Translate this page into:
Frizzled receptors (FZD) play multiple cellular roles in development, in diseases, and as potential therapeutic targets
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Frizzled receptors (FZDs) are 7-pass transmembrane proteins and members of the G protein-coupled receptor (GPCRs) superfamily. They are classified as WNT receptors and show specific and dynamic expression during different embryonic development stages of invertebrates and vertebrates. They are required in the regulation of developmental processes such as cell specification, cell polarity, and neural patterning in Drosophila, zebrafish, Xenopus, mice, humans, and other animals. The dysfunction of FZDs causes several diseases, including cancer, neural tube defects, and neural degeneration. This review focuses on the structure, signaling, and WNT binding specificity of FZDs as well as their roles in development and diseases, including cancer, embryonic defects, and neurodegenerative disorders. Furthermore, the use of FZDs as potential therapeutic targets for various human diseases is discussed.
Keywords
Frizzled
FZD
Wnt signaling
Development
Cancer
Neurodegenerative disorders
Embryogenesis
Neural tube defects
Gene expression
1 Introduction
FZD receptors have been identified in invertebrates and vertebrates. Four FZDs have been found in Drosophila and C. elegans. In vertebrates (chickens, mice, and humans), 10 FZDs have been reported (van Amerongen and Nusse, 2009). FZD receptors are divided into four groups according to their amino acid sequence similarity and are numbered from 1 to 10 (FZD1–10) (Huang and Klein, 2004).
FZDs are members of the G protein-coupled receptor (GPCRs) superfamily and are 7-pass transmembrane proteins (Barnes et al., 1998; Wang et al., 2006a) (Fig. 1). The length of FZD proteins can range from 500 to 700 amino acids. FZD structure consists of an N-terminal extracellular cysteine-rich domain (CRD), a linker region, seven hydrophobic domains, and a C-terminal intracellular domain (Barnes et al., 1998; MacDonald and He, 2012; Huang and Klein, 2004; Wang et al., 1996). The length of the CRD is approximately 120 amino acids; it is smaller in all known FZDs and is responsible for WNT binding with great affinity (Bhanot et al., 1996; Dann et al., 2001). The KTXXXW motif in the C-terminal is crucial for the stimulation of the canonical WNT pathway (MacDonald and He, 2012; Umbhauer et al., 2000) (Fig. 1).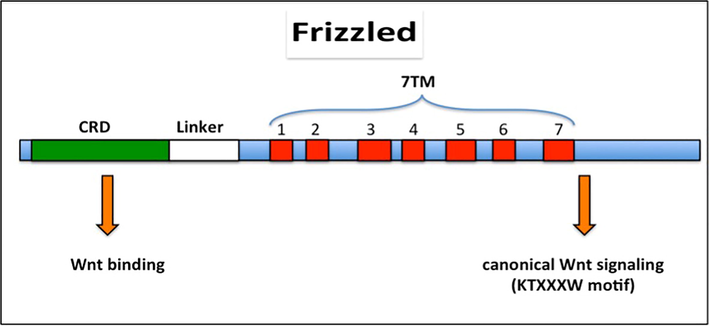
Schematic illustration of Frizzled (FZD) structure. The cysteine-rich domain (CRD) is an extracellular domain in which WNTs bind to FZDs and is followed by the linker region and seven transmembrane (7TM) domains. The C-terminal is the intracellular domain where the KTXXXW motif is located.
FZDs are expressed on the surfaces of WNT-responsive cells and act as receptors for WNT ligands (Bhanot et al., 1999). FZDs were first identified in Drosophila by showing that FZD is capable of WNT binding; the overexpression of DFz2 results in the activation of canonical Wnt in non-sensitive Wnt cell cultures (Bhanot et al., 1996). Moreover, depletion of DFz1/2 yielded the same results as those reported in Wingless mutants (Bhanot et al., 1996; Bhat, 1998). In Xenopus, Fz receptors interact with Wnts in axis duplication assays (Deardorff et al., 2001; Garcia-Morales et al., 2009; Yang-Snyder et al., 1996). In addition, several studies have shown that FZD CRDs are needed for WNT binding (Hsieh et al., 1999; Wu and Nusse, 2002; Cadigan et al., 1998; Rulifson et al., 2000; Zhang and Carthew, 1998). For example, the mechanism of WNT–FZD interactions are confirmed and explained through the crystal structure of Fz8-CRD and XWnt8-mFZD8CRD (Hsieh et al., 1999; Dann et al., 2001; Janda et al., 2012). Therefore, FZD receptors are essential for WNT activation and signal transportation.
It is important to mention that FZDs can bind several ligands, such as secreted Frizzled-related proteins (SFRPs) and R-spondin, but mainly interact with 19 WNTs (Bhat et al., 2007; Rodriguez et al., 2005; Nam et al., 2006). Wnts (Wingless-type MMTV integration site) belong to a family of conserved glycoproteins. Wnt signalling activation is dependent on the binding of FZDs and the low-density lipoprotein-related receptor protein (LRP5/6) (Liu et al., 2003; Li and Bu, 2005). The canonical Wnt signalling pathway consists of several components, including 19 Wnts, 10 FZDs (FZD1-10) a co-receptor; LRP5/6; dishevelled (Dvl), a cytoplasmic protein β-catenin, destruction complex; glycogen synthase kinase 3β (GSK-3β); adenomatous polyposis coli (APC); Axin proteins and casein kinase I (CKI) and nuclear transcription factors of the lymphoid enhancer factor/T-cell factor (LEF/TCF) family as well as Wnt target genes. Furthermore, canonical Wnt signalling is inhibited by antagonists such as sFRP, WNT Inhibitory Factor 1 (WIF-1), Cerberus and Dickkopf-related protein 1 (DKK) (Gordon and Nusse, 2006; MacDonald and He, 2012). In FZD/WNT/β-catenin signalling, Wnt/FZD interaction with LRP5/6 and the process of phosphorylation results in β-catenin stabilisation and accumulation in the nucleus (Fig. 2A) (Gordon and Nusse, 2006). Then, β-catenin enters the nucleus and interacts with LEF/TCF DNA-binding transcription factors leading to the activation of Wnt target genes including Myc and Twin (van Amerongen and Nusse, 2009; Reya and Clevers, 2005).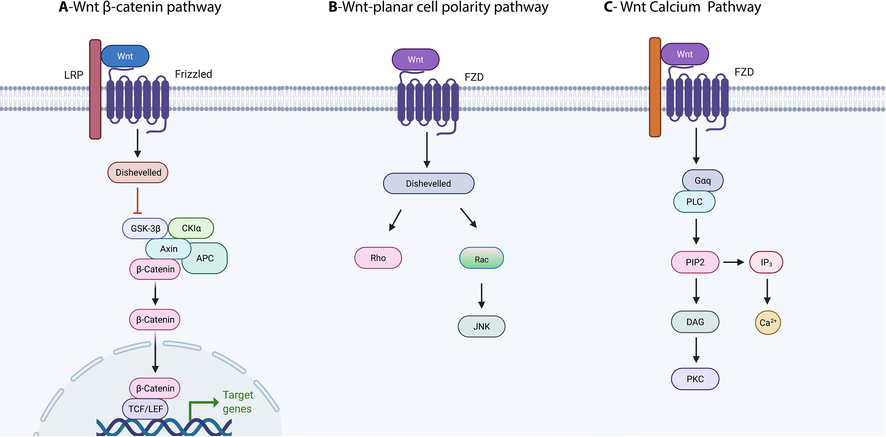
Wnt signalling Pathways. A) FZD/WNT/β-catenin signalling. B) FZD/WNT/PCP signalling. C) FZD/WNT/Ca2 + signalling. This Fig is created with Biorender.com.
In contrary, FZD/WNT/planar cell polarity (PCP) signalling is regulated in a β-catenin-independent manner, Wnt/FZD binding leads to Dvl recruitment (Komiya and Habas, 2008). Dvl recruitment activates Rho, which in turn activates (ROCK), through Daam1. Also, actin polymerization is mediated by Daam1 via the actin binding protein Profilin. Dvl is also involved in the activation of Rac, which activates the c-Jun N-terminal kinase (JNK) pathway (Fig. 2B) (Seifert and Mlodzik, 2007).
In FZD/WNT/Ca2+ signalling, FZDs binding is critical for increasing the level of intracellular calcium in cells, which leads to the stimulation of calcium-responsive enzymes, such as protein kinase C (PKC) (Fig. 2C) (Kohn and Moon, 2005; Slusarski et al., 1997).
2 Expression and function of FZDs during development:
FZD expression has been observed in several embryonic tissues and is required during embryogenesis (Alrefaei et al., 2021; Clevers, 2006; Medina et al., 2000). In Drosophila, the expression of DFz1 has been documented in the epidermis and wings (Adler and Lee, 2001). DFz2 expression has been detected during development in many cell types, such as the mesoderm and epidermis. In the late stages of development, expression of DFz2 was exclusively found in the dorsal vessel, hindgut, and central nervous system (Bhanot et al., 1996).
Genetic screening and RNA interference were used to study the function of FZDs in flies. Genetic manipulation of DFz1 results in defects in cell polarity in the dorsal epidermis, sensory bristles, and wings (Gubb and García-Bellido, 1982; Gray et al., 2011). Furthermore, deletion of DFz1 and DFz2 leads to severe abnormalities in embryo patterning, neuroblast specification, and heart formation (Bhat, 1998; Müller et al., 1999).
Nikaido et al., (2013) conducted a comprehensive analysis of fzd expression and investigated thair roles during development in zebrafish. They characterized 13 fzd members and illustrated that their expression patterns were dynamic and overlapping in zebrafish embryos. For example, fzd3a, fzd9b, and fzd10 expression overlapped in the dorsal neural tube, and fzd7b and fzd10 expression was detected in the border of the neural plate. This report showed that the knockdown of fzd7a and fzd7b affected the movement of convergent extension during gastrulation. Also, inhibition of fzd7a, fzd7b, and fzd10 by morpholino results in pronounced defects in mesodermal convergent extension.
Xenopus Frizzled receptors (XFzs) exhibit dynamic expression at different stages during development (See Tables 1). They are required in several biological events including neurogenesis, axis formation, and patterning. Some XFzs are expressed during neuronal generation; for instance, XFz2/3/4/7/10 expression has been detected in many neural tissues (Deardorff et al., 2001; Moriwaki et al., 2000; Shi and Boucaut, 2000; Wheeler and Hoppler, 1999). XFz2 expression was observed in the optic vesicles, somites, and eyes (Deardorff and Klein, 1999). XFz3 was found in the neural plate, the neural tube, and the eye, where it can regulate the formation of the eye (Rasmussen et al., 2001). XFz3 overexpression leads to ectopic eye formation, whereas the overexpression of a dominant-negative form of XFz3 reduces the expression of eye markers such as Pax6 and Otx2, causing malformation of the eyes in Xenopus embryos. It was also reported that XFz3 inhibition by morpholino affects neural crest induction (Deardorff et al., 2001). In addition, XFz4 expression was found during gastrulation and in the forebrain (Shi and Boucaut, 2000).
Tissues
FZD2
FZD3
FZD4
FZD7
FZD8
FZD10
Spemann organiser
X
Neural tissues
X
X
X
X
X
X
Otic vesicle
X
Eye
X
X
Somite
X
Heart
X
XFz7 expression was detected in several tissues, including the neural crest, neural tube, and heart (Wheeler and Hoppler, 1999). It has been indicated that XFz7 is involved in early patterning, proliferation, and morphogenesis (Medina et al., 2000; Zhang et al., 2013). Consistent with these results, overexpression and knockdown of XFz7 have been shown to affect neural crest development and neural tube patterning (Abu-Elmagd et al., 2006). XFz8 expression is detected in early developing cells including the Spemann organiser (Deardorff et al., 1998). The injection of xFz8 RNA into ventral blastomeres resulted in secondary axis formation, and it interacted with XWnt8 (Deardorff et al., 1998). This study concluded that XFz8 is a receptor for Wnt and is required for the morphogenesis of developing embryos. XFz10 is expressed in the neural ectoderm and in the neural fold at early stages of development (Wheeler and Hoppler, 1999). XFz10 is detected in the central nervous system where it is strongly expressed in the midbrain and hindbrain and in the dorsal neural tube and promotes sensory neural formation (Moriwaki et al., 2000; Garcia-Morales et al., 2009).
In mice, FZD expression is required during development and adulthood, and it exhibits complex and specific expression patterns (Borello et al., 1999; Summerhurst et al., 2008) (see Table 2). FZD expression is detected by in situ hybridisation during early embryogenesis (5.5 days). For instance, FZD5 and FZD8 expression has been observed in the visceral endoderm, and FZD7 expression is present in the epiblast during gastrulation (Lu et al., 2004a; Lu et al., 2004b; Kemp et al., 2007).
Tissues
FZD1
FZD3
FZD4
FZD5
FZD6
FZD7
FZD8
FZD9
FZD10
Visceral endoderm
X
X
The epiblast
X
X
Somite
X
X
X
X
X
X
Eye
X
X
X
X
X
Neural tube
X
X
X
X
X
Hippocampus
X
X
X
Borello et al. (1999) analysed the expression profile for FZD in mice during the segmentation stage using in situ hybridisation. They found that FZD is expressed in several tissues, including neural tube, limb bud, and somite tissues. For example, FZD1/3/6/7/8/9 expression is detected in developing somites and in the neural tube, except for FZD6. FZD3 expression is specific to the dorsal neural tube, whereas FZD7 expression is limited to the intermediate-ventral region of the neural tube. FZD4/5 expression is found in the ventral telencephalon. Moreover, Borello et al. (1999) showed that some FZDs (1/3/5/6) are expressed in the developing eye, indicating a role for these receptors during eye formation.
In addition, FZD3, FZD5, and FZD8 are found in the developing mouse hippocampus (Davis et al., 2008). FZD10 is highly expressed during embryogenesis in central nervous system including neural tube and the brain (Kemp et al., 2007; Yan et al., 2009). The specific and dynamic expression of FZDs in several embryonic tissues indicates the essential function of these receptors during development.
Several developmental defects have been reported in FZD knockout mice. For example, FZD1/2 mutants show obvious defects in the palate closure and ventricular septum (Yu et al., 2010). Moreover, Loss of FZD2 leads to defects in branching morphogenesis were observed in developing lung epithelium (Kadzik et al., 2014). Knockout of FZD3 leads to severe abnormalities in axon development in the central nervous system in mice (Hua et al., 2014; Wang et al., 2006b). The loss of FZD4 mutant resulted in many defects in the cochlea, cerebellum, cerebellar degeneration and retina (Xu et al., 2004; Wang et al., 2001). Knockout of FZD5 led to yolk defects, resulting in embryonic lethality (Ishikawa et al., 2001). Moreover, FZD5 conditional loss-of-function led to several abnormalities in the eye, such as increased cell death in the ventral retina (Liu and Nathans, 2008). It has been reported that FZD6 loss resulted in a polarity defect in which hair was mis-orientated in mice (Guo et al., 2004). FZD3/6 double mutants exhibited many defects in tissue closure, in which the eyelid and neural tube failed to close (Wang et al., 2006b). Furthermore, the polarity and patterning of inner ear sensory hair cells are affected in FZD3/6 double mutants. The loss of FZD9 led to abnormal B-cells, which indicates that FZD9 regulates B-cell development (Ranheim et al., 2005). These phenotypes clearly indicate that FZDs are required for development in mouse embryos. However, the specific WNT ligands that bind to each FZD to regulate embryonic development must be investigated in more details in vivo.
3 Fzds and WNT specificity:
WNT–FZD specificity is complex and poorly understood for several reasons, including the capability of FZDs to activate many pathways. For example, FZD7 can activate both canonical and non-canonical WNT signalling, as shown in chick somites and other systems (Medina et al., 2000; Gros et al., 2009). Moreover, some WNTs can bind to many FZDs, and FZDs can interact with more than one WNT ligand (19 WNTs can bind to 10 FZDs) (Hsieh et al., 1999; Carmon and Loose, 2010). For instance, WNT9a is able to bind to the CRD of FZD4, FZD7, and FZD9, as indicated by assays of immunoprecipitation in COS7 cells (Matsumoto et al., 2008). Furthermore, redundancy has been reported in FZD receptors, which makes it difficult to investigate the independent function for each FZD. In Drosophila, DFz1 and DFz2 function redundantly, and both genes must be mutated to produce a wingless-like phenotype (Chen and Struhl, 1999; Müller et al., 1999). Genetic studies in mice have revealed redundancy in the roles of FZD1 and FZD2, as well as in those of FZD3 and FZD6 (Wang et al., 2006b; Yu et al., 2010).
By contrast, few studies have shown the selectivity of specific WNTs and their receptors. For instance, in Drosophila, DFz2-CRD has a stronger binding affinity for Wg (10-fold) than does Fz1-CRD (Rulifson et al., 2000). Moreover, DFz1 has a primary role in PCP signalling, whereas DFz2 does not have a role in polarity (Rulifson et al., 2000).
In Xenopus, axis duplication assays have provided several examples for WNT–FZD selectivity. For example, XWnt5a only causes axis duplication when it is co-injected with xFz5, but the effect was not observed with other xFzs (He et al., 1997). This could indicate that xFz5 is a receptor for XWnt5a and transduces its activity in axis induction. Genetic manipulation of xFz10 revealed its interaction with Wnt1 but not Wnt3a, as shown by axis duplication assays (Garcia-Morales et al., 2009). Furthermore, xFz8 is able to interact with XWnt8, resulting in the induction of complete axes in Xenopus (Deardorff et al., 1998). Also, XWnt8 protein can bind with xFz8 protein, and xFz8-CRD interacts with XWnt8 with higher affinity compared with other examined xFz (Janda et al., 2012).
In zebrafish, fzd3a interacts with Wnt8b and regulated the formation of forebrain commissures, and their independent knockdown resulted in the same commissural defect phenotypes (Hofmeister and Key, 2013).
Luciferase assays have been applied to elucidate WNT–FZD interactions in several cell lines. FZD9 (RFz9) strongly activates TCF transcription (Wnt/β-catenin reporter) when it is co-expressed with WNT2, but not when it is co-expressed with other WNTs, as shown in 293 T cells (Karasawa et al., 2002). This shows that FZD9 is a receptor for WNT2 and transduces its function through canonical WNT signalling. Another study using the same cell line found that WNT2 was able to increase TCF activity more than 25-fold when it was co-transfected with FZD8 and 15-fold with FZD9, but not with the other eight FZDs (Bravo et al., 2013).
Dijksterhuis et al., (2015) characterized a novel cell line called the mouse myeloid progenitor (32D). This cell line does not express FZD receptors, but it expresses LRP5 and LRP6. Thus, the authors generated an overexpressing cell line for each FZD (FZD2, FZD4, and FZD5) and studied their roles and selectivity with multiple WNTs (WNT3a, WNT4, WNT5a, and WNT9b). Surprisingly, only WMT3a increased β-catenin stability and induced LRP6 phosphorylation (PS) in a dose-dependent manner in 32D/FZD2, 4 and 5 cells. However, high concentrations of WNT5a induced β-catenin stability in FZD5-expressing 32D cells, but not in FZD2- or FZD4-expressing cells. This indicates that WNT5a preferentially binds to FZD5. The authors also revealed that phosphorylation (PS) of Dishevelled 2 (DVL2) and DVL3 was affected by WNT–FZD selectivity. For example, stimulation of WNT3a and WNT5b led to the formation of PS-DVL3 but not PS-DVL2 in FZD5-expressing 32D cells. This evidence clearly indicates that WNT–FZD pair binding has various biological activities in this cell line. Thus, this cell line and other approaches could help elucidate the receptor–ligand interactions and selectivity and contribute to the investigation of the downstream effects of each WNT–FZD pair. In addition, studies of WNT–FZD signalling are important to address WNT/FZD-related diseases, such as cancer, neural tube defects, and neurodegenerative disorders.
4 WNT/FZD signalling and diseases
4.1 Fzds and cancer
Wnt/FZD dysfunction leads to several kinds of diseases, including cardiac hypertrophy, neural degeneration, neural tube defects, and cancers (Luo et al., 2007; Malaterre et al., 2007; Ueno et al., 2013; Wang et al., 2006b; Zeng et al., 2018). Wnt/FZD signalling is involved in the regulation of cell proliferation, and WNT members are highly expressed in human cancers, such as breast cancer, lung cancer, and colon cancer (van Amerongen and Nusse, 2009; Bravo et al., 2013; Gurney et al., 2012). Furthermore, high FZD expression has been reported in several human cancers (Ueno et al., 2008). For instance, FZD1 is highly expressed in colon cancer (Holcombe et al., 2002), and high expression of FZD3 has been detected in lymphoma cells in chronic lymphocytic leukaemia cells (Lu et al., 2004a; Lu et al., 2004b; Qiang et al., 2003). FZD5 is highly expressed in prostate and kidney cancer (Janssens et al., 2004; Thiele et al., 2011). High expression of FZD7 is found in more than six colorectal cancer lines (Ueno et al., 2008). FZD8 is upregulated in many types of lung cancer (Bravo et al., 2013). Furthermore, FZD10 shows strong expression in colon cancer, sarcoma, and lung cancer (Terasaki et al., 2002; Nagayama et al., 2005).
Many knockdown approaches have been used to target FZDs in different cell lines. FZD7 siRNA inhibits invasion activity and cell viability in colorectal cancer lines (Ueno et al., 2008). The targeting of FZD8 by shRNA resulted in significant inhibition of the proliferation of lung cancer cell lines (Wang et al., 2012). In addition, FZD8 shRNA reduced tumour growth in vivo, as shown in xenograft mouse models. Notably, both studies reported that the knockdown of either FZD7 or FZD8 affected canonical WNT signalling. Furthermore, FZD10 siRNA suppressed tumour growth in synovial sarcomas cell lines (Nagayama et al., 2005). Because FZDs are involved in several human cancers, targeting these receptors could be a valuable tool for combatting cancer. For example, the 3D structure of h FZD CRD proteins can be used in prediction of specific targets and in drug discovery.
In addition, antibodies have been used against FZDs in cancerous cells. MAb 92-13 antibody selectivity binds to FZD10-overexpressing tumours, which facilitates the targeting of these tumours by radioimmunotherapy (Fukukawa et al., 2008). OMP-18R5 antibody (a human IgG2 isotype) has been characterized and is able to bind to five FZDs (FZD1, 2, 5, 7, and 8) through a conserved epitope (Gurney et al., 2012). OMP-18R5 reduces canonical WNT signalling by preventing WNT ligands from binding to FZD receptors. This antibody inhibits human tumours, including breast, pancreatic, and colon cancer. Moreover, OMP-18R5 exhibits strong synergy with several chemotherapy drugs, including Taxol.
Furthermore, microRNAs have been used to regulate FZD expression and their biological activities (Ueno et al., 2013). For example, FZD1 expression is reduced by miR-204 overexpression (Li et al., 2011), and miR-23b regulates FZD7 in colon cancer cells (Zhang et al., 2011). Therefore, other microRNAs should be identified as they could improve cancer treatment and reduce tumour growth that results from overexpression of FZDs and/or other WNT components.
4.2 WNT/FZD signalling and developmental defects
Wnt/FZD signalling has been implicated in various developmental defects in human including nonsyndromic cleft lip, familial exudative vitreoretinopathy and neural tube defects (NTDs) (MacDonald et al., 2009). For example, rare variants of FZD6 (c.1843_1844insA, p.Arg405Gln) has been reported to contribute to NTDs in human as shown by computational analysis (De Marco et al., 2011). NTDs result from the failure of neural tube closure during primary neurulation in the brain or spinal cord (Greene and Copp, 2009). Furthermore, FZD6 (a rare variant in intron 1) is involved in nonsyndromic cleft lip and palate as reported in an African-American family (Cvjetkovic et al., 2015). Mutations of FZD4 are linked with familial exudative vitreoretinopathy (Robitaille et al., 2002; Toomes et al., 2004). Therefore, genetic modified mice are used as a model in defining the role of Wnt/FZD in developmental defects in mammal. FZD knockout mice have uncovered that FZD mutations lead to several embryonic defects and some of them mimic the defects that reported in human embryos. For example, FZD4 mutations affect the development of retinal capillaries in mice and human (Xu et al., 2004; Wang et al., 2001). In addition, neural tube fails to close in Fz3−/−;Fz6−/− mouse embryos and causes NTD phenotypes (Wang et al., 2006b; Yu et al., 2010).
Thus, components of the WNT signalling pathway, including FZDs, play important roles in the process of development. Understanding FZDs fundamental biological functions in developmental defects could lead to develop better diagnoses of embryonic malformations or enhance preventive measures during pregnancy.
4.3 WNT/FZD signalling and neurodegenerative disorders
The disruption of WNT/FZD signalling is involved in neurodegenerative disorders such as Alzheimer’s disease and Huntington’s disease (Galli et al., 2014; Salinas, 2012; Serafino et al., 2020). In Alzheimer’s disease (AD), it was reported that β-catenin levels are inhibited in the brain, whereas WNT/β-catenin inhibitors (GSK-3β) are active (Pei et al., 1999). The stimulation of WNT/β-catenin signalling can prevent β-amyloid peptide (Aβ)-induced neurotoxicity in cultured rat hippocampal neurons (De Ferrari et al., 2003; Alvarez et al., 2004). It has been shown that WNT3a is able to activate WNT/β-catenin signalling through FZD1 (not FZD2) and has a protective effect against Aβ toxicity in PC12 cells and hippocampal neurons (Chacón et al., 2008). Overexpression of FZD1 along with WNT3a results in a significant increase in cell survival and prevents β-catenin degradation and caspase-3 activation in the same cell line. This protective effect could be a result of WNT/β-catenin signalling activation, which in turn suppresses WNT inhibitors, such as DKK1 and GSK-3β. Interestingly, these inhibitors are shown to be overexpressed in AD brains (Galli et al., 2014; Inestrosa and Varela-Nallar, 2014). Therefore, WNT/FZD signalling could potentially be used as a therapeutic target for neurodegenerative diseases.
5 Conclusions
This review focused on FZD expression in different models; the roles of FZD during development; WNT/FZD signalling specificity; and FZD involvement in cancer, neural tube defects, and neuronal diseases. FZDs are classified as receptors for WNTs and participate in the regulation of several biological processes, including embryogenesis, cell specification, and growth of neural synapses. In addition, FZD overexpression has been detected in several types of cancers, which results in increased cell proliferation and metastasis. Therefore, understanding FZD function, binding selectivity, and regulation will help to exploit them as potential therapeutic targets for cancer treatment and other diseases. Many studies mentioned in this review demonstrated that different approaches can be used to target FZDs in cancer cells, such as microRNAs, antibodies, and siRNAs. Although these approaches are promising, further investigation is required to improve their selectivity and ensure their safety for human use.
Furthermore, FZD signalling has important functions during neural tube development, as demonstrated by NTD phenotypes in FZD1/2 and FZD3/6 mutant mice. Therefore, FZD expression and function during embryogenesis is crucial, and FZDs could be used as biomarkers for neural tube defects during pregnancy.
Finally, WNT/FZD signalling shows a proactive function against Aβ toxicity. This suggests that canonical WNT/FZD signalling has the potential to be used as a therapeutic target for neuronal degeneration.
Acknowledgments
I would like to thank Professor Andrea Münsterberg and Professor Grant Wheeler (Professors of Developmental Biology at University of East Anglia) for their valuable comments and suggestions in this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Frizzled7 mediates canonical Wnt signaling in neural crest induction. Dev. Biol.. 2006;298:285-298.
- [CrossRef] [Google Scholar]
- Frizzled signaling and cell-cell interactions in planar polarity. Curr. Opin. Cell Biol.. 2001;13:635-640.
- [CrossRef] [Google Scholar]
- Expression analysis of chick Frizzled receptors during spinal cord development. Gene Expr. Patterns GEP. 2021;39:119167
- [CrossRef] [Google Scholar]
- Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp. Cell Res.. 2004;297:186-196.
- [CrossRef] [Google Scholar]
- Frizzled proteins constitute a novel family of G protein-coupled receptors, most closely related to the secretin family. Trends Pharmacol. Sci.. 1998;19:399-400.
- [CrossRef] [Google Scholar]
- A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225-230.
- [CrossRef] [Google Scholar]
- Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Dev. Camb. Engl.. 1999;126:4175-4186.
- [Google Scholar]
- frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell. 1998;95:1027-1036.
- [CrossRef] [Google Scholar]
- Structure-function analysis of secreted frizzled-related protein-1 for its Wnt antagonist function. J. Cell. Biochem.. 2007;102:1519-1528.
- [CrossRef] [Google Scholar]
- Differential expression of the Wnt putative receptors Frizzled during mouse somitogenesis. Mech. Dev.. 1999;89:173-177.
- [CrossRef] [Google Scholar]
- Frizzled-8 receptor is activated by the Wnt-2 ligand in non-small cell lung cancer. BMC Cancer. 2013;13:316.
- [CrossRef] [Google Scholar]
- Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767-777.
- [CrossRef] [Google Scholar]
- Development of a bioassay for detection of Wnt-binding affinities for individual frizzled receptors. Anal. Biochem.. 2010;401:288-294.
- [CrossRef] [Google Scholar]
- Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Abeta oligomers. J. Cell. Physiol.. 2008;217:215-227.
- [CrossRef] [Google Scholar]
- Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Dev. Camb. Engl.. 1999;126:5441-5452.
- [Google Scholar]
- Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480.
- [CrossRef] [Google Scholar]
- Regulatory variant in FZD6 gene contributes to nonsyndromic cleft lip and palate in an African-American family. Mol. Genet. Genomic Med.. 2015;3:440-451.
- [CrossRef] [Google Scholar]
- Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86-90.
- [CrossRef] [Google Scholar]
- Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Develop.. 2008;3:32.
- [CrossRef] [Google Scholar]
- Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol. Psychiatry. 2003;8:195-208.
- [CrossRef] [Google Scholar]
- FZD6 is a novel gene for human neural tube defects. Hum. Mutat.. 2011;33:384-390.
- [CrossRef] [Google Scholar]
- Xenopus frizzled-2 is expressed highly in the developing eye, otic vesicle and somites. Mech. Dev.. 1999;87:229-233.
- [CrossRef] [Google Scholar]
- Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Dev. Camb. Engl.. 1998;125:2687-2700.
- [Google Scholar]
- A role for frizzled 3 in neural crest development. Dev. Camb. Engl.. 2001;128:3655-3663.
- [Google Scholar]
- Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J. Biol. Chem.. 2015;290:6789-6798.
- [CrossRef] [Google Scholar]
- Radioimmunotherapy of human synovial sarcoma using a monoclonal antibody against FZD10. Cancer Sci.. 2008;99:432-440.
- [CrossRef] [Google Scholar]
- Deficient Wnt signalling triggers striatal synaptic degeneration and impaired motor behaviour in adult mice. Nat. Commun.. 2014;5:4992.
- [CrossRef] [Google Scholar]
- Frizzled-10 promotes sensory neuron development in Xenopus embryos. Dev. Biol.. 2009;335:143-155.
- [CrossRef] [Google Scholar]
- Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem.. 2006;281:22429-22433.
- [CrossRef] [Google Scholar]
- Planar Cell Polarity: Coordinating Morphogenetic Cell Behaviors with Embryonic Polarity. Dev. Cell. 2011;21:120-133.
- [CrossRef] [Google Scholar]
- Development of the vertebrate central nervous system: formation of the neural tube. Prenat. Diagn.. 2009;29:303-311.
- [CrossRef] [Google Scholar]
- WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature. 2009;457:589-593.
- [CrossRef] [Google Scholar]
- A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol.. 1982;68:37-57.
- [Google Scholar]
- Frizzled6 controls hair patterning in mice. Proc. Natl. Acad. Sci. U. S. A.. 2004;101:9277-9281.
- [CrossRef] [Google Scholar]
- Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. U. S. A.. 2012;109:11717-11722.
- [CrossRef] [Google Scholar]
- A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652-1654.
- [CrossRef] [Google Scholar]
- Frizzled-3a and Wnt-8b genetically interact during forebrain commissural formation in embryonic zebrafish. Brain Res.. 2013;1506:25-34.
- [CrossRef] [Google Scholar]
- Expression of Wnt ligands and Frizzled receptors in colonic mucosa and in colon carcinoma. Mol. Pathol. MP. 2002;55:220-226.
- [CrossRef] [Google Scholar]
- Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl. Acad. Sci. U. S. A.. 1999;96:3546-3551.
- [CrossRef] [Google Scholar]
- Frizzled3 is required for the development of multiple axon tracts in the mouse central nervous system. Proc. Natl. Acad. Sci. U. S. A.. 2014;111:E3005-3014.
- [CrossRef] [Google Scholar]
- The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol.. 2004;5:234.
- [CrossRef] [Google Scholar]
- Wnt signaling in the nervous system and in Alzheimer’s disease. J. Mol. Cell Biol.. 2014;6:64-74.
- [CrossRef] [Google Scholar]
- Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Dev. Camb. Engl.. 2001;128:25-33.
- [Google Scholar]
- Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59-64.
- [CrossRef] [Google Scholar]
- Alteration of frizzled expression in renal cell carcinoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med.. 2004;25:161-171.
- [CrossRef] [Google Scholar]
- Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc. Natl. Acad. Sci. U. S. A.. 2014;111:12444-12449.
- [CrossRef] [Google Scholar]
- Frizzled-9 is activated by Wnt-2 and functions in Wnt/beta -catenin signaling. J. Biol. Chem.. 2002;277:37479-37486.
- [CrossRef] [Google Scholar]
- Expression of Frizzled5, Frizzled7, and Frizzled10 during early mouse development and interactions with canonical Wnt signaling. Dev. Dyn. Off. Publ. Am. Assoc. Anat.. 2007;236:2011-2019.
- [CrossRef] [Google Scholar]
- Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439-446.
- [CrossRef] [Google Scholar]
- Wnt signal transduction pathways. Organogenesis. 2008;4:68-75.
- [CrossRef]
- Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci.. 2011;52:2999-3007.
- [CrossRef] [Google Scholar]
- LRP5/6 in Wnt signaling and tumorigenesis. Future Oncol. Lond. Engl.. 2005;1:673-681.
- [CrossRef] [Google Scholar]
- An essential role for frizzled 5 in mammalian ocular development. Dev. Camb. Engl.. 2008;135:3567-3576.
- [CrossRef] [Google Scholar]
- A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol. Cell. Biol.. 2003;23:5825-5835.
- [CrossRef] [Google Scholar]
- The mouse frizzled 8 receptor is expressed in anterior organizer tissues. Gene Expr. Patterns GEP. 2004;4:569-572.
- [CrossRef] [Google Scholar]
- Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. U. S. A.. 2004;101:3118-3123.
- [CrossRef] [Google Scholar]
- Wnt signaling and human diseases: what are the therapeutic implications? Lab. Investig. J. Tech. Methods Pathol.. 2007;87:97-103.
- [CrossRef] [Google Scholar]
- Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb. Perspect. Biol.. 2012;4
- [CrossRef] [Google Scholar]
- Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell. 2009;17:9-26.
- [CrossRef] [Google Scholar]
- Wnt-Frizzled signalling and the many paths to neural development and adult brain homeostasis. Front. Biosci. J. Virtual Libr.. 2007;12:492-506.
- [CrossRef] [Google Scholar]
- Wnt9a secreted from the walls of hepatic sinusoids is essential for morphogenesis, proliferation, and glycogen accumulation of chick hepatic epithelium. Dev. Biol.. 2008;319:234-247.
- [CrossRef] [Google Scholar]
- Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech. Dev.. 2000;92:227-237.
- [CrossRef] [Google Scholar]
- Isolation of Xenopus frizzled-10A and frizzled-10B genomic clones and their expression in adult tissues and embryos. Biochem. Biophys. Res. Commun.. 2000;278:377-384.
- [CrossRef] [Google Scholar]
- Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Dev. Camb. Engl.. 1999;126:577-586.
- [Google Scholar]
- Therapeutic potential of antibodies against FZD 10, a cell-surface protein, for synovial sarcomas. Oncogene. 2005;24:6201-6212.
- [CrossRef] [Google Scholar]
- Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem.. 2006;281:13247-13257.
- [CrossRef] [Google Scholar]
- A systematic survey of expression and function of zebrafish frizzled genes. PloS One. 2013;8:e54833
- [CrossRef] [Google Scholar]
- Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol.. 1999;58:1010-1019.
- [CrossRef] [Google Scholar]
- Frizzled 9 knock-out mice have abnormal B-cell development. Blood. 2005;105:2487-2494.
- [CrossRef] [Google Scholar]
- Regulation of eye development by frizzled signaling in Xenopus. Proc. Natl. Acad. Sci. U. S. A.. 2001;98:3861-3866.
- [CrossRef] [Google Scholar]
- Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat. Genet.. 2002;32:326-330.
- [CrossRef] [Google Scholar]
- SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat. Neurosci.. 2005;8:1301-1309.
- [CrossRef] [Google Scholar]
- Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol. Cell. 2000;6:117-126.
- [Google Scholar]
- Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb. Perspect. Biol.. 2012;4:a008003
- [CrossRef] [Google Scholar]
- Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet.. 2007;8:126-138.
- [CrossRef] [Google Scholar]
- Targeting the Wnt/β-catenin pathway in neurodegenerative diseases: recent approaches and current challenges. Expert Opin. Drug Discov.. 2020;15:803-822.
- [CrossRef] [Google Scholar]
- Xenopus frizzled 4 is a maternal mRNA and its zygotic expression is localized to the neuroectoderm and trunk lateral plate mesoderm. Mech. Dev.. 2000;94:243-245.
- [CrossRef] [Google Scholar]
- Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410-413.
- [CrossRef] [Google Scholar]
- 3D representation of Wnt and Frizzled gene expression patterns in the mouse embryo at embryonic day 11.5 (Ts19) Gene Expr. Patterns GEP. 2008;8:331-348.
- [CrossRef] [Google Scholar]
- Frizzled-10, up-regulated in primary colorectal cancer, is a positive regulator of the WNT - beta-catenin - TCF signaling pathway. Int. J. Mol. Med.. 2002;9:107-112.
- [Google Scholar]
- Expression profile of WNT molecules in prostate cancer and its regulation by aminobisphosphonates. J. Cell. Biochem.. 2011;112:1593-1600.
- [CrossRef] [Google Scholar]
- Mutations in LRP5 or FZD4 Underlie the Common Familial Exudative Vitreoretinopathy Locus on Chromosome 11q. Am. J. Hum. Genet.. 2004;74:721-730.
- [Google Scholar]
- Frizzled homolog proteins, microRNAs and Wnt signaling in cancer. Int. J. Cancer. 2013;132:1731-1740.
- [CrossRef] [Google Scholar]
- Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia N. Y. N. 2008;10:697-705.
- [CrossRef] [Google Scholar]
- The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J.. 2000;19:4944-4954.
- [CrossRef] [Google Scholar]
- Towards an integrated view of Wnt signaling in development. Dev. Camb. Engl.. 2009;136:3205-3214.
- [CrossRef] [Google Scholar]
- Structure-function analysis of Frizzleds. Cell. Signal.. 2006;18:934-941.
- [CrossRef] [Google Scholar]
- Frizzled-8 as a putative therapeutic target in human lung cancer. Biochem. Biophys. Res. Commun.. 2012;417:62-66.
- [CrossRef] [Google Scholar]
- The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. Off. J. Soc. Neurosci.. 2006;26:2147-2156.
- [CrossRef] [Google Scholar]
- Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J. Neurosci. Off. J. Soc. Neurosci.. 2001;21:4761-4771.
- [Google Scholar]
- A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem.. 1996;271:4468-4476.
- [CrossRef] [Google Scholar]
- Two novel Xenopus frizzled genes expressed in developing heart and brain. Mech. Dev.. 1999;86:203-207.
- [CrossRef] [Google Scholar]
- Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J. Biol. Chem.. 2002;277:41762-41769.
- [CrossRef] [Google Scholar]
- Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883-895.
- [CrossRef] [Google Scholar]
- Expression of Frizzled10 in mouse central nervous system. Gene Expr. Patterns GEP. 2009;9:173-177.
- [CrossRef] [Google Scholar]
- A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr. Biol. CB. 1996;6:1302-1306.
- [CrossRef] [Google Scholar]
- Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Dev. Camb. Engl.. 2010;137:3707-3717.
- [CrossRef] [Google Scholar]
- Frizzled Receptors as Potential Therapeutic Targets in Human Cancers. Int. J. Mol. Sci.. 2018;19:E1543.
- [CrossRef] [Google Scholar]
- Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat. Commun.. 2011;2:554.
- [CrossRef] [Google Scholar]
- Interactions between Wingless and DFz2 during Drosophila wing development. Dev. Camb. Engl.. 1998;125:3075-3085.
- [Google Scholar]
- Different thresholds of Wnt-Frizzled 7 signaling coordinate proliferation, morphogenesis and fate of endoderm progenitor cells. Dev. Biol.. 2013;378:1-12.
- [CrossRef] [Google Scholar]







