Formulation of biofertilizers from oil palm empty fruit bunches and plant growth-promoting microbes: A comprehensive and novel approach towards plant health
⁎Corresponding author. chongkp@ums.edu.my (Khim Phin Chong)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
-
The potential of utilizing empty fruit bunches (EFB) as biofertilizer for sustainable agriculture were discussed.
-
The advantages, mechanisms, and effects of liming material were described.
-
Microbial approaches for releasing nutrient components available in EFB biomass for plant growth and development were described.
-
Previous literatures on EFB biocomposting were analysed.
-
Characterization of plant growth-promoting microbes (PGPM).
Abstract
Our heavy reliance on chemical fertilizers for agricultural practices has fostered the development of a vast industry that is producing chemicals that are toxic not only for humans but also for the environment. Biofertilizers are microbial formulations containing native plant growth-promoting microorganisms (PGPM) which have the potential to enhance plant growth either directly or indirectly by producing different types of phytohormones, iron-binding metabolites (siderophores), and solubilizing soil nutrients and minerals. The positive impacts on crop growth and development were documented by many researchers while using biofertilizers. Thus, biofertilizers offer enormous promise for sustainable agriculture, particularly in the face of climate change. Despite the growing interest in this technology, its entire potential remains untapped. This review collectively describes the potential use of empty fruit bunches (EFB) biomass as a biofertilizer for sustainable agricultural practices and the roles of plant growth-promoting microbes (PGPM) in plant growth and development. Attempts were also made to give insights into the oil palm industry in Malaysia and the nutrient profile of EFB biomass. We concluded that more research, fund and development activities are needed to improve traits of beneficial microbes that will potentially enhance the biological pathway of different biocompounds production and find solutions for the current issues related to converting EFB biomass into biofertilizers.
Keywords
Biofertilizer
Malaysian oil palm industry
Empty fruit bunches
Sustainability
Plant nutrients
Plant growth-promoting microbes
1 Introduction
The Malaysian oil palm industry is one of the most significant lignocellulosic waste producers for the past few decades. More than 90% of its total agricultural biomass waste is generated from 5.87 million hectares of oil palm plantations (MPOB, 2020; Loh, 2017). Among the oil palm biomass produced by the Malaysian oil palm industry, empty fruit bunches (EFB) comprise 20–22 percent of the solid by-products generated during palm oil production, making it the most notable waste produced by this colossal industry (Tahir et al., 2019; Han and Kim, 2018; Loh, 2017). Such oil palm waste production has nevertheless presented a massive problem of waste management and various environmental issues. Composting the biodegradable matter that can be recycled back to the environment, like biofertilizers, is an efficient way of utilizing EFB biomass (Kour et al., 2020). This alternative method can perhaps turn this large quantity of biomass into a profitable and sustainable resource such as biofertilizer that can be used on oil palm plantation areas or as a market product (Truckell et al., 2019). Biofertilizers are microbial formulations containing native plant growth-promoting microorganisms (PGPM) which have the potential to enhance plant growth either directly or indirectly by producing different types of phytohormones, iron-binding metabolites (siderophores), and solubilizing soil nutrients and minerals (Soumare et al., 2020; Kour et al., 2020). Microbial biofertilizer is made up of several different types of bacteria, fungus, and blue-green algae, all which have a symbiotic interaction with plants. According to research, the application of biofertilizers can enhance the yield of diverse crops by around 25% while reducing the usage of inorganic nitrogen and phosphorus fertilizers by approximately 25–50% and 25%, respectively (Aloo et al., 2020). Thus, biofertilizers offer enormous promise for sustainable agriculture, particularly in the face of climate change. Despite the growing interest in this technology, its entire potential remains untapped. Converting EFB biomass will be beneficial for the environment and economically viable for the oil palm industry.
On the contrary, according to the Food and Agriculture Organization (FAO), the world population will reach 9.8 billion by 2050, up from 7.6 billion now. (Soumare et al., 2020; Kumar et al., 2019). This rapid increase in the world population will increase the food demand somewhere between 59% and 98% by 2050. Another estimation from the FAO indicates rice, wheat, corn, and soybean production all need to increase by 60% by 2050 to meet the demand (FAO, 2018; FAO, 2017). This increasing food demand will shape agricultural practices in a way that our civilization has never witnessed before in its history (Gouel and Guimbard, 2019). Conventional farming practices play a significant role in fulfilling a rising population's nutritional needs. This has resulted in a growing dependence on chemical fertilizers and pesticides for higher productivity (Singh et al., 2019; Bhardwaj et al., 2014). Chemical fertilizers are inorganic substances of entirely or partially synthetic origin produced, consisting of a known amount of nitrogen, phosphorus, and potassium (Kumar et al., 2019; Vandana et al., 2017). In most established agriculture sectors, chemical fertilizers are usually applied regularly to supply nutrients to the planted crops. The application of these fertilizers at the recommended rates usually gives quick responses to the crops' physiological growth and yield. However, the use of chemical fertilizers has various adverse effects on the environment. Besides the acceleration of soil acidification, chemical fertilizers also contribute to the risk of contaminating groundwater and the surrounding environment. It also weakens plant roots, thereby putting them at risk of harmful diseases (Singh et al., 2019; Mahanty et al., 2017; Chun-Li et al., 2014). As the global climate is changing and the loss of natural resources, the future remains unclear as to if conventional agriculture will be able to sustain this increasing food demand in the coming years (Singh et al., 2019). We could produce more food by destroying even more forests to create new farmland, use more chemical fertilizers, or figure out how to do it on the land we have right now, using a more efficient and environment-friendly way (FAO, 2018). The approach to sustainable farming has been strongly sought as a result of these difficulties. The use of biofertilizers can help to achieve sustainable farming because they protect the soil ecosystem through a variety of mechanisms such as nitrogen fixation, potassium, and phosphate solubilization, and decomposition of organic materials in the soil (Arif et al., 2020). A further advantage of biofertilizers over chemical fertilisers is that they are more affordable for marginal and small-scale farms. In this regard, attempts have been made to develop a high-quality biofertilizer using EFB of oil palm, rich in nutrients, to assure biosafety.
In Malaysia, biocomposting of EFB biomass would be the best practical solution since the end-product could be directly applied in oil palm plantations. This will lower greenhouse gas emissions and reduce the demand for chemical fertilizers, thus saving valuable resources (Abdullah et al., 2017). In addition, it will reduce the possibility of pests, weed seeds, or parasites spreading into the plantation area, while direct application of EFB increases these risks. Much attention has been drawn to the issue of sustainability, and technical advancements in solid waste management have been introduced in order to minimize the creation of needless material and waste disposal costs (Begum et al., 2019). Agricultural biomass consists of rich nutrients that, if handled correctly, may be put to good use in a variety of applications. As a source of high-quality organic matter, they may be processed to eliminate pathogens before being utilised to feed the soil (Dimkpa, 2016). In order to create useful organic matter that may be utilized as biofertilizers or additions to improve the soil structure, waste materials are being recycled and reprocessed (Kour et al., 2021). Modern agricultural industries have become more conscious of the need to embrace a much more environmentally friendly approach in providing plant nutrition and the conservation of soil fertility (Ai May et al., 2020; Ariana and Candra, 2017). In recent years, various studies were carried out to evaluate the most appropriate method for managing different types of solid waste in the oil palm industry, including the EFP, and turning this abundant biomass into biofertilizer is the most appropriate solution (Alizadeh et al., 2014). Microbial communities especially plant growth-promoting microbes (PGPM), produce various biomolecules, which enhance the breaking down of the lignocellulosic components in EFB biomass. These microbes also help convert all the macronutrients and micronutrients available in the EFB biomass from an inaccessible form to an accessible form for the plants (Mącik et al., 2020; Kuan et al., 2016). PGPM play an important role in regulating numerous biological processes, including the breakdown of organic matter, the availability of different plant nutrients such as iron and magnesium as well as phosphorus, potassium, and nitrogen (Mącik et al., 2020). Microbial inoculants have long been regarded as an essential element of unified nutrient management, which leads to sustainability.
Since the primary aim of biofertilizer is to improving overall soil quality and enhancing plant growth whether via direct or indirect mechanisms, there are several reviews on the influence of biofertilizers on soil and plant health. However, no comprehensive review has been reported which describe the potential of utilizing EFB biomass as a biofertilizer. It is not enough to emphasize just soil impact and plant health alone, and a thorough assessment must also be given on the utilization of agricultural biomass as biofertilizer for a futuristic sustainable agriculture. The review first collectively describes what is biofertilizer and way it is important for sustainable agriculture. It then critically evaluates the Malaysian Oil Palm Industry, its overall waste production during oil palm cultivation and the nutrient profile of EFB biomass. It also assesses the possibility of utilizing EFB biomass as biofertilizer. Then it characterizes the roles of PGPM in plant growth and development. Finally, it summarises significant findings and suggests areas of ambiguity regarding successful application of biofertilizer and proposes important areas for future research on improving PGPM traits of beneficial microbes that will potentially enhance the biological pathway of different biocompounds production.
2 Biofertilizer: Why is it necessary for sustainable agriculture?
Biofertilizers can easily be referred to as living fertilizers because, unlike chemical fertilizers, biofertilizers have a combination of beneficial microbes such as bacteria and fungi (Thomas and Singh, 2019; Alori et al., 2017). In a broad sense, the term biofertilizer can also include every organic plant growth resource made accessible for plant development via microbial community associations or interactions (Mącik et al., 2020; Mahanty et al., 2017; Bhardwaj et al., 2014). Microbes are tiny but exceptionally useful (Soumare et al., 2020; Nath et al., 2018). Microorganisms can provide all the 16 essential elements plants need for healthy growth and development. Microbes present in biofertilizer can provide all these elements, especially nitrogen, phosphorus, and potassium. These elements are abundantly present in the soil or environment, but it is an inaccessible form. Microbial activities convert these unusable elements into a usable form for plants (Mącik et al., 2020; Kuan et al., 2016). Biofertilizer's introduction will enhance the biodiversity that makes up all sorts of beneficial bacteria and fungi (Prasad et al., 2017; Ritika and Utpal, 2014). These microbial communities benefit the plant's development (Kour et al., 2020; Yadav and Sarkar, 2019). Biofertilizers improve soil fertility and crop production in sustainable agriculture as an alternative to conventional fertilizers (Zaidi et al., 2017). Adding beneficial PGPM to agricultural activities began approximately 60 years ago. It is now evident that such beneficial microbes may improve plant tolerance to unfavorable environmental changes such as water and nutrient shortage and pollution of heavy metals (Mendoza-arroyo et al., 2020; Ritika and Utpal, 2014). Such potential biological fertilizers will play a very significant role in soil biodiversity and productivity. Producing chemical fertilizers is very costly and challenging to fulfil the current demand. This created a need for the development of biofertilizers as a potential replacement for chemical fertilizers. Biofertilizers will also protect the ecosystem in the farmers' community's interests as an environmentally sustainable and better economic output (Qu et al., 2019).
In the coming decades, PGPMs are expected to become widely utilized bioinoculants around the world and it will play a significant role in boosting the nutrient and yield status of agroecosystems by decreasing the reliance on pesticides and synthetic fertilizers in conventional agriculture (Mącik et al., 2020; Singh et al., 2019). PGPM stimulates the development of plants via a variety of methods, both direct and indirect. It is important to distinguish between direct and indirect mechanisms (Fig. 1). Direct mechanisms include processes such as P solubilization and N fixation, as well as the production of phytohormones, siderophores, and vitamins. Indirect mechanisms include those mechanisms that do not directly promoting growth but do so through the path of synthesis. For example, ACC deaminase activity, antibiotic synthesis, cell wall disintegrating enzymes, and increased systemic resistance are all examples of indirect methods (Khanna et al., 2021; Mącik et al., 2020). More detail explanation of mechanism of PGPMs both direct and indirect is given in section below.
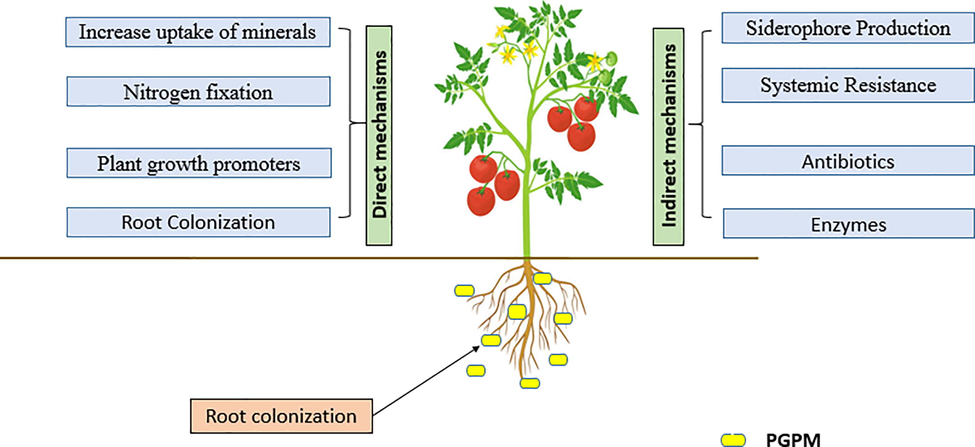
- A schematic descriptive mechanism of various traits shown by Plant Growth-Promoting Microbes present in biofertilizers that acts as bioinoculants and promote plant growth.
2.1 Types of biofertilizers
Biofertilizers used in organic farming can be classified depending on the type of active microorganisms it contains. The different types of biofertilizers include but are not limited to be:
-
Nitrogen fixing biofertilizers: This class of biofertilizers symbiotically fix atmospheric nitrogen and help balancing N-levels in the soil. Nitrogen deficiency is a limiting factor for plant development, as plants need a specific quantity of nitrogen in the soil in order to flourish (Kuan et al., 2016; Defez et al., 2017). In order to get the best results for each soil type, the kind of nitrogen biofertilizer to be used must be determined by the crop being grown on the land. Legume crops benefit from the use of Rhizobia, whereas non-legume crops benefit from the use of Azotobacter or Azospirillum, sugarcane benefits from the use of Acetobacter and blue green algae benefits from the use of Azolla in lowland rice paddies (Singh et al., 2019; Vandana et al., 2017).
-
Phosphorus solubilizing biofertilizers: The element phosphorus, like nitrogen, has the same effect on plant development. Incorporating P-biofertilizers into the soil may help it achieve its optimal P-level and can also assist to rectify low P-levels in the soil. In contrast to nitrogen biofertilizers, the use of P-biofertilizers is not reliant on the crops that are grown on the land (Mendoza-Arroyo et al., 2020; Kalayu, 2019; Vandana et al., 2017).
-
Compost Biofertilizers: Cellulolytic fungus cultures and Phosphotika and Azotobacter cultures are appropriate biofertilizers for compost usage. Vermicompost is another example of compost biofertilizer which is a 100% pure organic fertiliser with organic nitrogen, phosphorus, potassium, organic carbon, sulphur, hormones, vitamins, enzymes and antibiotics that assist to increase crop quality and quantity.
3 The oil palm industry in Malaysia
The Oil palm (Elaeis guineensis) is a primary plantation crop in Malaysia. Malaysia currently ranks second at global palm oil production after Indonesia (Begum et al., 2019; Tahir et al., 2019; European Palm Oil Alliance, 2016). Malaysia has ideal climatic conditions for oil palm crop cultivation which fostered a rapid expansion of the oil palm industry (Begum et al., 2019: Tiemann et al., 2018). Taking a look at the most productive parts of Malaysia, it is clear that, the oil palm crop requires a relative humidity of at least 85%, an average of 5 h of sunlight each day, and at least 2000 mm annual rainfall spread uniformly throughout the year with little or no dry season in order to achieve optimal growth and production (Tiemann et al., 2018). In addition, steady average temperatures between 24C and 28C appear to have optimal conditions, with seasonal fluctuations of less than 6C. Average temperatures below 17C will reduce growth by more than half, and no growth will occur anymore at maximum daily temperatures of 15C. The crop usually thrives at altitudes below 400 m in tropical lowlands (Tiemann et al., 2018: Alam et al., 2015). In Malaysia, oil palm is primarily cultivated, which is a hybrid between the dura and pisifera. The oil palm tree can grow 7–15 m high depending on various conditions. The oil palm leaves look like feathers, emerging from both sides of the frond. The oil palm fronds can grow up to 5 m. Within three years of planting, the oil palm tree can begin to produce fruits, and after 5–6 months, fruits are mature enough for harvesting. The fruits are in bunches or known as fresh fruit bunches (FFB), weigh 15–30 kg, and depending on the condition, can reach up to 50 kg (Awalludin et al., 2015). FFB consisted of the oily pericarp, shell, and edible oil contenting palm kernel. The average economic lifespan of an oil palm tree is 25–30 years. Therefore, oil palm cultivation is the best option for many countries since it can produce a high volume and quality of edible oil in a limited area (Foong et al., 2019).
Palm oil has evolved to be the world's most significant oil produced and consumed. Palm oil is everywhere in our daily life, as food palm oil is used for frying, and it is also an ingredient in many processed foods. In addition, it is used in dietary supplements, medicine, cosmetics, dye, soaps, waxes, lubricants, and ink (MPOB, 2021). The world population has increased, leading to a further increase in the production of palm oil. The use of palm oil has risen exponentially in the last ten years, leading to the use of palm oil from 6% to 28%. To meet this increasing global demand, more farming land and rain forest in Malaysia are converted to oil palm plantations (Begum et al., 2019). Oil palm cultivation began in Malaysia nearly a century ago. In 1871, the British planted the first oil palm tree in Malaysia, and oil palm was used in those days as ornamental plants (Onoja et al., 2019). In 1917 in Tenamaran Estate, Selangor, Malaysia, began its first commercial palm oil production (Alam et al., 2015; Basiron, 2007). Oil palm planting statistics reveal that Malaysia only had 54,000 ha of oil palm plantation area back in the 1960s. The cultivated area of oil palm has risen exponentially since then. The oil palm plantations have occupied 5.87 million hectares of Malaysian soil in 2020 (MPOB, 2020). Abdullah et al. (2017) stated that the demand for Malaysian palm oil commodities is exponentially increasing in India, China, and Europe. One of the major agricultural products exported from Malaysia is refined and crude palm oil (Abdul-Hamid et al., 2020; Truckell et al., 2019). According to the Malaysia Palm Oil Board (MPOB), in the year 2020, total Malaysian exports of palm oil and other oil palm products amounted to 26.73 million tonnes, contributing to the total export revenue of approximately USD 17.88 billion. With the rapid advancement of the palm oil industry, sustainability development throughout the sector is extremely difficult. Therefore, there is an interest in incorporating sustainable development task forces within the frameworks of palm oil companies which include key elements, such as economic, environmental and social factors (Tiemann et al., 2018). In Malaysia, for example, a Roundtable on Sustainable Palm Oil (RSPO) has been formed to regulate the sustainability of the palm oil sector, describing key elements of legal, economic viability, environmental and public policy via policies known as RSPO Principles and Criteria (Begum et al., 2019). Although this sector is regarded as financially secure, the oil palm industry may enhance its sustainability by improving various aspects of oil palm cultivation and mill processing.
4 The oil palm biomass
Oil palm biomass is an inevitable by-product of the oil palm industries during trimming, harvesting, replanting, and milling activities (Basiron, 2007). Being the world's second-largest palm oil producer, Malaysia has a diverse range of palm biomass generated as by-products from its vast oil palm industry (Fig. 2). Oil palm trunks (OPT), oil palm fronds (OPF) generally produce at plantations area, empty fruit bunches (EFB), palm kernel shells (PKS), mesocarp fibers (MF), and palm oil mill effluent (POME) is usually produced during mill operation of the FFB (Abdul-Hamid et al., 2020; Onoja et al., 2019). Nevertheless, utilization of oil palm biomass as value-added products is minimal due to the increased cost of labor, transportation, distribution, and storage. Thus, in most cases, these abundant resources are left to decompose in the plantation area. It is estimated that by 2020, Malaysian oil palm industries would produce 100 million tonnes of solid oil palm biomass. Such oil palm waste production has nevertheless presented a massive problem of waste management and various environmental issues (Awalludin et al. 2015).
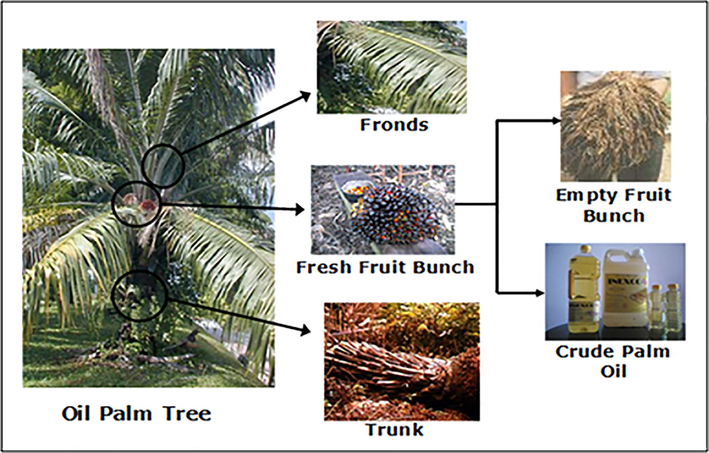
- Simplified representation of biomass and product produced from an oil palm tree (Wan Daud and Law, 2011). The fresh fruit bunches (FFB) are collected from the plantation area during harvesting season. In the oil palm mill, the FFB is sterilized, and fruits are removed from the bunches, so the empty fruit bunches (EFB) become residues. While oil palm trunks (OPT) and oil palm fronds (OPF) generally produce at plantations during maintenance pruning and replanting. After various milling operations, crude palm oil is extracted from the oil palm fruits.
The necessary waste management steps are reducing waste generation to a minimal level, recycling, and safe disposal. In Malaysia, the oil palm industry implements environment-friendly waste management strategies recommended by the Department of Environment Malaysia and the MPOB. EFB is now utilized in the plantation areas for better weed control, preventing soil erosion, and retaining soil moisture (Begum et al., 2019; Awalludin et al., 2015). Oil palm biomass can be converted into high-value bioproducts such as biofuels, biofertilizers, bioplastics, biosugars, biochemicals, and biomaterials. When various economically valuable alternatives that will benefit both the environment and the oil palm industries are available, steps should be taken to utilize the oil palm biomass for a sustainable bioeconomy.
5 Empty fruit bunches (EFB)
EFB is lignocellulosic biomass formed once oil palm fruits are separated from the FFB during palm oil production. EFB comprises 20–22 percent of the solid by-products generated during palm oil production, making it the most notable waste produced by the oil palm industries (Tahir et al., 2019; Han and Kim, 2018; Samiran et al., 2015). Previously, EFB was used in oil palm mills as a fuel source to create steam, which leads to greenhouse gas emissions. Although using EFB as boiler fuel is less feasible due to its high moisture content (Ahmad et al., 2019). Sometimes it returned to the plantation area to decay naturally and prevent soil erosion. Considering that EFB is generated in large quantities in oil palm mills, it is an ideal residue for recycling as a biofertilizer.
5.1 Lignocellulosic components of empty fruit bunches
The lignocellulosic biomass is a plant-based organic compound with a highly complex structure. The lignocellulosic compound consists of three main components known as cellulose, hemicellulose, and lignin. Cellulose is the most abundant organic compound found in the face of the Earth as it contains a high amount of plants (Law et al., 2007; Glazer and Nikaido, 2007). It consists of glucose molecules linked together by β-(1,4)-glycosidic bonds, which form the cellobiose as the basic repeating unit (Onoja et al., 2019). Hemicellulose is another element present along with cellulose in almost all terrestrial cellular plant structures. It comprises pentoses, hexoses, and uronic acids, which are firmly ramifications, non-crystalline heteropolysaccharides. Lignin is a group of complex organic polymers that form necessary structural materials in the supporting tissues of vascular plants. Lignin is phenolic polymers cross-linked in chemistry. Generally, softwoods contain higher lignin than hardwoods (Glazer and Nikaido, 2007). Such biomass, traditionally discarded as waste, is currently used as valuable sources of feed, biofuel, biofertilizer, biochemicals and used in the paper and pulp industry (Onoja et al., 2019). The lignocellulose components of EFB are lignin, cellulose, and hemicellulose, illustrated in Table 1. The amount of each lignocellulosic component varies depending on various environmental conditions.
| Lignocellulosic content (%) based on the dry weight | |||
|---|---|---|---|
| Cellulose (%) | Hemicellulose (%) | Lignin (%) | References (%) |
| 44.2 | 33.5 | 20.4 | Rosli et al., 2017 |
| 40–50 | 20–30 | 20–30 | Alizadeh et al., 2014 |
| 50 | 30 | 20 | Loh, 2017 |
5.2 Nutrient content of empty fruit bunches
Studies show EFB contains a sufficient number of macronutrients such as carbon (C), hydrogen (H), nitrogen (N), sulfur (S), oxygen (O), and phosphorous (P), which is vital to sustaining the healthy development of plants are illustrated in Table 2 (Ai May et al., 2020). In addition, micronutrients such as copper (Cu), boron (B), manganese (Mn), and iron (Fe), also present relatively smaller quantities in EFB as illustrated in Table 3.
| Macronutrient content (%) based on the dry weight | ||||||
|---|---|---|---|---|---|---|
| C (%) | H (%) | N (%) | S (%) | O (%) | P (%) | References |
| 45.00 | 6.40 | 0.25 | 1.06 | 47.30 | – | Samiran et al. (2015) |
| 48.79 | 7.33 | 0.70 | – | 0.68 | – | Hamzah (2008) |
| 47.65 | 3.2 | 1.82 | 0.36 | 44.97 | – | Idris et al. (2012) |
| 48.72 | 7.86 | 0.25 | 2.21 | 48.18 | 2.03 | Loh (2017) |
| Micronutrient's content (%) based on the dry weight | |||||
|---|---|---|---|---|---|
| Cu (mg/kg) | B (mg/kg) | Zn (mg/kg) | Mn (mg/kg) | Fe (mg/kg) | References |
| 23 | 10 | 51 | 48 | 473 | Hayawin et al. (2014) |
| 26 | – | 71 | 88 | 210 | Gandahi and Hanafi (2014) |
Scientists reported EFB contains almost all 16 essential elements, which are known as plant nutrients. All these nutrients are necessary for plant growth, fighting off diseases, pests, and reproducing. Scientists have divided these nutrients into two categories, macronutrients, and micronutrients, according to the relative amount that plants need (Table 4). It is hard to tell whether all these essential nutrients are available in soil or not. Thus, EFB is a perfect source for composting materials. Due to its high nutrient profile and capacity to hold large amounts of water, aerobic microbial composting is suitable for making inaccessible nutrients in EFB accessible (Gandahi and Hanafi, 2014).
| Nutrient Category | Nutrient | Percentage of plant | Major function in plants |
|---|---|---|---|
| Macronutrient | Carbon (C) | 45 | Plant structures. |
| Hydrogen (H) | 6 | pH regulation, water retention, synthesis of carbohydrates. | |
| Nitrogen (N) | 1.75 | Protein/amino acids, chlorophyll, cell formation. | |
| Sulphur (S) | 0.03 | Protein, amino acid, vitamin, and oil formation. | |
| Oxygen (0) | 45 | Respiration, energy production, plant structures. | |
| Phosphorous (P) | 0.25 | Cell formation, protein synthesis, fat, and carbohydrate metabolism. | |
| Micronutrient | Copper (Cu) | 0.0001 | Enzyme activity. |
| Boron (B) | 0.0001 | Enzyme activity. | |
| Zine (Zn) | 0.002 | Enzyme activity. | |
| Manganese (Mn) | 0.005 | Enzyme activity and pigmentation. | |
| Iron (Fe) | 0.01 | Enzyme development and activity. |
One of the most effective ways to address all of the difficulties associated with conventional EFB handling techniques is through the use of a biological-based composting process, which has been in use for many years and produces a variety of valuable end products.
6 Conversion of empty fruit bunches into biofertilizer
One of the most effective ways to address all of the difficulties associated with traditional EFB biomass management is a biological-based composting process that has been introduced for a very long time to create valuable end-products (Wei et al., 2016). Biocomposting is an aerobic process that relies on the presence of a diverse microbial population to break down the organic components of EFB and release nutrients available in EFB and destroy pathogens. During biocomposting, lignocellulosic components are broken down by the aerobic thermophilic microbes naturally present in biomass to produce nutrient-rich humus-like materials (biofertilizer). The biocomposting process can be divided into three stages: high-rate composting, stabilization, and maturation (Meyer-Kohlstock et al., 2013). Long formation time and low nitrogen content are some of the common issues faced while composting EFB biomass. Several factors can lead to these issues, including temperature, pH, moisture content, agitation, conductivity, and EFB characteristics such as size, initial C:N ratio, and nutrient contents.
It is worth noting that most of the literature reported that the EFB biomass takes about 2–3 months to complete the composting process. However, Siddiquee et al. (2017) reported a 30-day composting process which significantly reduces the EFB composting time. With the assistance of two strains of Trichoderma to composting the EFB biomass. The same researchers also found that the EFB compost showed a high amount of macronutrients (N, P, and K), which improves soil acidity and electrical conductivity. In a separate report, Lim et al. (2015a) reported the effects of microbial inoculation additive to enhance the efficiency of EFB decomposition and at the end of the composting process lower C:N ratio, increase pH value (∼8), microbial population, and degradation of organic acids. Another study by Lim et al. (2015b) investigated the physicochemical properties of EFB compost while inoculated with effective microorganisms. The EFB composting process took approximately two months. The microbial-treated EFB compost experienced an increase in the mineral content (Mg, Ca, B, and K), pH (∼8), and loss of total organic carbon (10.8%). They concluded that the addition of effective microorganisms speeds up the EFB composting process.
In another study, Wei et al. (2016) compared how the final quality of EFB compost was affected by the different temperatures, aeration rates, and reaction time during the composting process. The team mixed urea as a nitrogen source and fresh compost as inoculum with EFB samples and carried out the composting process for 42 days. Temperature and reaction time were observed to have a significant effect on moisture content. All three factors have a major impact on carbon loss. Nitrogen content was influenced by aeration intensity, reaction time, and the temperature-reaction-time relationship. Total ion changes over time revealed a strong association with conductivity (Pearson correlation coefficient of 0.853), with the most significant decrease in C/N ratio (from 30.2:1 to 17.6:1) obtained at a temperature of 40 °C and aeration rate of 0.4 L/min kg. The findings of this study will help the oil palm industry improve the quality of EFB composts produced in a short period of maturation and with a low C:N ratio. Tahir et al. (2019) experimented with isolating and identifying competent fungi that can naturally compost EFB biomass. The results showed that the changes in the physical characteristic of EFB were correlated to fungal growth. They concluded that one of the strategies for rapid composting is the inclusion of competent lignocellulolytic fungi in the composting process combined with an appropriate EFB composting method. In a similar study, Ichriani et al. (2018) reported the growth and yield of maize while applying EFB biochar mixed with phosphate-solubilizing fungi. They stated that the positive effects on maize growth and yield were recorded.
Trisakti et al. (2017) studied the effect of frequency on the rate of composting of EFB mixed with activated liquid fertilizer (ALOF) in a basket composter for 40 days, with the 3rd day showed the best results. The best EFB + ALOF compost characteristic was pH 9.0; moisture content (MC) 57.24%; water holding capacity (WHC) 76%; C:N ratio 12.15%; P 0.58%; and K 0. 95%. In an extensive study, Trisakti et al. (2018) experimented with producing EFB compost mixed with ALOF in a basket composter for ornamental plant cultivation. After 40 days data revealed that the characteristic of EFB + ALOF compost were pH 9.0; MC 52.59%; WHC 76%; C:N ratio 12.15; N 1.96%; P 0.56%; and K 0.95%. They also reported that the best-growing medium was for cactus, sansevieria, and anthurium was EFB + ALOF compost-sand-husk rice mixture at 1:3:1; 1:1:1; and 1:0:1; respectively. Hau et al. (2020) conducted a study to examine the effects of EFB + POME based biofertilizer mixing with various organic wastes to enhance biofertilizer nutrient content. They mixed EFB + POME with fishmeal, bonemeal, and bunch ash in a 1:1 wt ratio. It took approximately 40 days to achieve maturation of the EFB mixed composts with a pH of 6.3. It was reported that all batches of EFB mixed compost experienced an increase in N, P, and K. They concluded that EFB based biofertilizers could be mixed with other organic wastes to increase their quality and nutrient availability.
In another study, Yahya et al. (2010) examined the composting of EFB and decanter cake (DC) slurry by incorporating POME in an ordered turning process. They observed that applying a DC slurry improved the rate of the EFB composting process. The compost (EFB and DC slurry) matured after 51 days of composting and included 46.4% N, 17.9% P, 17.7% P, and Ca 23.1% compared to compost without a DC. Finally, the physicochemical modifications of an oil palm biomass during vermicomposting were investigated by Nahrul Hayawin et al. (2010). According to the results, vermicomposting of EFB is a suitable method for recycling oil palm wastes into value-added vermicompost. The overall organic carbon, C/N ratio, and pH value decreased during the phase, while the N, P, and K proportions improved. Furthermore, the heavy metal amount rose but not above the nutrient range in the vermicompost.
7 Characterization of plant growth-promoting microbes present in EFB biofertilizer
Once applied to the plants or soil, the PGPM present in the biofertilizer colonizes the plants' roots largely and encourages plant development by accumulating nutrients for the host plants (Kour et al., 2020; Mishra et al., 2017). All these different microorganisms flourish in the soil, particularly in the plants' rhizosphere, and a very significant number of such microbes have a functional link. The rhizosphere is a tiny layer of soil that adheres to the root surface of plants. (Soumare et al., 2020; Naik et al., 2019). Plant roots release various organic compounds that attract the microbial community, and they colonize the plant's rhizosphere. The soil dwelling microorganisms present in the rhizosphere comprise of different taxa such as bacteria, arbuscular mycorrhizal fungi (AMF), actinomycetes, and cyanobacteria (Singh et al., 2019; Kaushal and Wani, 2016). Furthermore, high lignocellulosic compounds, which can be used as a microbial energy source for the biosynthesis process, make EFB a natural habitation for different beneficial microbes (Dermiyati et al., 2020; Rosli et al., 2017). For example, Ariana and Candra (2017) isolated 430 microbes from EFB as part of their study. In another study, Lai et al. (2017) reported that 34 bacterial isolates were extracted from EFB compost. Besides, Harith et al. (2014) obtained 24 bacterial isolates from EFB biomass. These microbial communities directly or indirectly help plant growth, preventing diseases or stress (Singh et al., 2019; Babalola, 2010). Important characteristics of PGPM include the production of organic acids, phytohormones, antibiotics, siderophores, volatile bacterial compounds, solubilization of phosphorus, and nitrogen-fixing, as illustrated in Table 5.
| Traits | Role | Microbial Species | References |
|---|---|---|---|
| Phosphate Solubilization | Organic Acid Production | Bacillus amyloliquetaciens | Chen et al. (2006) |
| Phosphatase Production | Burkholderia cepacian | ||
| Serratia marcescens | |||
| Nitrogen Fixation | Symbiotic | Rhizobium phaseoli | Vandana et al. (2017) |
| Non-Symbiotic | Gluconacetobacter diazotrophicus | ||
| Phytohormones | IAA Production | Bacillus licheniformis | Nandi et al. (2015) |
| Phoma glomerata | |||
| Biocontrol | Siderophore Production | Pseudomonas aeruginosa | Vandana et al. (2017) |
| Antibiotic Production | Pseudomonas fluorescens | ||
| Induced Systemic Tolerance | ACC Deaminase Production | Achromobacter piechaudii | Choi et al. (2014) |
| Penicillium citrinum |
As a result of the constant communication that occurs between the plant and its microbiota, it makes feasible to manipulate the microbes present, which in turn can influence the development of the plants defence against pests and diseases, thereby increasing total yield (Arif et al., 2020; Aloo et al., 2020). Several direct and indirect methods by which PGPM contribute to the promotion and development of plants have been identified (Fig. 3). Plant endogenous hormone levels are modulated by certain PGPM, which produce phytohormones such as auxins, gibberellins, and cytokinins, among other things. In addition, several PGPM species, such as Pseudomonas spp. and Arthrobacter spp., and Bacillus spp. secrete ACC deaminase, which breaks down ACC, an intermediate in the production of the plant stress hormone ethylene, resulting in reduced stress levels in plants growing in less-than-optimal conditions (e.g., salinity, drought, or heavy metal toxicity) (Arif et al 2020; Khanna et al., 2019a). Plant growth-promoting effects by the PGPM, such as N fixing and phosphorus solubilization, as well as ACC deaminase and auxin synthesis, have been shown in wheat and soybean. On 32 different plant species, including maize, tomato, melon, and pepper, Xia et al. (2015) examined the impact of isolated bacterial endophytes on their growth and development. Of the isolates examined, 61% were found to increase tomato growth, while 50–64% were shown to increase biomass production.
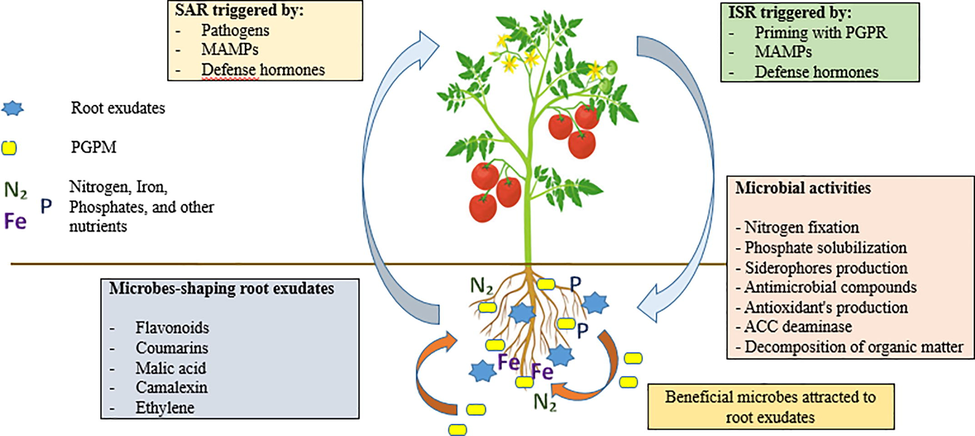
- Simplified representation of beneficial plant-microbial interactions. Microbial activities benefit plants in terms of physical growth, disease resistance, and stress tolerance, resulting in up to a fourfold increase in biomass output. microbe-associated molecular patterns (MAMPs) generated from pathogens or beneficial PGPM can be used to achieve both systemic acquired resistance and induced systemic resistance to plant diseases., and resistance can also be induced through the use of MAMPs from beneficial PGPM (Arif et al., 2020).
7.1 Role in phosphate fixation
Phosphorus (P) is a vital macronutrient for plants' growth and development (Kour et al., 2020; Rafi et al., 2019). Excessive use of traditional P-fertilizers may cause surface and groundwater contamination, waterway eutrophication, reduction of soil fertility, and accumulation of toxic components (Mendoza-Arroyo et al., 2020; Kalayu, 2019; Vandana et al., 2017). Phosphate-solubilizing capacity is well established in various bacterial genera, including Pseudomonas, Bacillus, Micrococcus, Enterobacter, Virgibacillus, and Azospirillum (Mukhtar et al., 2020). Many researchers reported that PGPM present in biofertilizer solubilize phosphate utilizing various techniques for converting inaccessible phosphate in empty fruit bunches of oil palm into accessible forms without harming the environment and promoting growth productivity of many crops (Soumare et al., 2020; Singh et al., 2019). Some bacteria have shown the production of organic acids to be consistent with the dissolution in Gram-negative bacteria of mineral glucose with gluconic acid (GA) (Vandana et al., 2017). In 1948, Pikovskaya documented the solubilization of insoluble P by microbess. In soil, P interacts with other metallic elements such as Fe, Al, and Ca ions to produce ferrous phosphate, aluminium phosphate, and calcium phosphate, respectively. The release of organic acids by phosphate solubilizing microorganisms (PSMs) initiates the chelation reaction. As a result, the bound P to other metallic elements is released and made available to plants. Several studies have shown that in addition to protecting antibiotics, and anti-pilot products, the PSMs can solubilize K, improve N fixation, and develop plant growth regulators, including auxin cytokininines and gibberellins. PSMs synthesize growth hormones such as auxins, cytokinins, and gibberellins, which promote cell division, differentiation, shoot growth, root development, flowering, germination, and xylem differentiation (Kour et al., 2021; Puri et al. 2020). Bacillus tequilensis, a novel strain, has been shown to secrete plant growth hormones such as abscisic acid, auxin, and gibberellins, and its inoculation in soybean has been shown to increase shoot biomass, leaf ultrastructure, and photosynthetic pigment under heat stress. A study conducted by Joe et al. (2016) reported Acinetobacter sp. and Bacillus sp., isolated from Phyllanthus amarus, have phosphate solubilizing and salt-tolerant properties. In a different study, Inagaki et al. (2015) found a higher concentration of phosphorus in the leaf tissue of maize while using several strains of plant growth-promoting rhizobacteria (PGPR) as inoculants in acidic sandy soil.
7.2 Role in nitrogen fixation
Nitrogen (N) is another essential macronutrient for the growth and development of plants. Although it is the seventh most common element in our entire universe, comprising about 78% of the earth atmosphere yet it is also the most limiting available nutrient for the plant due to insoluble forms (Singh et al., 2019; Vandana et al., 2017) Ammonium and nitrate are the two forms in which plants uptake N from their environment. Microbes that can transform atmospheric or environmental N into ammonium or nitrate are nitrogen-fixers or diazotrophs (Singh et al., 2019; Kuan et al., 2016). Scientists have reported that many PGPR present in biofertilizers have this transforming ability to convert insoluble N in their environment into a soluble form that plants can absorb. To increase the growth and yield of agricultural production, these groups of microbes can be used to enhancing the quality of biofertilizers (Defez et al., 2017). The use of chemical nitrogen fertilizer has many disadvantages compared to biofertilizers. It requires a significant amount of fossil fuels to produce chemical nitrogen fertilizer, which contributes more greenhouse gas emissions, thus causing climate change. Therefore, using biofertilizers instead of chemical nitrogen fertilizers is more sustainable.
7.3 Role in phytohormones production
Approximately 80% of PGPM produce phytohormones include auxins such as indole-3-acetic acid (IAA), cytokinins and gibberellins (GAs), ethylene, and abscisic acid (ABA), which stimulate plant physical growth via different physiology and biochemical activities to ensure the healthy life cycle of plants (Waadt et al., 2015; Wani et al., 2016). These phytohormones play an essential role in plant growth and function and contribute to defense against pests and specific abiotic stress management (Kruasuwan and Thamchaipenet, 2018). IAA, the most common physiological active phytohormone (Vandana et al., 2017). The production of IAA is a significant feature of rhizospheric microbes that stimulates plant growth and encourages it (Bhardwaj et al., 2014). The research dealt with isolating, characterizing, and identifying microbes from the rhizospheric soil that produces indole acetic acid. According to Hsu (2010), indole acetic acid can be produced as a secondary metabolite by isolated bacteria from rhizosphere regions of different crops due to the abundant availability of substrates. Indole acetic acid leads to the growth of longer roots with an increased amount of root and lateral root hairs involved in absorbing nutrients.
7.4 Role in siderophore production
For both plants and microbes, Iron (Fe) is a crucial element. Due to its complex insoluble forms, iron found in the environment, and the empty fruit bunches are not accessible to plants. Several PGPR microbes have evolved to produce siderophores which convert insoluble iron into soluble so that plants can fulfil their iron requirement for better growth and development (Dimkpa, 2016). Siderophores are secondary metabolites of low molecular weight formed by iron-deficient microbes, which provide iron for the organism (Kumar et al., 2019; Dimkpa, 2016; Singh et al., 2019). Many siderophores play a Fe scavenging function secreted by a group of PGPR microbes (Leventhal et al., 2019; Vandana et al., 2017). Many PGPR, such as P. aeruginosa, have been reported to produce siderophores which also have a prominent role in plant growth and biocontrol activities against chili disease. Similar findings were also recorded by Bindu and Nagendra (2016) in a paddy plantation where biofertilizer was inoculated with P. aeruginosa (Singh et al., 2019).
7.5 Role in antibiotic production
Every year, all over the world, farmers are facing a significant yield loss due to weeds, pests, and plant pathogens such as bacteria, protozoa, fungi, and viruses (Vandana et al., 2017). Traditional pest control strategies are limited significantly when disease-causing agents frequently change their genome to create pesticide resistance. In this situation, PGPR microbes can offer an effective solution. PGPR in biofertilizers is environmentally friendly. While producing phytohormones for plant growth, they also indirectly produce antibiotics with inhibitory effects on pathogenic organisms. These antibiotics secreted by PGPM have antifungal, antibacterial, and anthelmintic properties, which can destroy or weaken the pathogen (Vandana et al., 2017). Some of the antibiotics reported having these characteristics are phenazines, pyrrolnitrin, and cyclic lipopeptides (Singh et al., 2019).
7.6 Role in induced systemic tolerance
There are numerous ways in which abiotic stress affects plants, which results in changes to the plant's growth, development, and physiology. When faced with stress signals like as drought, infections, heavy metals, salt, and heat, all plants react by producing a variety of metabolites that help them regulate their metabolic rate (Khanna et al., 2019a). Microorganisms present in biofertilizer have broad characteristics, such as promoting plant growth and production via the development of positive interactions with and survival under severe circumstances. They stimulate plant development by boosting the synthesis of hormones and metabolites as well as improving the absorption of nutrients. PGPM develops methods to reduce various abiotic stressors, such as heavy metals, drought, salt and temperature. Bacilus, Pseudomonas alacaligenes, P. fluorescens, P. aeruginosa, Methylobacterium, Burkholderia are among the stress resistant organisms (Khanna et al., 2020). Induced systemic tolerance (IST) is the induction of abiotic stress tolerance regulated by microorganisms (Vandana et al., 2017; Khanna et al., 2019b). It is still unknown how the molecular mechanism of plant and microbial communication is associated with IST. Some PGPM is reported to produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme, which cleaves ACC. ACC is a precursor of ethylene to ammonia and α-ketobutyrate. Gupta and Pandey (2019) have stated that ACC deaminase activity has a vital role in plant growth, protection under unfavourable environmental conditions, and symbiotic programs. In addition, the ACC has a crucial role in controlling bacterial colonization by modulating plant immune responses in the rhizosphere, endophytes, and phyllosphere.
8 Plant responses to biofertilizers
The technology that goes into the manufacturing of biofertilizers is very economical and straightforward when compared to chemical fertilizers (Alori et al., 2017). In the past several decades, significant advancements have been achieved in the study and use of various biofertilizers. The scope of this study includes the variety of PGPM, as well as the commercial issues and technology for the manufacture of biofertilizers for agricultural and environmental sustainability, among other topics. Newer technologies, including as coating, co-encapsulation, fermentation, lyophilization, and inoculation, are now being developed for this purpose (Arif et al., 2020). Examples include: The development of fertilizers derived from chemical, organic, and microbial sources (biofertilizers) is expected to gain widespread adoption in the future for environmentally benign and economically successful agriculture. A lot of assumptions are imposed on biofertilizers. Biofertilizers consist of living organisms like bacteria and fungi; therefore, their performance varies depending on the surrounding environment. Furthermore, the influence on the plants is slow in comparison with chemical fertilizers. Consequently, the results are supposed to be inconsistent.
Biofertilizers are often portrayed as even more expensive as synthetic fertilizers due to the shortage of skills and technology to produce biofertilizers by utilizing abundant palm oil waste (Chun-Li et al., 2014). Recent ground-breaking advancements in molecular research on plant-pathogen interactions and genomics can help understand the necessary protocols to overcome these shortages. Nevertheless, many researchers have reported positive outcomes while using a variety of PGPR microbes with biofertilizers. The usage of different biofertilizers is an essential part of agricultural activity in recent days. The successful introduction of the biofertilizer depends on several important factors such as inoculums size, types, in which state biofertilizer is applied to field (solid, liquid, or powder), the competition between microbes in biofertilizers with the native niche for survival. The farmer's knowledge about the proper usages of biofertilizers is also an essential factor for a positive outcome.
9 Commercialization of biofertilizers
A century ago, biofertilizers were first commercialized, when “Nitragin” was registered for plant bioinoculation with Rhizobium sp. Biofertilizers account for around 5% of the overall fertilizer industry, and over 150 products based on microbial strains agricultural use were registered. Table 6 lists some of the most popular biofertilizers used throughout the globe. Rhizobial bioinoculants have become the most common microbial inoculants in recent years, accounting for about 79 percent of global demand. P-solubilizing biofertilizers account for 15% of the worldwide market, whereas other bioinoculants, such as mycorrhizal fungi, account for 7% (Mącik et al., 2020). In 2020, the global biofertilizers market reached a value of $2.3 billion dollars and is predicted to hit $3.28 billion by 2027 (Research and Markets, 2021; Fortune Business Insights, 2021). As the global demand for organic food increases, so does the market for biological fertilizers. On the basis of geography, the global biofertilizers market is segmented into the following regions: North and South America; Europe; Asia-Pacific; Latin Biofertilizers have the fastest expanding market in Asia-Pacific.
| Name of company | Name of product | Active microbial inoculants | Mechanisms | Compatible crops |
|---|---|---|---|---|
| Embrafos | BioAtivo | N-fixer and P-solubilizer | Increase soil N and P levels by fixing atmospheric N and insoluble P and make it available to plants. | All crops |
| The Tokachi Federation of Agricultural Cooperatives | R-Processing seeds | Rhizobia sp inoculated the legume seeds | Production of indole acetic acid, siderophore, N-fixation and P-solubilization. | Soybeans, azuki beans, and Phaseolus beans |
| Hyper-coating seeds | Rhizobia within the capsule of calcium carbonate | |||
| EM Research Organization) | EM Bokashi | Lactobacillus plantarum, L. casei, L. fermentum, and R. palustris | Transfer phosphate to the root cortex from the soil. These are wide-ranging bio-fertilizers | Soybeans, azuki beans, and Phaseolus beans |
| China Bio-Fertilizer AG | Xin Sheng Li | K-solubilizing bacteria (Bacillus mucilaginosus) and a P-solubilizing bacteria (B. subtilis) | Solubilize K by secreting organic acids that break down silicates, remove metal ions and make them accessible to plants. Transfer P to root cortex from the soil | Maize, alfalfa, onion |
| Vanadis Bioscience Pty. Ltd. | Catapult ™ | VAM plus 2 species of Bacillus | Solubilize insoluble forms of P by selecting organic acids and reducing the pH | Legumes and cereal crops |
10 Conclusion and future perspectives
This review updates our understanding of microbial activities to convert biodegradable waste into valuable biofertilizer, how microbes can improve biofertilizer quality by converting inaccessible nutrients in EFB into an accessible form for plants and critically propounds on their future prospects for sustainable crop production. Chemical fertilizers are proven effective and convenient for agricultural production and disease management, but they are a severe threat to public health and the environment. Currently, there is a big gap between chemical fertilizer's demand and production. Therefore, converting EFB biomass into biofertilizer and using it for agricultural practices will be a more sustainable approach for modern agriculture in the context of their cost, environment-friendly nature, and profitable conversion of oil palm biomass. There has been a substantial increase in the health, growth, and yield of plants due to the application of biofertilizer. PGPM stimulates in a variety of ways, both direct and indirect. The Malaysian government should give more support and encourage the usage of EFB biomass from the palm oil industry for biofertilizer production. Developing biofertilizers using EFB biomass will have a significant impact on the country's stainable agricultural economic development. It will also contribute to the protection of the environment and public health. The government should encourage more organic farming by offering special incentives to the local farmers and private sector and educate them about the benefits of using biofertilizers. Government and privately funded research and development (R&D) activities need to increase to find new opportunities and solutions to current issues related to converting EFB into biofertilizers. Molecular biotechnological tools can improve traits of beneficial microbes that can enhance the biological pathway of various biomolecules such as phytohormones, antibiotics, enzymes, Volatile compounds productions, and many more. A better knowledge of the whole process of PGPM may aid in the development of more specialized strains capable of working in more unfavourable and variable circumstances.
Acknowledgement
The authors wish to thank Universiti Malaysia Sabah for the financial assistance via Skim Dana Khas (SDK0128-2020 and SDK0079-2019).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Impeding challenges on industry 4.0 in circular economy: Palm oil industry in Malaysia. Comput. Oper. Res.. 2020;123:105052.
- [Google Scholar]
- Sustainable manufacturing practices in Malaysian palm oil mills. J. Manuf. Technol. Manag.. 2017;28(3):278-298.
- [Google Scholar]
- The outlook of the production of advanced fuels and chemicals from integrated oil palm biomass biorefinery. Renew. Sustain. Energy Rev.. 2019;109:386-411.
- [Google Scholar]
- Investigation of pesticidal ability of humic acid derived from palm oil Empty Fruit Bunch (EFB) vermicompost. Int. J. Recycl. Organ. Waste Agric.. 2020;9(3):237-247.
- [Google Scholar]
- Malaysian oil palm industry: prospect and problem. J. Food Agric. Environ.. 2015;13(2):143-148.
- [Google Scholar]
- Inoculation Technology for Trichoderma harzianum during Interaction with Oil Palm Elaeis guineensis Jacq. J. Pure Appl. Microbiol.. 2014;8(6):4541-4547.
- [Google Scholar]
- Aloo, B.N., Makumba, B.A., Mbega, E.R., 2020. Plant growth promoting rhizobacterial biofertilizers for sustainable crop production: The past, present, and future.
- Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol.. 2017;8:971.
- [Google Scholar]
- Isolation and characterization of lignocellulolytic microbes from oil palm empty fruit bunches (EFB) Sustinere: J. Environ. Sustain.. 2017;1(1):1-9.
- [Google Scholar]
- Plant microbiome engineering: expected benefits for improved crop growth and resilience. Trends Biotechnol.. 2020;38(12):1385-1396.
- [Google Scholar]
- An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew. Sustain. Energy Rev.. 2015;50:1469-1484.
- [Google Scholar]
- Beneficial bacteria of agricultural importance. Biotechnol. Lett.. 2010;32(11):1559-1570.
- [Google Scholar]
- Barker A.V., Pilbeam D.J., eds. Handbook of plant nutrition. CRC Press; 2015.
- Palm oil production through sustainable plantations. Eur. J. Lipid Sci. Technol.. 2007;109(4):289-295.
- [Google Scholar]
- Sustainability of Malaysian oil palm: a critical review. Int. J. Environ. Sustain. Dev.. 2019;18(4):409-429.
- [Google Scholar]
- Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact.. 2014;13(1):1-10.
- [Google Scholar]
- Siderophore production by Pseudomonas aeruginosa isolated from the paddy fields of Kuttanad, Kerala. Int. J. Sci. Res.. 2016;12:1577-1581.
- [Google Scholar]
- Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol.. 2006;34(1):33-41.
- [Google Scholar]
- Genome sequence of Bacillus amyloliquefaciens GB03, an active ingredient of the first commercial biological control product. Genome Announcements. 2014;2(5)
- [CrossRef] [Google Scholar]
- Present situation and future perspective of biofertilizer for environmentally friendly agriculture. Ann. Rep. 2014:1-5.
- [Google Scholar]
- Improved drought stress response in alfalfa plants nodulated by an IAA over-producing Rhizobium strain. Front. Microbiol.. 2017;8:2466.
- [Google Scholar]
- Abundance and characterization of microorganisms isolated from oil palm empty fruit bunches waste under aerobic, anaerobic, and facultative anaerobic conditions. Biodiversitas J. Biol. Divers.. 2020;21(9)
- [Google Scholar]
- Microbial siderophores: Production, detection and application in agriculture and environment. Endocytobiosis Cell Res.. 2016;27(2)
- [Google Scholar]
- European Palm Oil Alliance, 2016. The Palm Oil Story. Available at: https://issuu.com/epoa/docs/brochure_palm_oil_story_def_online_c6305acc5b0a30 (Accessed 30 December 2020).
- Food and Agriculture Organization (FAO), 2017. The Future of Food and Agriculture – Trends and Challenges. Rome. Available Online at: http://www.fao.org/PUBLICATIONS (Accessed 30 Dec 2020).
- Food and Agriculture Organization (FAO), 2018. The State of Agricultural Commodity Markets 2018. Agricultural Trade, Climate Change and Food Security. Rome. Available Online at: www.fao.org/PUBLICATIONS (Accessed 30 Dec 2020).
- Input–output optimisation model for sustainable oil palm plantation development. Sustain. Prod. Consump.. 2019;17:31-46.
- [Google Scholar]
- Fortune Business Insights, 2021. Biofertilizers Market Size, Share & COVID-19 Impact Analysis, By Type (Nitrogen Fixing, Phosphate Solubilizers, and Others), Microorganism (Rhizobium, Azotobacter, Azospirillum, Pseudomonas, Bacillus, VAM, and Others), Application (Seed Treatment, Soil Treatment, and Others), Crop Type, and Regional Forecast, 2020–2027. Available Online at: https://www.fortunebusinessinsights.com/industry-reports/biofertilizers-market-100413 (Accessed 2 Jun 2021).
- Bio-composting oil palm waste for improvement of soil fertility. Cham: Springer; 2014. p. :209-243.
- Microbial biotechnology: fundamentals of applied microbiology. Cambridge University Press; 2007.
- Nutrition transition and the structure of global food demand. Am. J. Agric. Econ.. 2019;101(2):383-403.
- [Google Scholar]
- ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol.. 2019;10:1506.
- [Google Scholar]
- Hamzah, M.M., 2008. The production of ecofiber from palm oil empty fruit bunch (EFB) (Doctoral dissertation, UMP).
- Han, J., Kim, J., 2018. Process simulation and optimization of 10-MW EFB power plant. In Computer Aided Chemical Engineering (Vol. 43, pp. 723-729). Elsevier.
- Harith, Z.T., Ibrahim, N.A., Yusoff, N.O.R.A.Z.I.L.A., 2014. Isolation and identification of locally isolated lignin degrading bacteria. J. Sustain. Sci. Manag. 9(2), 114–118.
- Mixed composting of palm oil empty fruit bunch (EFB) and palm oil mill effluent (POME) with various organics: an analysis on final macronutrient content and physical properties. Waste Biomass Valorization. 2020;11(10):5539-5548.
- [Google Scholar]
- The growth and reproduction of Eisenia fetida and Eudrilus eugeniae in mixtures of empty fruit bunch and palm oil mill effluent. Compost Sci. Utiliz.. 2014;22(1):40-46.
- [Google Scholar]
- Hsu, S., 2010. IAA production by Streptomyces scabies and its role in plant microbe interaction.
- Formulation of biochar-compost and phosphate solubilizing fungi from oil palm empty fruit bunch to improve growth of maize in an ultisol of Central Kalimantan. J. Ecol. Eng.. 2018;19(6):45-55.
- [Google Scholar]
- Combustion characteristics of Malaysian oil palm biomass, sub-bituminous coal and their respective blends via thermogravimetric analysis (TGA) Bioresour. Technol.. 2012;123:581-591.
- [Google Scholar]
- Maize initial growth with the inoculation of plant growth-promoting bacteria (PGPB) under different soil acidity levels. Aust. J. Crop Sci.. 2015;9(4):271-280.
- [Google Scholar]
- Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum & Thonn: Evaluation of plant growth promotion and antioxidant activity under salt stress. J. Appl. Res. Med. Aromat. Plants. 2016;3(2):71-77.
- [Google Scholar]
- Kalayu, G., 2019. Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int. J. Agron. (2019).
- Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol.. 2016;66(1):35-42.
- [Google Scholar]
- Plants-Nematodes-Microbes Crosstalk within Soil: A trade-off among friends or foes. Microbiol. Res.. 2021;248:126755.
- [CrossRef] [Google Scholar]
- Plant-Microbe Interactions under Adverse Environment. In: Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I. Singapore: Springer; 2020. p. :717-751.
- [Google Scholar]
- Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep.. 2019;9(1):1-14.
- [Google Scholar]
- Role of beneficial microorganisms in abiotic stress tolerance in plants. In: Plant Tolerance to Environmental Stress. CRC Press; 2019. p. :327-348.
- [Google Scholar]
- Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol.. 2020;23:101487.
- [CrossRef] [Google Scholar]
- Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and-mobilizing microbes: A review. Pedosphere. 2021;31(1):43-75.
- [Google Scholar]
- 1-Aminocyclopropane-1-carboxylate (ACC) deaminase-producing endophytic diazotrophic Enterobacter sp. EN-21 modulates salt–stress response in sugarcane. J. Plant Growth Regul.. 2018;37(3):849-858.
- [Google Scholar]
- Effects of organophosphate pesticides on siderophore producing soils microorganisms. Biocatal. Agric. Biotechnol.. 2019;21:101359.
- [CrossRef] [Google Scholar]
- Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE. 2016;11(3):e0152478.
- [CrossRef] [Google Scholar]
- Lai, C.M.T., Chua, H.B., Danquah, M.K., Saptoro, A., 2017, June. Isolation of thermophilic lignin degrading bacteria from oil-palm empty fruit bunch (EFB) compost. In: IOP conference series: materials science and engineering (Vol. 206, No. 1, p. 012016). IOP Publishing.
- Morphological and chemical nature of fiber strands of oil palm empty-fruit-bunch (OPEFB) BioResources. 2007;2(3):351-362.
- [Google Scholar]
- Why microbes secrete molecules to modify their environment: the case of iron-chelating siderophores. J. R. Soc. Interface. 2019;16(150):20180674.
- [CrossRef] [Google Scholar]
- A potential bioconversion of empty fruit bunches into organic fertilizer using Eudrilus eugeniae. Int. J. Environ. Sci. Technol.. 2015;12(8):2533-2544.
- [Google Scholar]
- Effects of microbial additive on the physiochemical and biological properties of oil palm empty fruit bunches compost. J. Eng. Sci. Technol.. 2015;5(1):10-18.
- [Google Scholar]
- The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers. Manage.. 2017;141:285-298.
- [Google Scholar]
- Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron.. 2020;162:31-87.
- [Google Scholar]
- Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res.. 2017;24(4):3315-3335.
- [Google Scholar]
- Malaysian Palm Oil Board (MPOB) (2021). Uses in Food and Non-Food Applications of Palm Oil. Available Online at: http://www.palmoilworld.org/applications.html (Accessed 01 Jun 2021)
- Malaysian Palm Oil Board (MPOB) (2020). Overview of the Malaysian Oil Palm Industry 2020. Available At: http://palmoilis.mpob.gov.my/V4/wp-content/uploads/2020/03/Overview_of_Industry_2018.pdf (Accessed 1 Jun 2021).
- Inorganic phosphate solubilization by a novel isolated bacterial strain Enterobacter sp. ITCB-09 and its application potential as biofertilizer. Agriculture. 2020;10(9):383.
- [Google Scholar]
- The value of composting in Germany-Economy, ecology, and legislation. Waste Manage.. 2013;33(3):536-539.
- [Google Scholar]
- Phylogenetic analysis of halophyte-associated rhizobacteria and effect of halotolerant and halophilic phosphate-solubilizing biofertilizers on maize growth under salinity stress conditions. J. Appl. Microbiol.. 2020;128(2):556-573.
- [Google Scholar]
- Exploring chemical analysis of vermicompost of various oil palm fibre wastes. Environmentalist. 2010;30(3):273-278.
- [Google Scholar]
- Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal. Agric. Biotechnol.. 2019;21:101326.
- [CrossRef] [Google Scholar]
- Pyrrolnitrin and hydrogen cyanide production by Pseudomonas chlororaphis strain PA23 exhibits nematicidal and repellent activity against Caenorhabditis elegans. PLoS ONE. 2015;10(4):e0123184.
- [CrossRef] [Google Scholar]
- Microbe-mediated enhancement of nitrogen and phosphorus content for crop improvement. In: Crop Improvement through Microbial Biotechnology. Elsevier; 2018. p. :293-304.
- [Google Scholar]
- Oil palm (Elaeis guineensis) biomass in Malaysia: the present and future prospects. Waste Biomass Valorization. 2019;10(8):2099-2117.
- [Google Scholar]
- In vitro and in vivo analyses of plant-growth-promoting potential of bacteria naturally associated with spruce trees growing on nutrient-poor soils. Appl. Soil Ecol.. 2020;149:103538.
- [CrossRef] [Google Scholar]
- Effect of organic manures and biofertilizer on plant growth, yield and quality of horticultural crop: A review. Int. J. Chem. Stud.. 2017;5(1):217-221.
- [Google Scholar]
- Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. Recent Dev. Appl. Microbiol. Biochem. 2019:223-233.
- [Google Scholar]
- Research and Markets, 2021. Biofertilizers Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021-2016. Available Online at: https://www.researchandmarkets.com/reports/5263929/biofertilizer-market-global-industry-trends (Accessed 1 Jun 2021).
- Biofertilizer, a way towards organic agriculture: A review. Afr. J. Microbiol. Res.. 2014;8(24):2332-2343.
- [Google Scholar]
- Chemical and physical characterization of oil palm empty fruit bunch. Malaysian J. Anal. Sci.. 2017;21(1):188-196.
- [Google Scholar]
- A review of palm oil biomass as a feedstock for syngas fuel technology. Jurnal teknologi. 2015;72(5)
- [Google Scholar]
- Effective composting of empty fruit bunches using potential Trichoderma strains. Biotechnol. Rep,. 2017;13:1-7.
- [Google Scholar]
- From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: status and needs. Front. Bioeng. Biotechnol.. 2020;7:425.
- [Google Scholar]
- Diversity and characterization of lignocellulolytic fungi isolated from oil palm empty fruit bunch, and identification of influencing factors of natural composting process. Waste Manage.. 2019;100:128-137.
- [Google Scholar]
- Microbial biofertilizers: types and applications. In: Biofertilizers for Sustainable Agriculture and Environment. Cham: Springer; 2019. p. :1-19.
- [Google Scholar]
- Trisakti, B., Lubis, J., Husaini, T., 2017, March. Effect of turning frequency on composting of empty fruit bunches mixed with activated liquid organic fertilizer. In: IOP Conference Series: Materials Science and Engineering (Vol. 180, No. 1, p. 012150). IOP Publishing.
- Trisakti, B., Mhardela, P., Husaini, T., Daimon, H., 2018, February. Production of oil palm empty fruit bunch compost for ornamental plant cultivation. In: IOP Conference Series: Materials Science and Engineering (Vol. 309, No. 1, p. 012094). IOP Publishing.
- Soil and transport factors in potential distribution systems for biofertilisers derived from palm oil mill residues in Malaysia. Comput. Electron. Agric.. 2019;166:105005.
- [CrossRef] [Google Scholar]
- Microbial biofertilizer: A potential tool for sustainable agriculture. In: Microorganisms for green revolution. Singapore: Springer; 2017. p. :25-52.
- [Google Scholar]
- Abscisic acid and other plant hormones: methods to visualize distribution and signaling. BioEssays. 2015;37(12):1338-1349.
- [Google Scholar]
- Oil palm fibers as papermaking material: Potentials and challenges. BioResources. 2011;6(1):901-917.
- [Google Scholar]
- Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J.. 2016;4(3):162-176.
- [Google Scholar]
- Effects of temperature, aeration rate and reaction time on composting of empty fruit bunches of oil-palm. Iranica J. Energy Environ.. 2016;7(2):156-162.
- [Google Scholar]
- Characterization of culturable bacterial endophytes and their capacity to promote plant growth from plants grown using organic or conventional practices. Front. Plant Sci.. 2015;6:490.
- [Google Scholar]
- Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol.. 2019;37(1):89-93.
- [Google Scholar]
- Effect of adding palm oil mill decanter cake slurry with regular turning operation on the composting process and quality of compost from oil palm empty fruit bunches. Bioresour. Technol.. 2010;101(22):8736-8741.
- [Google Scholar]
- Microbes for Legume Improvement. Cham: Springer International Publishing; 2017. p. :175-197.
- [CrossRef]







