Translate this page into:
Fly ash properties, characterization, and applications: A review
⁎Corresponding author at: Department of Chemistry, College of Science, King Saud University, Riyadh, Saudi Arabia. salterary@ksu.edu.sa (Seham S. Alterary)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Fly ash (FA) is the principal industrial waste byproduct from the burning of solid fuels. FA is a powdery solid that is constituted mostly of unburned carbon (UC), metal oxides (Si, Fe, Ca, and Al), and other inorganic substances. UC is an inexpensive source of activated carbon that plays an important role in FA adsorption capacity. Due to the broad variability in its composition, FA characterization is challenging. Accordingly, FA is categorized into class F, and class C according to the maximum and minimum % of SiO2, Al2O3, Fe2O3, and SO3. X-ray diffraction, and fluorescence, and scanning microscopy with an energy dispersive spectroscopy are the common techniques employed to characterize FA. FA was used to remove hazardous contaminants, organic and inorganic chemicals, and dyes from wastewater. Furthermore, investigations revealed that FA has promising potential beneficial usage in the construction industry, particularly in cement and concrete production. FA has been added to cement in a reduced nanosize form giving good durability and minimizing concrete pores size to resist adverse environment. In this article, significant properties, characterization methods and, applications of FA were summarized.
Keywords
Fly ash
Nano
Unburned carbon
Hazardous contaminants
Coal combustion
- ASTM
-
American society for testing and material
- EDXA
-
Energy dispersive x-ray spectroscopy
- FA
-
fly ash
- LOI
-
Loss on ignition
- SEM
-
scanning electron microscope
- UC
-
Unburned carbon
- XRD
-
X-ray diffraction
- XRF
-
X-ray fluorescence
Abbreviations
1 Introduction
Coal fly ash is primarily produced in power plants as a byproduct of coal combustion. As shown in Fig. 1, the by-products of coal ash combustion include fly and bottom ash, boiler slag, and flue gas. FA particles, the main by-product, are fine powdery particles, and heterogeneous. During coal combustion, FA particles were carried usually aside by the aid of flue gas and detained by precipitators either electrostatic or mechanical. Molten droplets formed by non-organic matter in the coal at a temperature of around 1500 °C were then solidified. This process forms solid glassy, and hollow spheres with a smooth surface, and their size range from 0.5 to 200 μm. FA composition differs drastically according to the burned coal type, conditions, cooling control, and combustion. The broad diversity of the FA constitution makes it an arduous material to characterize. Therefore, FA was classified into two main groups class C and class F fly ash as claimed by the American Society for Testing and Materials (ASTM) (ASTM, 2008). Class F fly ash is pozzolanic brought out from either anthracite or bituminous burning of coal. The total amount of SiO2, Al2O3, and Fe2O3 must be greater than 70%. Class C ashes are pozzolanic, and cementitious produced from sub-bituminous or lignite coal burning. The overall quantity of Fe2O3, Al2O3, and SiO2 must be greater than 50% as stated by the ASTM. The bulk of metal oxides content represents the basis for most of the characterization techniques for FA class C, and F. Yet, FA with more than 20% calcium oxide was classified as class C, and lower calcium content as class F (Bentz et al., 2011). Furthermore, we can distinguish between classes C and F based on the percentage CaO, with class C being greater than 10% and class F is less than 10%.
Coal ash by-products resulting from the coal combustion.
FA, as mentioned earlier, is a pozzolanic material that is silica or silica-alumina based, allowing its incorporation in concrete as a partial substitution for cement (McCarthy et al., 2019). FA is composed of three main components: (1) organic materials, (2) inorganic materials constituted of amorphous and crystalline phase, and (3) fluid materials present in both organic and inorganic materials (ASTM and Materials, 2011). UC in FA indicates inefficiency in the combustion process representing an obstacle to the beneficial utilization in diverse applications. Moreover, UC characteristics determined the rank and type of coal (Hower et al., 2017). Remarkably, FA is utilized in many applications especially in the industry of concrete. Furthermore, FA is implemented in wastewater treatment, synthesis of zeolites, soil stabilization, and nano fly ash to improve its properties (Mushtaq et al., 2019a, 2019b). Fig. 2 showed the number of publications per year since 2000 according to google scholar explaining the tremendous interest increase using FA in many applications including wastewater treatment, soil amelioration, synthesis of zeolites, concrete industry, and ceramic industry. Coal production is not limited to one region worldwide. Moreover, the produced coal is used within its home country; only around 15% according to the world coal association is destined for the international market. Fig. 3 explains the differences in the amount of coal produced in 2018 worldwide per region. The FA is characterized utilizing a discrete approach including scanning electron microscopy (SEM) with energy dispersive spectroscopy (EDAX), X-ray fluorescence (XRF), X-ray diffraction (XRD), and particle size distribution. This review article provides a survey on FA composition, applications, and characterization techniques.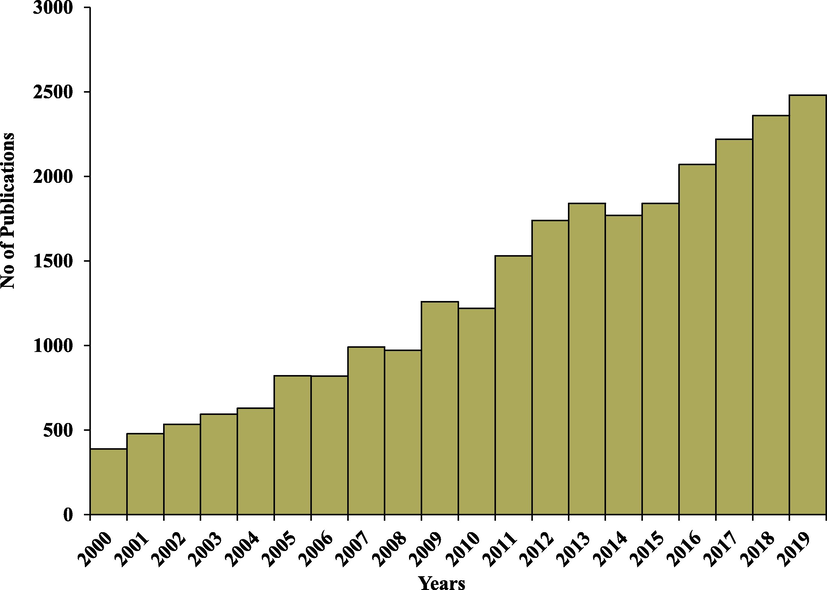
Number of publications of fly ash per year from 2000 to 2019 according to google scholar.
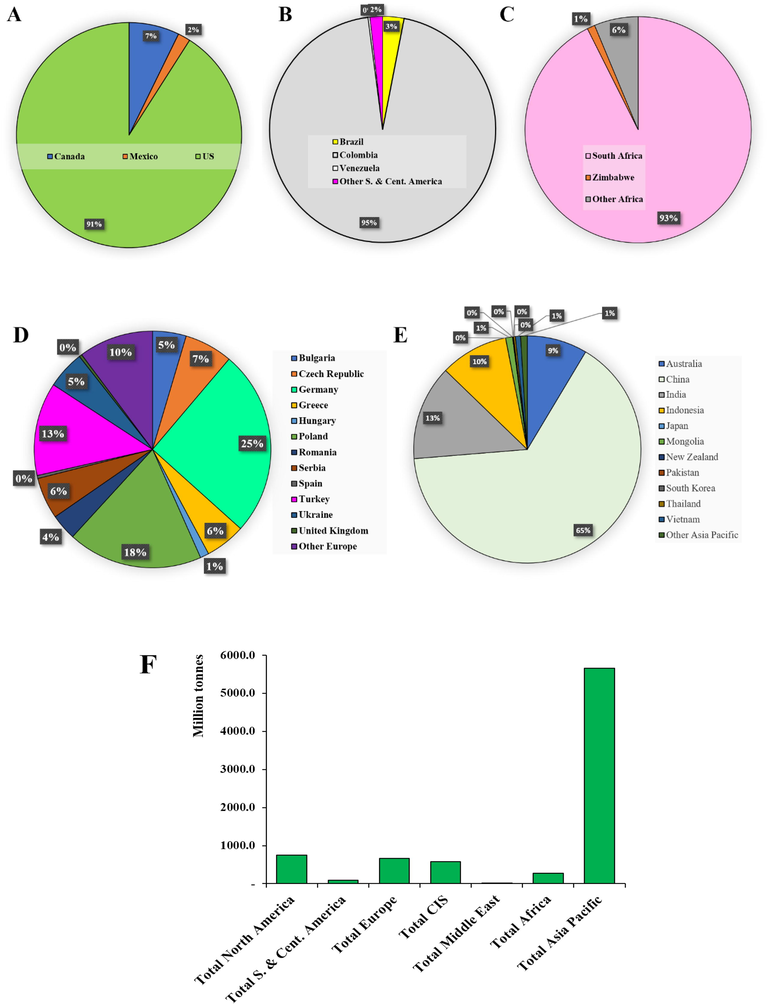
Explain the differences in the amount of coal production worldwide in 2018 whereas (A) Total North America, (B) Total S. & Cent. America, (C) Total CIS, (D) Total Europe, (E) Total Asia, and (F) is a comparison between all the totals in addition to the middle east region. This data is according to the Statistical Review of World Energy.
2 Fly ash composition
The FA mineralogical makeup is mainly represented by the crystalline and glass mineral components in the inorganic amorphous phase as well as partially or unburned black carbon (Dai et al., 2012). According to the formation time, FA is classified into primary, secondary, and tertiary phases. Primary phases encounter no change. During the combustion of coal, the secondary phases including the oxides and silicates are formed. Moreover, the tertiary phases are created through the transport of FA comprising portlandite and gypsum. The principal elements of FA are trace elements, silicon, oxygen, calcium, aluminum, carbon, iron, magnesium, potassium, hydrogen, titanium, sodium, phosphorus, nitrogen, and barium (Vassilev and Vassileva 2007). The cooling rate of FA particles affects the inorganic matter formation. A fast-cooling results in the formation of small glassy particles, while slow cooling results in large crystalline particles. The inorganic matter consisting of amorphous and crystalline phases represents the bulk composition of FA. FA is classified and categorized also according to its oxide content. The CaO, SiO2, Al2O3, and Fe2O3 make up most of FA. As CaO increases, SiO2, Al2O3, and Fe2O3 decrease. However, when CaO increases, the alkalis including Na2O and K2O as well as SO3 increase. Moreover, the density of the fly ash can be determined from iron and carbon content. Thus, when the C content increase in FA, its density decrease, and the more Fe, the more the density of FA. Additionally, the water demand and workability in fly ash are affected by the carbon content affects. The carbon content differences between FA classes are performed by calculating the loss on ignition (LOI). LOI is higher when more unburnt carbon is present. Consequently, Class F (LOI) is higher than Class C. Fig. 4 summarizes the main differences between class C and F as claimed by ASTM (Hower et al., 2001).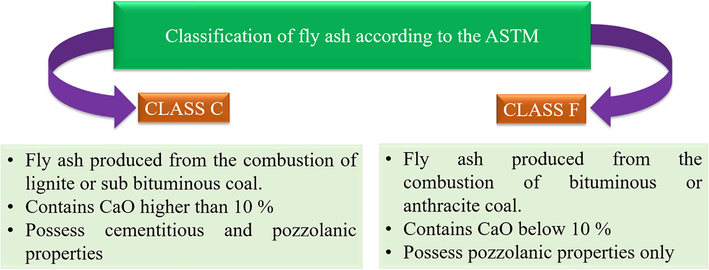
FA classification according to ASTM illustrating the main differences.
2.1 Crystalline phases
FA crystalline phases account for 5 to 50% of its mass. The crystalline phases consist of ten phases. Anhydrite (CuSO4), Merwinite, Periclase, and C3A are found in class C fly ash. In addition to mullite, melite, quartz, hematite (Fe2O3), magnetite (Fe3O4), and lime. Anhydrite results from O2, SO2, and CaO presence in the furnace, and it increases by the increase of SO3. Moreover, anhydrite is mainly involved with the formation of ettringite. Mullite is not reactive and is found more in Class F fly ash. However, quartz is present in all fly ash types. Melite and merwinite are related to MgO content which is a solidified solution of gehlenite and akermanite. Periclase and lime are crystalline MgO, and CaO, respectively. Lime has a crucial role in taking part in hydration reactions as it is found in all FA types. Lime also increases the pH to greater than 12 by dissolving. C3A is responsible for sulfate expansion. Crystalline phases in FA are recognized by using XRD to differentiate between the reactive and stable phases. The most exposed reactive phases to sulfate attack include C3A, anhydrite, and periclase, while in sulfate or hydration reactions stable phases do not occur.
2.2 Amorphous phases
Amorphous phase particles are circular with a diameter size range in size within 1 to 5 μm that are formed by quenching particles causing their disorder. This disorder ends up in a crystal structures lack, making them difficult to characterize. A low level of calcium in parent coal builds up aluminosilicate glass, while a high calcium content results in calcium aluminosilicate glass. The latter is considered as a known reactive phase in FA Class C lead to its high reactivity compared to class F. total amorphous phases are known by deducting total crystalline from the total FA mass (Ash, Kruse et al.)
2.3 Black carbon (Unburned carbon)
Unburned carbon (UC) shows inefficiency in the combustion process. UC represents a barrier to the advantageous FA applications in a diversity of applications (Styszko-Grochowiak et al., 2004). Coal-derived UC characteristics are the main function for determining the type, the rank of coal, combustion conditions, and the coal feed size. The UC utilization as a substitute for cement is restricted by its potential of air-entraining agent absorption. Accordingly, limitations were imposed to decide the tolerable UC concentrations. However, several benefits may result in UC utilization processes making it a valuable part of fly ash with a diverse range of applications (BartoĖová et al., 2011). These potential properties of UC include: (a) the adsorption of organic compounds like dyes, phenols, biphenyls polychlorinated, constituents of petroleum, along with herbicides (b) the technique for the capture of valuable and toxic elements especially the flue gas mercury, and (c) an inexpensive source of activated carbons, cokes in the metallurgical industry, production of graphite manufacture, and, returning carbon to the boiler (BartoĖová et al., 2011; Batra et al., 2011).
3 Fly ash testing and characterization techniques
3.1 X- ray diffraction (XRD)
XRD is applied to identify qualitatively the fly ash crystalline phases. XRD works by using electrons to hit the solid sample which then emits x-rays. Once hitting a crystal structure, different behavior for the x-rays can be examined. Some diffract based on the crystallinity of the structure; however, other types of x-rays will perforate deep inside the sample up to colliding with a crystal. The X-ray instrument scan angles of scattered and diffracted patterns as well as measuring their intensities to create a pattern. A diffractogram is constructed by plotting the produced patterns to link diffraction angle to diffracted intensity.
3.2 X-ray fluorescence (XRF)
XRF is used to evaluate bulk oxide contents in all types of fly ash. Besides, XRF is frequently employed to find out the amorphous phase content. This was performed as explained earlier by deducting the crystalline phase from the content of oxide measured. FA is excited with excessive energy x-rays which emit either fluorescent or secondary x-rays. both quantitatively together with qualitatively identification of FA oxide contents employed secondary x-rays (Kruse et al., 2013).
3.3 Particle size distribution
Laser diffraction is usually employed in FA particle size measurement. Laser diffraction instruments employ laser for drawing FA particle size distribution as well as another sample type. In the light beam electron cloud is removed by an electron causing light scattering due to the interaction of the material and the light beam, and then that light is re-emitted. This scattering is resulted from diffraction, refraction, and reflection of the light due to this reaction. However, the light also can be absorbed (Veranth, et al., 2000). Based on the diffraction pattern, the machine analyzes the interaction of the light and the material particles and matches the analyzed pattern according to a mathematically calculated model. The model was roughly calculated by employing the Fraunhofer or Mie theory. The diffracted light beam is focused using a Fourier transform lens to obtain electric signals from incident energy. Particle size is related to the angle between the incident and diffracted light is related (Cyr et al., 2001). The recommended dispersive agent is isopropyl alcohol due to its increased reproducibility and high viscosity (Ferraris, et al., 2006). A broader particle size distribution indicates a greater packing density of the mixture and hence, resulting in greater workability (Joshi et al., 1984; Lohtia and Joshi, 1996).
3.4 Scanning electron microscopy (SEM)
SEM is utilized to identify FA amorphous phase composition. SEM works by using an electron beam that penetrates deep into a sample with about 1 μm. The beam of the SEM centre toward a point, followed by signals reading by detectors that were then converted into intensities to create an image on the attached computer. In a secondary electron signal image, surface topography information is produced by these signals. The signals are created due to the hitting of valence electrons by a beam, which is accordingly emitted from the atom. The valence electrons are attracted to a detector. The latter produces a signal which is proportional to the number of attracted electrons (Chancey 2008).
An energy-dispersive x-ray analyser (EDXA) was performed using a computer program to point count the particles of the amorphous phases. This happened when x-rays are emitted, as the beam hits the specimen surface. These emitted x-rays are characteristic of the sample’s elemental composition. On that account, the EDXA technique is used to identify the elemental composition of the amorphous particles. The amorphous particles are spherical particles with a size range from 1 to 5 μm. in diameter. A built-in measuring tool is used to identify the size of the amorphous particles. Only particles with diameter sizes from 1 to 5 μm. in diameter were selected for the EDAX point analysis. However, analysis of amorphous particles with a size ranges from 150 to 200 μm. constituted a comprehensive analysis of the fly ash sample. These particles were investigated quantitatively for eight main elements: iron, oxygen, calcium, aluminium, potassium, sodium, carbon, and silicon. These eight elements were converted into oxides, and then each point was plotted for further analysis on a ternary diagram (Kruse et al., 2012, 2013; Sambangi, 2021).
4 Applications of fly ash
4.1 Synthesis of zeolites
Zeolite synthesis is gaining great interest as one of the most important and effective ways for FA usage. Thus, is due to composition similarities between fly ash and some volcanic materials. The latter is the precursor for naturally occurring zeolites. Zeolite synthesis is developed under alkaline conditions using hydrothermal crystallization (Querol et al., 2002). Although, quartz and mullite present in ash are difficult to dissolve and inert. Zeolite synthesis by an alkali hydrothermal reaction is performed on three main steps, dissolution, condensation, and crystallization (Yao and Potential Environmental Impacts. Nova Science Publishers, 2013; Yao et al., 2015). The hydrothermal reaction of FA in LiOH·H2O solution was tested and found to promote the dissolution of inert phases by acting as a strong activator (Yao and Potential Environmental Impacts. Nova Science Publishers, 2013). Zeolites ABW type phases contents augment at the expense of mullite and quartz with alkaline solution concentration increase. But recently, the method used was improved by utilizing more advanced treatments counting on the alkaline fusion followed by hydrothermal treatment, and sometimes by using microwave-assisted zeolite synthesis treatment (Rayalu, et al., 2000; Querol et al., 2002; Yao et al., 2015). Na-X zeolite from coal fly ash has been manufactured by FA fusion with NaOH preceding hydrothermal reaction (Yao et al., 2009). Zeolite Li-ABW is Another type fabricated by alkaline fusion followed by hydrothermal treatment in a medium of LiOH·H2O (Yao et al., 2009).
4.2 Concrete industry
In the last decades, fly ash-based geopolymer has arisen as a propitious new cement alternative in the field of construction and building materials. FA has been widely used as a raw material substitute in the construction industry or as an additive in the cement industry. FA Class C has both cementitious (high CaO content) and pozzolanic properties, while Class F has only pozzolanic properties (Hou, et al., 2013). The fly ash pozzolanic properties render it beneficial for the replacement of cement in concrete (Nonavinakere and Reed, 1995; Yao et al., 2015). The FA silica reacts with calcium hydroxide released by CaO hydration producing Ca2SiO4 hydrate (González et al., 2009). The bleeding of freshly mixed concrete decreased, and its workability improved by the inclusion of FA in the binder. The hydration heat can be reduced by a partial cement replacement with Class F and hence, the concrete cracking risk in the early stages (González et al., 2009; Sarker, 2009; Yao et al., 2015). FA can ameliorate the long-term concrete durability by decreasing the ingress of aggressive agents such as Cl- (Nath, 2011). Hardened FA/concrete manifests augmented strength with low permeability when appropriately designed (Taylor, 1997; Maroto-Valer et al., 2001). Furthermore. Production costs can be reduced by the partial replacement of cement with fly ash. Generally, the amount of fly ash to be added ranges from 15 to 35 wt% and can even reach 70 wt% for concrete in constructions such as walls, parking lots, and pavements. Yet, it can reach 80 wt% in autoclaved aerated concrete (Dilmore, 2001). In India, about 45.5% of FA produced was used in the cement and concrete industry in 2011, while in China approximately 41% were used (Yao et al., 2015). The use of high levels of low lime FA as a cement component in the concrete industry has been tested and the results showed that FA levels up to 45% could be added with Portland cement leading to a range of practical concrete strengths design, even though to some extent a reduction in the early strength occurred (McCarthy, 2005).
4.3 Water treatment
The demand for obtaining clean water from untreated wastewater from the pollution of groundwater and industries, has increased due to the need for a sustainable society. Accordingly, cheap treatment methods for water lead to the interest in recycling fly ash waste. Fly ash can be used in an effective way to treat domestic wastewater with simple techniques and low-cost adsorbents (Akar, 2010; Aljerf, 2018a, 2018b). Fly ash wastewater treatment methods are economical, environmentally friendly, and efficient (Mushtaq et al., 2019). As mentioned earlier, unburned carbon in fly ash allows the adsorption of organic compounds such as phenols, dyes, toxic metals, herbicides, petroleum constituents, and other inorganic pollutants from wastewater (Hower et al., 2017). A severe warning to public health is toxic heavy metals in wastewater (Wang et al., 2016; Aljerf, 2018a, 2018b; Tauanov et al., 2018). Adsorption has been a simple and efficient technique among many removal methods for the uptake of heavy metal ions by using fly ash as a low cost as well as effective adsorbent. The first reported employment of fly ash for the removal of heavy metals from industrial wastewater was interpreted at 1975 by Gangoli et al (Wang, 2006). The dispersion of fly ash in water results in an alkaline pH from 10 to 13. At high pH, the fly ash becomes negatively charged allowing the removal of heavy metal ions by precipitation and electrostatic adsorption from water (Cho et al., 2005). Fly ash can be modified or transformed into new materials for heavy metal ions adsorption. The high content of Al2O3 and SiO2 in class F fly ash make it suitable for conversion to other substrates with high-capacity adsorption. Class F fly ash has been chemically altered into a substrate having a surface with high polarity by hydrothermal treatment and showed a complex adsorption process from a pollutant system containing multi cations (cadmium, lead, and zinc together) (Visa et al., 2012). The adsorption application area can be expanded by the modification of fly ash to synthetic zeolites with various metals, metal oxides, and hydroxides (Goscianska, et al., 2018; Tauanov et al., 2018). Synthetic zeolites derived from fly ash contain a zeolite fraction which is carrying a negative charge, and a non-zeolite fraction containing Fe2O3, Al2O3, and CaO. These fractions are responsible for the removal of anionic and cationic heavy metals pollutants. The zeolite fraction is responsible for cationic pollutants removal, while the non-zeolites fraction is responsible for the removal of anionic pollutants from water (Xie et al., 2014). Zeolites have unique physical and chemical properties such as thermal stability, crystallinity, and ion exchange capacity (Nascimento et al., 2009). Removal of metal ions by synthetic zeolite, zeolite NaeP1, zeolite X, Ag-doped derived zeolite, and zeolite material synthesized by hydrothermal modification has been reported in the literature (Jha et al., 2009; Nascimento et al., 2009; Cardoso et al., 2015; Visa et al., 2016; Tauanov et al., 2018). Another interesting application of fly ash in wastewater is fly ash-based membrane filters. Ceramic, inorganic, and porous membranes have received great attention due to their high chemical, thermal, and mechanical stability, rugged structure, low energy consumption, environmental friendliness, long time of operation with high selectivity, separation efficiency, and the ability of membrane regeneration by backflushing (Dong et al., 2006; Jedidi et al., 2009; Lim et al., 2009; Abadi, et al., 2011; Cao et al., 2014). The main disadvantage of the ceramic membrane is the limited availability of raw materials such as ZrO2, SiO2, Al2O3, TiO2, and other oxides as well as high cost confined their practical applications over the polymeric membrane (Van Gestel et al., 2006, 2008; Cao et al., 2014). In light of the high content of SiO2 and Al2O3 in fly ash, many efforts have been made for the fabrication of microporous inorganic membrane filters from it for large volumes of effluents treatment (Dong et al., 2009; Cao et al., 2014).
4.4 Ceramic industry
Fly ash contains a great number of metal oxides Al2O3, SiO2, Fe2O3, and CaO. For the ceramic industry, the FA metal oxides are considered as low-cost materials to be used. Also, the FA fine powdery structure makes it suitable to be combined directly and easily with almost no pretreatment in ceramic pastes (Erol et al., 2008). Researchers tested the production of stirred materials and glass-ceramics from fly ash based on the temperature activation of the raw FA. The basis of this glass or ceramic manufacture is due to the variations in the temperature and coregents directing their final form. Glass-ceramic materials based on Al2O3, Li2O, and SiO2 ternary systems have significant industrial applications due to their negative and low thermal expansion coefficient. Still, the product is very expensive by the usage of these high-grade reagents (Yao et al., 2015). Recently, coal bottom ash has been used to prepare Li2Al2Si3O10 and the results showed that the thermal expansion coefficient value was 18% smaller than for the Li glass–ceramic fabricated commercially (Kniess et al., 2007). Further, Li2Al2Si3O10 was prepared using FA as a precursor and they found that the product was with small grain size and well crystallized (Yao et al., 2012). Cordierite occurs in 3 different polymorphic forms and has an orthorhombic structure. The form with high temperature is known as indialite. Cordierite ceramic is considered a candidate for structural materials due to its low thermal expansion coefficient, high refractoriness, and low dielectric constant. Cordierite is employed in many applications including gas turbine engines heat exchangers, catalyst carriers for purification of exhaust gas, refractory metal coatings, and electronic packing materials. Recently, FA is used in the manufacture of cordierite instead of kaolinite (He et al., 2005; Yao et al., 2015).
4.5 Fly ash impact on soil systems
FA can find a better application for improvement of degraded/marginal soil if combined with organic amendments such as organic compost, press mud, cow and farmyard manure, sewage and paper factory sludge, and crop residues (Sajwan et al., 2003; Kumpiene et al., 2007; Shen et al., 2008). Few beneficial combined effects of FA and organic matter possess few advantageous properties on the soil. FA is lethal for pathogens in the sludge, reduced the availability of heavy metal, and enhance soils by its better texture, higher nutrient concentrations, mass moisture content, higher porosity, and content of fine-grained minerals improved the biological activity in the soil. A major limitation for the use of FA in agriculture boron toxicity which inhibits microbial respiration. But this problem can be prevented by mixing alkaline FA with highly carbonaceous acidic material to make compost for soil treatment (Tripathi et al., 2000; Adriano et al., 2002).
Dolomite and lime are used commonly for soil acidity amelioration. However, they are not environmentally friendly and take a long time to improve soil‘s physical properties and structure. The physicochemical properties of FA including high water holding capacity, source of essential plant nutrients, favorable pH, low bulk density, and clay-sized particles. Moreover, the high CO2 emission during calcite calcination to produce lime can be reduced when using FA instead of lime decreasing global warming (Tripathi et al., 2000; Ram et al., 2006; Pandey et al., 2009). Few beneficial combined effects of FA and organic matter on soil have been found such as reduced heavy-metal availability and killing pathogens in the sludge improved soils through higher nutrient concentrations, better texture, lower bulk density, higher porosity, and mass moisture content and higher content of fine-grained minerals enhanced the biological activity in the soil reduced the leaching of major nutrients and beneficial for vegetation (Wong, 1995; Rautaray et al., 2003; Sajwan et al., 2003; Kumpiene et al., 2007; Shen et al., 2008). The changes occurring in the soil pH are based on the pH fly ash, the fly ash neutralizing capacity and the buffering capacity of the soil. As a result, FA can be utilized for soil buffering by being alkaline (Molliner, 1982; Manoharan et al., 2010). Additionally, due to the high content of CaO (≥10%), Class C fly ash can raise pH and accordingly has been applied at absurdly high rates. On the other hand, Class F ashes contain mostly low CaO content lower than 10% creating potential limitations in soil acidity amelioration. When two applications of acidic and alkaline FA (Class F) were tested, results revealed a low ameliorating effect on soil acidity, except for ash with a relatively high CaCO3 equivalent. This alkaline ash raised the soil pH by 2 units with additions at 36 tonnes/ha. The amelioration of soil pH ability by FA has a massive role in leading vast areas of soils, including wastelands, non-productive lands, and mine spoils (Ram, 2010, 2014). Besides, FA contains useful nutrients for the soil including S, P, Cu, K, Ca, Zn, Mg, and Mg enhancing plant growth. Researchers have found more and more interesting and advantageous benefits for the use of FA in soil including aeration, improving the soil texture, percolation, reducing soil bulk density, and works as an insecticide due to the presence of Silica (Ram et al., 2006, 2007; Basu, et al., 2009; Ram, 2010, 2014; Shaheen et al., 2014).
5 Conclusion
FA the main by-product resulted from coal combustion attained the interest of many scientists nowadays due to its powerful properties and applications. Fly as is classified to Class C and Class F where the loss of ignition in class F is higher than Class C. Also, Class C fly ash contains a higher amount of CaO. FA was used to eliminate toxic materials, organic and inorganic compounds, and for the removal of dyes in wastewater treatment. In addition, FA has potential beneficial uses in construction industries, especially in cement and concrete manufacturing. FA has been added to cement in a reduced nanosize form giving good durability and minimizing concrete pores size to resist adverse environment. Zeolite synthesis was one of the most important and effective ways for FA applications. Moreover, FA was used for the improvement of degraded soil. FA is lethal for pathogens in the sludge, reduced the availability of heavy metals, and enhances the soil by its better texture.
Acknowledgment
“The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education “in Saudi Arabia for funding this research work through the project number IFKSURP-2020-135.”
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abadi, S. R. H., et al. (2011). “Ceramic membrane performance in microfiltration of oily wastewater.” 265(1-3): 222-228.
- Adriano, D., et al. (2002). “Effects of high rates of coal fly ash on soil, turfgrass, and groundwater quality.” 139(1-4): 365-385.
- Akar, S. T. and R. J. C. E. J. Uysal (2010). “Untreated clay with high adsorption capacity for effective removal of CI Acid Red 88 from aqueous solutions: Batch and dynamic flow mode studies.” 162(2): 591-598.
- Aljerf, L. J. D. i. b. (2018). “Data of thematic analysis of farmer׳ s use behavior of recycled industrial wastewater.” 21: 240-250.
- Aljerf, L. J. J. o. e. m. (2018). “High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: kinetics and equilibrium study.” 225: 120-132.
- ASTM, N. J. E. U. A. S. f. T. and Materials (2011). “C1157/C1157M, Standard Performance Specification for Hydraulic Cement, West Conshohocken, Pa.”
- BartoĖová, L., et al. (2011). “On unburned carbon in coal ash from various combustion units.”
- Basu, M., et al. (2009). “Potential fly-ash utilization in agriculture: a global review.” 19(10): 1173-1186.
- Batra, V. S., et al. (2011). “Value‐added products from unburned carbon in bagasse fly ash.” 6(5): 787-793.
- Comparison of ASTM C311 strength activity index testing versus testing based on constant volumetric proportions. J. ASTM Int.. 2011;9(1):1-7.
- [Google Scholar]
- Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc.. 2014;34(13):3181-3194.
- [Google Scholar]
- Cardoso, A. M., et al. (2015). “Synthesis of zeolite Na-P1 under mild conditions using Brazilian coal fly ash and its application in wastewater treatment.” 139: 59-67.
- Chancey, R. T. (2008). “Characterization of crystalline and amorphous phases and respective reactivities in a class F fly ash.”
- Cho, H., et al. (2005). “A study on removal characteristics of heavy metals from aqueous solution by fly ash.” 127(1-3): 187-195.
- “Particle size distribution of fine powders by LASER diffraction spectrometry. Case of Cementit. Mater.“. 2001;34(6):342-350.
- [Google Scholar]
- “Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit. 2012;90::72-99.
- Dilmore, R. M. and R. D. J. J. o. e. e. Neufeld (2001). “Autoclaved aerated concrete produced with low NO x burner/selective catalytic reduction fly ash.” 127(2): 37-50.
- Fabrication and characterization of low cost tubular mineral-based ceramic membranes for micro-filtration from natural zeolite. J. Membr. Sci.. 2006;281(1–2):592-599.
- [Google Scholar]
- Dong, Y., et al. (2009). “Reaction-sintered porous mineral-based mullite ceramic membrane supports made from recycled materials.” 172(1): 180-186.
- Erol, M., et al. (2008). “Characterization of sintered coal fly ashes.” 87(7): 1334-1340.
- Ferraris, C. F., et al. (2006). “Particle size distribution by LASER diffraction spectrometry: application to cementitious powders.” 100.
- Fly ashes from coal and petroleum coke combustion: current and innovative potential applications. 2009;27:976-987.
- Goscianska, J., et al. (2018). “Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash.” 513: 72-81.
- He, Y., et al. (2005). “Characterization of α-cordierite glass-ceramics from fly ash.” 120(1-3): 265-269.
- Hou, P.-k., et al. (2013). “Effects of colloidal nanosilica on rheological and mechanical properties of fly ash–cement mortar.” 35(1): 12-22.
- Hower, J. C., et al. (2001). “An approach toward a combined scheme for the petrographic classification of fly ash.” 15(5): 1319-1321.
- Jedidi, I., et al. (2009). “Elaboration of new ceramic microfiltration membranes from mineral coal fly ash applied to waste water treatment.” 172(1): 152-158.
- Jha, V. K., et al. (2009). “Zeolite formation from coal fly ash and heavy metal ion removal characteristics of thus-obtained Zeolite X in multi-metal systems.” 90(8): 2507-2514.
- Joshi, R., et al. (1984). “Scanning electron microscopy and X-ray diffraction analysis of various size fractions of fly ash.” 43.
- Kniess, C., et al. (2007). “Dilithium dialuminium trisilicate phase obtained using coal bottom ash.” 353(52-54): 4819-4822.
- Characterizing Fly Ash. The University of Texas, CTR Technical Report. 2012;6648(1)
- [Google Scholar]
- Kruse, K., et al. (2013). Characterizing fly ash.
- Kumpiene, J., et al. (2007). “Stabilization of Pb-and Cu-contaminated soil using coal fly ash and peat.” 145(1): 365-373.
- Lim, G.-T., et al. (2009). “Fabrication of a silica ceramic membrane using the aerosol flame deposition method for pretreatment focusing on particle control during desalination.” 238(1-3): 53-59.
- Mineral admixtures. Concrete admixtures handbook: Elsevier; 1996. p. :657-739.
- Manoharan, V., et al. (2010). “Assessments of Class F fly ashes for amelioration of soil acidity and their influence on growth and uptake of Mo and Se by canola.” 89(11): 3498-3504.
- Maroto-Valer, M., et al. (2001). “Characterization of differing forms of unburned carbon present in fly ash separated by density gradient centrifugation.” 80(6): 795-800.
- McCarthy, M. and R. J. F. Dhir (2005). “Development of high volume fly ash cements for use in concrete construction.” 84(11): 1423-1432.
- Pozzolanas Pozzolanic Mater.. 2019;363
- Molliner, A. and J. J. P. S. C. S. S. Street (1982). “Effect of fly ash and lime on growth and composition of corn (Zea mays L.) on acid sandy soils.” 41: 217-220.
- Possible applications of coal fly ash in wastewater treatment. J. Environ. Manage.. 2019;240:27-46.
- [Google Scholar]
- Mushtaq, F., et al. (2019). “Possible applications of coal fly ash in wastewater treatment.” 240: 27-46.
- Nascimento, M., et al. (2009). “Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method.” 88(9): 1714-1719.
- Nath, P. and P. J. P. E. Sarker (2011). “Effect of fly ash on the durability properties of high strength concrete.” 14: 1149-1156.
- Fly ash enhanced metal removal process. Inc, Lancaster, PA (United States): Technomic Publishing Co.; 1995.
- The Indian perspective of utilizing fly ash in phytoremediation, phytomanagement and biomass production. 2009;90:2943-2958.
- Ram, L. and R. J. E.-S. R. Masto (2014). “Fly ash for soil amelioration: a review on the influence of ash blending with inorganic and organic amendments.” 128: 52-74.
- Ram, L., et al. (2006). “Management of mine spoil for crop productivity with lignite fly ash and biological amendments.” 79(2): 173-187.
- Ram, L. C. and R. E. J. J. o. E. M. Masto (2010). “An appraisal of the potential use of fly ash for reclaiming coal mine spoil.” 91(3): 603-617.
- Ram, L. C., et al. (2007). “Leaching behavior of lignite fly ash with shake and column tests.” 51(7): 1119-1132.
- Rautaray, S., et al. (2003). “Effect of fly ash, organic wastes and chemical fertilizers on yield, nutrient uptake, heavy metal content and residual fertility in a rice–mustard cropping sequence under acid lateritic soils.” 90(3): 275-283.
- Rayalu, S., et al. (2000). “Highly crystalline faujasitic zeolites from flyash.” 77(1-3): 123-131.
- Sajwan, K., et al. (2003). “Assessing the feasibility of land application of fly ash, sewage sludge and their mixtures.” 8(1): 77-91.
- Sambangi, A. and E. J. M. T. P. Arunakanthi (2021). “Fresh and mechanical properties of SCC with fly ash and copper slag as mineral admixtures.” 45: 6687-6693.
- Sarker, P. K. J. M. and structures (2009). “Analysis of geopolymer concrete columns.” 42(6): 715-724.
- Shaheen, S. M., et al. (2014). “Opportunities and challenges in the use of coal fly ash for soil improvements–a review.” 145: 249-267.
- Soil improvement with coal ash and sewage sludge: a field experiment. 2008;53:1777-1785.
- Styszko-Grochowiak, K., et al. (2004). “Characterization of the coal fly ash for the purpose of improvement of industrial on-line measurement of unburned carbon content.” 83(13): 1847-1853.
- Tauanov, Z., et al. (2018). “Synthetic coal fly ash-derived zeolites doped with silver nanoparticles for mercury (II) removal from water.” 224: 164-171.
- Taylor, H. F. (1997). Cement chemistry, Thomas Telford London.
- Tripathi, R., et al. (2000). Reclamation of fly ash land fills by successive plantation, soil amendments and/or through biotechnological approach, Final Technical Report. Directorate of Environment, UP, India (unpublished ….
- Van Gestel, T., et al. (2006). “ZrO2 and TiO2 membranes for nanofiltration and pervaporation: Part 1. Preparation and characterization of a corrosion-resistant ZrO2 nanofiltration membrane with a MWCO< 300.” 284(1-2): 128-136.
- Van Gestel, T., et al. (2008). “ZrO2 and TiO2 membranes for nanofiltration and pervaporation: Part 2. Development of ZrO2 and TiO2 toplayers for pervaporation.” 318(1-2): 413-421.
- “A new approach for the classification of coal fly ashes based on their origin, composition, properties. and behaviour.“. 2007;86(10–11):1490-1512.
- [Google Scholar]
- Veranth, J. M., et al. (2000). “Measurement of soot and char in pulverized coal fly ash.” 79(9): 1067-1075.
- Visa, M., et al. (2016). “Behavior of the new composites obtained from fly ash and titanium dioxide in removing of the pollutants from wastewater.” 388: 359-369.
- Visa, M., et al. (2012). “Fly ash adsorbents for multi-cation wastewater treatment.” 258(17): 6345-6352.
- Wang, F., et al. (2016). “Mechanisms and roles of fly ash compositions on the adsorption and oxidation of mercury in flue gas from coal combustion.” 163: 232-239.
- Wang, S. and H. J. J. o. h. m. Wu (2006). “Environmental-benign utilisation of fly ash as low-cost adsorbents.” 136(3): 482-501.
- Wong, J. J. E. T. (1995). “The production of artificial soil mix from coal fly ash and sewage sludge.” 16(8): 741-751.
- Xie, J., et al. (2014). “Synthesis and properties of zeolite/hydrated iron oxide composite from coal fly ash as efficient adsorbent to simultaneously retain cationic and anionic pollutants from water.” 116: 71-76.
- YAO, Z.-t., et al. (2009). “Hydrothermal synthesis of sodalite from coal fly ash and its property characterization [J].” 2.
- Yao, Z., et al. (2015). “A comprehensive review on the applications of coal fly ash.” 141: 105-121.
- Yao, Z., et al. (2012). “Dilithium dialuminium trisilicate crystalline phase prepared from coal fly ash.” 21(6): 877-881.
- Yao, Z. J. F. A. S., Applications and N. Y. Potential Environmental Impacts. Nova Science Publishers (2013). “Generation, characterization and extracting of silicon and aluminium from coal fly ash.” 3-58.







