Further ascertaining the microstructure of D. officinale, it was observed that under the scanning electron microscope, the extraction process of volatile compounds could lead to a slight change of the microstructure of the solid particles of D. officinale, which may lead to changes in the structure of D. officinale and the uniformity of particles as well as other subtle changes. Whether this was due to the chemical changes caused by extraction or the physical changes caused by the extraction process remains to be further explored.
Acknowledgements
This project is supported by the Science and Technology Project of Hunan Province, China (2016NK2154), and the National Natural Science Foundation of China (31172257).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Formulation and characterization of silk sericin-pva scaffold crosslinked with genipin. Int. J. Biol. Macromol.. 2010;47l:668-675.
- [Google Scholar]
- Structural features and bioactivities of the chitosan. Int. J. Biol. Macromol.. 2011;49l:543-547.
- [Google Scholar]
- Analysis, occurrence and potential sensory significance of aliphatic aldehydes in white wines. Food Chem.. 2011;127l:1397-1403.
- [Google Scholar]
- Description and life cycle of a new didymium (myxomycetes) from arid areas of argentina and chile. Mycologia. 2009;101l:707-716.
- [Google Scholar]
- Ft-ir spectroscopic characteristics of differently cultivated bacillus subtilis. Microbiol. Res.. 2004;159l:257-262.
- [Google Scholar]
- Application of an automatic thermal desorption-gas chromatography-mass spectrometry system for the analysis of polycyclic aromatic hydrocarbons in airborne particulate matter. J. Chromatogr. A. 2009;1216l:1285-1289.
- [Google Scholar]
- Lythrum and peplis from the late cretaceous and cenozoic of north america and eurasia: New evidence suggesting early diversification within the lythraceae. Am. J. Bot.. 2011;98(11):1801-1815.
- [Google Scholar]
- Identification of genes involved in biosynthesis of mannan polysaccharides in dendrobium officinale by rna-seq analysis. Plant Mol. Biol.. 2015;88l:219-231.
- [Google Scholar]
- Effect of combined administration of ginger (zingiber officinale roscoe) and atorvastatin on the liver of rats. Phytomedicine. 2010;17l:1076-1081.
- [Google Scholar]
- Assessment of multicomponent hydrogel scaffolds of poly (acrylic acid-2-hydroxy ethyl methacrylate)/gelatin for tissue engineering applications. J. Biomater. Appl.. 2013;27l:848-861.
- [Google Scholar]
- Characterization of dandelion species using h-1 nmr- and gc-ms-based metabolite profiling. Analyst.. 2011;136l:4222-4231.
- [Google Scholar]
- Preparation and properties of epoxy resin-coated micro-sized ferrosilicon powder. Materials Research-Ibero-American. J. Mater.. 2016;19l:889-894.
- [Google Scholar]
- Genetic diversity analysis and conservation of the endangered chinese endemic herb dendrobium officinale kimura et migo (orchidaceae) based on aflp. Genetica. 2008;133l:159-166.
- [Google Scholar]
- Lukic I, Radeka S, Grozaj N, Staver M, Persuric D, 2016. Changes in physico-chemical and volatile aroma compound composition of gewurztraminer wine as a result of late and ice harvest. Food Chem, 196l, 1048–57.
- Research on regional clustering and two-stage SVM method for container truck recognition. Discrete Continuous Dyn. Syst. Ser. S. 2019;12:1117-1133.
- [Google Scholar]
- Discovering metabolic indices for early detection of squash (cucurbita maxima) storage quality using gc-ms-based metabolite profiling. Food Chem.. 2016;196l:1150-1155.
- [Google Scholar]
- Adsorption Characteristics of sulfur powder by bamboo charcoal to restrain sulfur allergies. Saudi J. Biol. Sci.. 2017;24:103-107.
- [Google Scholar]
- Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi J. Biol. Sci.. 2017;24:399-404.
- [Google Scholar]
- Composition of the cuticle of developing sweet cherry fruit. Phytochemistry. 2007;68l:1017-1025.
- [Google Scholar]
- Vitamin e as a functional and biocompatibility modifier of synthetic hemodialyzer membranes: An overview of the literature on vitamin e-modified hemodialyzer membranes. Am. J. Nephrol.. 2012;35l:559-572.
- [Google Scholar]
- Combined transcription factor profiling, microarray analysis and metabolite profiling reveals the transcriptional control of metabolic shifts occurring during tomato fruit development. Plant J.. 2011;68l:999-1013.
- [Google Scholar]
- Brewing and volatiles analysis of three tea beers indicate a potential interaction between tea components and lager yeast. Food Chem.. 2016;197l:161-167.
- [Google Scholar]
- Infrared, raman and magnetic resonance spectroscopic study of sio2:C nanopowders. Nanoscale Res. Lett.. 2017;12l:292.
- [Google Scholar]
- A sem evaluation of a 6% hydrogen peroxide tooth whitening gel on dental materials in vitro. J. Dent.. 2004;32(Suppl 1l):35-39.
- [Google Scholar]
- A deep Q-learning network for ship stowage planning problem. Pol. Marit. Res.. 2017;24:102-109.
- [Google Scholar]
- Nanotrap and mass analysis of aromatic molecules by phenyl group-modified nanoparticle. Anal. Chem.. 2011;83l:1370-1374.
- [Google Scholar]
- Investigation on the antidepressant effect of sea buckthorn seed oil through the gc-ms-based metabolomics approach coupled with multivariate analysis. Food. Funct.. 2015;6l:3585-3592.
- [Google Scholar]
- Development of a screening method for the analysis of organic pollutants in water using dual stir bar sorptive extraction-thermal desorption-gas chromatography-mass spectrometry. Talanta. 2011;87l:152-160.
- [Google Scholar]
- Vitamins c and e: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med.. 2011;51l:1000-1013.
- [Google Scholar]
- Moscatilin, a bibenzyl derivative from the india orchid dendrobrium loddigesii, suppresses tumor angiogenesis and growth in vitro and in vivo. Cancer Lett.. 2010;292l:163-170.
- [Google Scholar]
- Van Oort P M, Nijsen T, Weda H, Knobel H, Dark P, Felton T, Rattray N J, Lawal O, Ahmed W, Portsmouth C, Sterk P J, Schultz M J, Zakharkina T, Artigas A, Povoa P, Martin-Loeches I, Fowler S J, Bos L D, Breathdx C, 2017. Breathdx - molecular analysis of exhaled breath as a diagnostic test for ventilator-associated pneumonia: Protocol for a european multicentre observational study. BMC Pulm Med, 17l, 1.
- Wallace P K, Arey B, Mahaffee W F, 2011. Subsurface examination of a foliar biofilm using scanning electron- and focused-ion-beam microscopy. Micron, 42l, 579-585.
- Extraction of organic materials from red water by metal-impregnated lignite activated carbon. J. Hazard Mater.. 2011;197l:352-360.
- [Google Scholar]
- Chemical characterization of auricularia auricula polysaccharides and its pharmacological effect on heart antioxidant enzyme activities and left ventricular function in aged mice. Int. J. Biol. Macromol.. 2010;46l:284-288.
- [Google Scholar]
- Transcriptome analysis of dendrobium officinale and its application to the identification of genes associated with polysaccharide synthesis. Front Plant Sci.. 2016;7l:5.
- [Google Scholar]







