Translate this page into:
Fisetin suppresses 1,2-dimethylhydrazine-induced colon tumorigenesis in Wistar rats via enhancing the apoptotic signaling pathway
⁎Corresponding author at: Department of Anorectal, The Fourth People's Hospital of Shaanxi Province, No. 512 Xianning East Road, Xincheng District, Xi'an City, Shaanxi Province 710043, China. wangqingkui2018@sina.com (Qingkui Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present research exploration is intended to inspect the chemotherapeutic potency of fisetin in 1,2‑dimethylhydrazine (DMH)-stimulated colon carcinoma in Wistar rats. The 20 mg/kgb·wt of DMH was injected to the investigational rats via subcutaneous route once in a week for 15 weeks to induce colon cancer in a rat model. Fisetin at 50 mg/kgb·wt dose were administered throughout the experimental period. Biochemical methods were employed to study the antioxidant, lipid peroxidation, Phase-I & II enzymes. Pro-inflammatory cytokines and apoptotic proteins were measured using Elisa methods. Colon tissue damages were examined using hematoxylin and eosin staining. Our result suggested that fisetin treatment efficiently decreased the tumor incidence andnumber by restoring the antioxidant status, phase-II enzymes and inhibiting the actions of phase-I enzymes and lipid peroxidative events in DMH-induced colon tumorigenesis model. Further, we noticed that fisetin treatment significantly inhibited the pro-inflammatory cytokine production. Moreover, we observed that fisetin treatment remarkably inhibited the anti-apoptotic Bcl-2 and induced pro-apoptotic Caspase-9 and Caspase-3 expression, thereby induces apoptosis in DMH-induced colon tumorigenesis model. From this observation, we conclude that fisetin (50 mg/kgb·wt) can efficiently prevent chemically induced colon cancer by enhancing antioxidant status and modulating inflammatory and apoptotic signalling events.
Keywords
Fisetin
DMH
Colon cancer
Antioxidant
Caspase-9
Apoptosis
1 Introduction
The International Agency for Research on Cancer (IARC) recently reported that the global cancer burden were ascended to 18.1million new incidences and 9.6million deaths in 2018 including all cancers from 185 countries (Bray et al., 2018). Next to the lung and breast cancer, the colorectal cancer is a largely reported (1.8 million incidences, 10.2% of the total in 2018), and is second ranked on mortality (881 000 deaths, 9.2%), due to poor prognosis (Bray et al., 2018; Wong et al., 2019).
Almost 70% of colon cancer incidences were sporadic cases which were mediated through environmental reasons like dietary styles, physical activity, smoking tobacco and excess alcohol consumption (Vieira et al., 2017). Colon cancer is a complex and multistage event instigated from epithelial cells through aberrant crypts and adenoma to sarcomas finally metastasis. In addition, oxidative stress was potentiating to interrupt signalling cascades that linked to initiation and malignant transformation. Increased accumulation of reactive oxygen species (ROS), a characteristic feature during oxidative-stress has been involved in a aberrant crypt foci development and the pre-cancerous lesions in the colon cancer (Prasad et al., 2017). Hence, the inhibition of ROS alongside the alleviation of signaling connected to cell multiplication believed an efficient strategy to prevent the colon cancer (Liu et al., 2017).
The long persistence of inflammation promotes the development of carcinogenic events via activating molecular signalling pathways (Wu et al., 2013). Moreover, a number of studies have been confirmed that colon tumors exhibit constitutive expression of transcription factors being influenced in essential component of multiple inflammatory pathways, namely signal transducer and stimulator of transcription-3 (STAT-3) and Nuclear factor-κB (NF-κB). The nonsteroidal anti-inflammatory drugs have been proven as a promising agent in colon cancer clinical trials. Unfortunately, they carry severe gastrointestinal side effects (Derry et al., 2017). Incidentally, the recognition of potential natural-based bioactive agents that down-regulates the inflammation and/or up-regulates apoptosis can be a harmonizing and beneficial target in colon cancer chemoprevention (Song et al., 2015).
Preclinical studies clearly addressing the possible benefits of flavonoids-rich diet in the colon cancer treatment. Fisetin (3,3′,4′,7-tetrahydroxyflavone), is largely occurs in fruits and vegetables, like apple, grape, cucumber, and onion (Khan et al., 2013). Fisetin is reported to possess various pharmacological activity including antioxidant (Kumar, 2016), free radical scavenger (Kang et al., 2014), anti-inflammatory (Kim et al., 2015), cardio protective (Shanmugam et al., 2018), anti-viral (Lin et al., 2012), antidiabetic (Prasath et al., 2013), anti-carcinogenic (Meng et al., 2017), anti metastatic activity (Pal et al., 2016). This exploration was intended to inspect the remedial benefits of fisetin against the DMH stimulated colon carcinoma in Wistar rats.
2 Materials and methods
2.1 Chemicals
Fisetin and DMH were procured in Sigma Aldrich Co. (USA). The ELISA test kits were procured from R&D Biosystem, China. Whole other chemicals utilized in the study were of diagnostic range.
2.2 Experimental design
The male Wistar rats (130–150 g) were utilized in this investigation and all investigations were done via adopting the laws of international Law on Animal Care and Use. All rats were sustained in controlled situations of like 12 h light/dark sequence, 50% moisture, 25 ± 2 °C temperature, and the rats were supplemented with pellet diet, water ad libitum. The entire rats randomly alienated into four groups with six rats in every. The group-I were regarded as control. The group-II animals received DMH at 20 mg/kgb·w. dose via subcutaneously once a week for 15 weeks. The group-III received 50 mg/kg b.w. of fisetin along with DMH. The group-IV animals received 50 mg/kg b·w. of fisetin alone. All animals fasted overnight prior to the last day of investigational schedule then sacrificed and blood and tissue samples were gathered for various investigations.
2.3 Bodyweight and growth rate changes
Bodyweight and growth during the investigational period were constantly measured and recorded. From the initial day the animals were weighed, subsequently once a week until animals sacrifice. The growth rate was determined via utilizing the below given formula:
2.4 Histopathological studies
Colon tissues of normal and investigational animals were fixed in 10% neutral formalin immediately after removal. 2–5 µm thick sections were taken for further studies. Hematoxylin and eosin (H&E) were employed to study tissue damages as described earlier (Jørgensen et al., 2017).
2.5 Estimation of lipid peroxidation
The thiobarbituric acid reactive substances (TBARS) status was inspected. (Katerji et al., 2019) The level of conjugated dienes (CD) was studied according to the technique of Rao and Recknagel (1969). (Rao and Recknagel, 1969) Jiang et al. (1991) (Jiang et al., 1991) method was used for lipid peroxides measurement.
2.6 Biochemical estimation
The cytochrome b5 and cytochrome-P450 (EC. 1.14.14.1) statuses were investigated as described earlier by the technique of Ferreon et al. (2007) (Ferreon et al., 2007). The SOD activity measurement performed biochemically followed by the technique of Kakkar et al., (1984) (Kakkar et al., 1984). The enzymatic function of catalase was inspected based on the way of Sinha (1972) (Sinha, 1972). The GSH content was calculated by previously described (Ngamchuea et al., 2017).
2.7 ELISA assay for cytokines and apoptosis
TNF-α, IL-6, IL-1β and apoptotic protein measurements were measured with an ELISA kit (R&D Biosystem, China) as described previously by Li X et al., (2015) (Li et al., 2015).
2.8 Statistical examination
Statistical investigation was done with the aid of SPSS tool. Data are portrayed as mean ± SD. The one-way ANOVA, subsequently Duncan Multiple Range test was employed to compare the variations among the data. Data are regarded as statistically significant if p < 0.05.
3 Results
3.1 Effect of fisetin on body weight changes in control and investigational animals
Table 1 depicts the changes in bodyweight and growth rate of normal and investigational animals. We noted a significant decrease in the growth and body weight of DMH-provoked experimental animals when compared with the control. In other hand, fisetin (50 mg/kg) treatment alleviated the loss in the growth rate and body weight of DMH-induced animals. There were no changes between normal and fisetin alone supplemented animals. Values are expressed as mean ± SD for six animals in each group. Values not sharing a common superscript (*, #) differ significantly at p < 0.05 (DMRT).
Groups
Initial body weight (g)
Final body weight (g)
Weight gain (g)
Growth rate (g)
Group I
131.54 ± 7.62
265.19 ± 10.42*
133.65 ± 8.39#
1.09 ± 0.04*
Group II
130.65 ± 8.26
187.34 ± 7.15*
56.69 ± 3.01#
0.62 ± 0.07*
Group III
129.69 ± 6.91
231.71 ± 12.75*
102.02 ± 8.58#
0.89 ± 0.01*
Group IV
132.87 ± 8.15
258.86 ± 11.64*
125.99 ± 7.14#
1.03 ± 0.06*
3.2 Effect of fisetin on colonic polyps in the colon of control and investigational animals
Our results clearly indicated that there were 100% of colonic polyps in the colon were noted in DMH stimulated experimental animals. Administration of 50 mg/kg of fisetin to DMH-incited rats was considerably diminished colonic polyps in the colon tissues by 72.19% Fig. 1. Moreover, we did not observe polyps in the untreated control and/or fisetin alone supplemented animals (Table 2).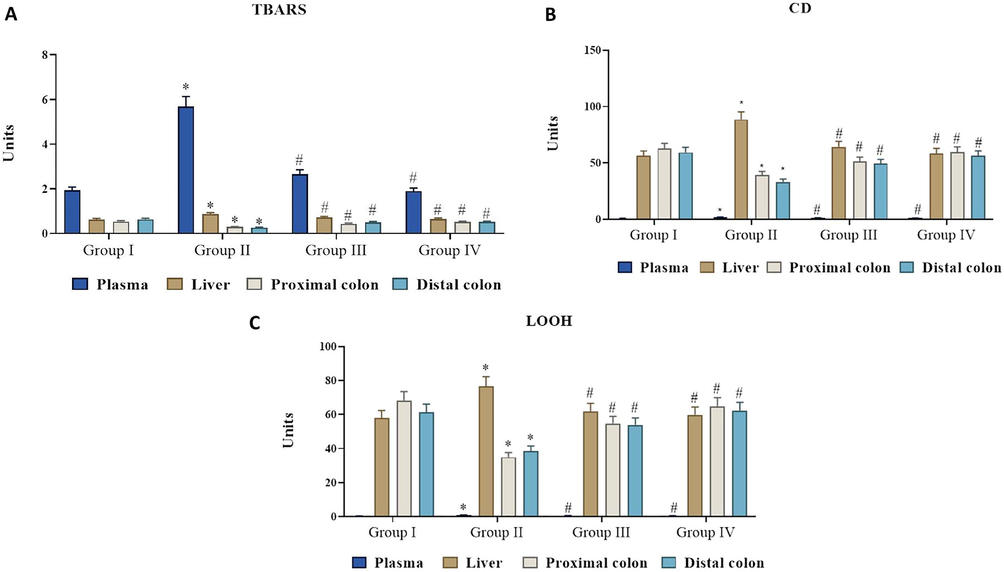
The effect of fisetin on enzymatic antioxidant levels in plasma, liver, proximal and distal colon of control and DMH – induced experimental animals Values are expressed as mean ± SD for six animals in each group. Values not sharing acommon superscript (*, #) differ significantly at p < 0.05 (DMRT).
Groups
No. of rats
No. of polyps bearing rats
Total no. of polyps
Average no. of polyps bearing rats*
Percentage incidence of polyps#
Percentage of polyps inhibition
Group I
6
0
0
0
0
nil
Group II
6
6
19
3.1
100
0
Group III
6
2
6
2.1
32.56
72.19
Group IV
6
0
0
0
0
nil
3.3 Effect of fisetin on enzymatic antioxidant level in control and investigational animals.
We noticed that DMH-induced experimental animals showed decreased levels of enzymatic antioxidants. This might be associated with DMH-induced excessive radical generation (Fig. 2). However, the fisetin (50 mg/kg) supplementation appreciably increased the SOD, CAT, GPx levels. There were no alterations in the SOD, CAT, GPx statuses of control and fisetin alone supplemented animals.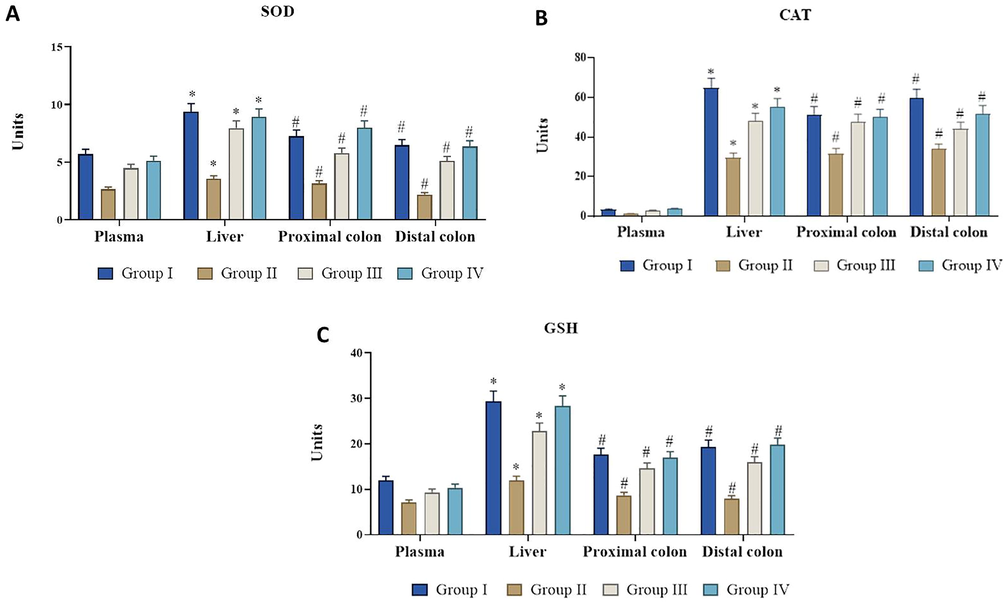
The inhibitory effect of fisetin on lipid peroxidative evnts in plasma, liver, proximal and distal colon. Values are expressed as mean ± SD for six animals in each group. Values not sharing acommon superscript (*, #) differ significantly at p < 0.05 (DMRT).
3.4 Effect of fisetin and DMH on lipid peroxidation byproducts.
The lipid hydroperoxide (LOOH), TBARS and CD levels were markedly augmented in the DMH alone-challenged rats than normal rats. The animals were supplemented with fisetin (50 mg/kg) efficiently lowered the LOOH, TBARS and CD levels in DMH-induced animals (Fig. 3). This might be associated with antioxidant properties of fisetin.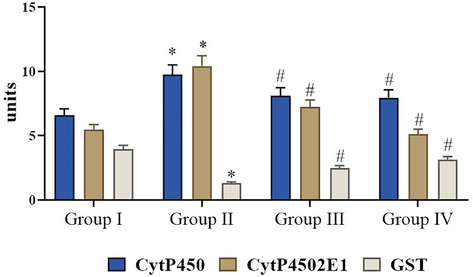
The effect of fisetin on phase I and phase II enzymes in the liver of control and DMH – induced experimental animals. Values are expressed as mean ± SD for six animals in each group. Values not sharing acommon superscript (*, #) differ significantly at p < 0.05 (DMRT).
3.5 Effect of fisetin on phase-I and phase-II enzymes in the liver
The functions of cytochrome-P450, cytochrome-b5 and GST were remarkably augmented in DMH induced experimental rats. However, fisetin (50 mg/kg) treatment significantly lowered the cytochrome b5, cytochrome P450 and GST compared to DMH induced experimental rats (Fig. 4). There were no changes in the phase-I and phase-II enzymes between normal and fisetin alone supplemented animals.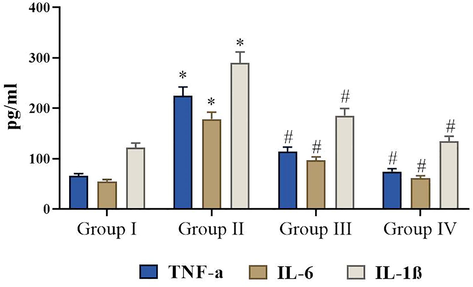
The effects of fisetin on the colonic pro-inflammatory cytokines. Values are expressed as mean ± SD from three independent experiments. Values notsharing a common superscript (*, #) differ significantly at p < 0.05 (DMRT).
3.6 Effects of fisetin on the colonic pro-inflammatory cytokines
In this investigation, we found the elevated statuses of these pro-inflammatory modulators like TNF-α, IL-1β and IL-6 in DMH induced experimental rats. However, fisetin (50 mg/kg) bodyweight treatment significantly inhibited the pro-inflammatory cytokines compared DMH-induced rats (Fig. 5). Meanwhile, there were no changes between the normal and fisetin alone supplemented animals.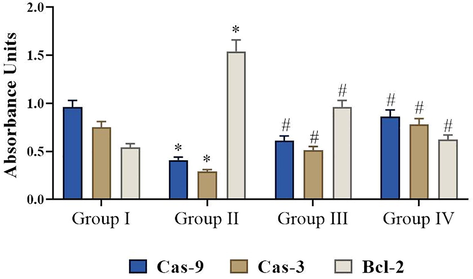
The effects of fisetin on the apoptotic signalling. Values are expressed as mean ± SD from three independent experiments. Values notsharing a common superscript (*, #) differ significantly at p < 0.05 (DMRT).
3.7 Effects of fisetin on the activities of Caspase-9, Caspase −3 and Bcl-2 expression
In this investigation, we noted the augmented statuses of antiapoptotic protein Bcl-2 and decreased expression of Caspase-9, Caspase-3 in DMH-induced experimental rats. Whereas, fisetin (50 mg/kg) treatment significantly inhibited the antiapoptotic protein Bcl-2 and enhanced the expression of Caspase-9, Caspase-3 while comparing it to DMH-induced rats (Fig. 6). Meanwhile, no significant alterations were noted between the normal and fisetin alone supplemented animals.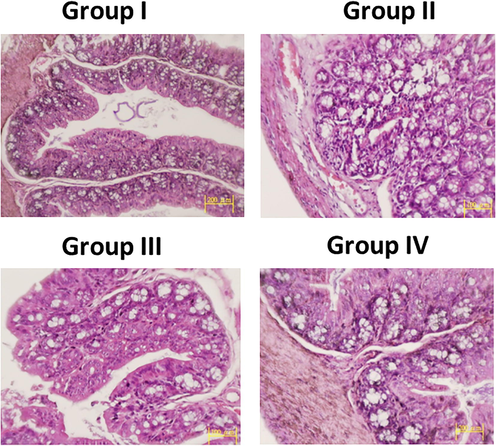
Shows the hematoxylin and eosin staining of colonic tissue of different experimentalrats. Colon tissues of control and fisetin alone treated rats showed normal crypt architecture and underlying muscularis mucosa, and the goblet cells are mostly adhered to epithelial cells. Colon tissue of DMH alone-treated rats showed infiltration of neoplastic cells and the submucosa indicating the genesis of malignant adenoma. Polystratification of cells and moderate dysplasia were also observed in the nearby tissues of DMH alone-administered rats.
4 Discussion
In this exploration, we investigated the chemotherapeutic potency of fisetin against chemically induced colon cancer model. We found that fisetin significantly reduced the number tumor formation in DMH-induced animals. Similarly, fisetin is reported to inhibit the tumor growth by 67% in lewis lung carcinoma-bearing mice (Touil et al., 2011) Free radicals are formed naturally in the body and play a vital role in normal cellular developmental processes (Loizzo and Tundis, 0000). However, the highly reactive nature of free radicals damages the cellular components, including nucleic acids, proteins and cellular membranes, when they are in enormous in amount. Naturally cells are evolved with antioxidant enzymes in order to protect from these free radicals (Obrador et al., 2019). In our study, we noticed that diminished levels of antioxidant enzymes like SOD, CAT, GSH and GST in DMH induced colon cancer bearing animals. It is well documented that biochemically most of the animal and human cancer cells exhibit low levels of antioxidant enzymes (Islam et al., 2019). Similarly, fisetin treatment regained the antioxidant profiles in different experimental models (Molfino et al., 2013). Hence, we conclude here that the antioxidant role of fisetin plays a major part in prevention of DMH-induced colon cancer.
Kikuchi et al., (2019) (Kikuchi et al., 2019) reported a number structurally related flavonoids, including fisetin and they found that fisetin effectively prevented the lipid peroxidative events by exerting dose-dependent antioxidative activity. Ravichandran et al., (2011) (Ravichandran et al., 2011) also found that fisetin inhibited lipid peroxidation levels in [B(a)P] stimulated lung carcinogenesis via restoring the anti-oxidants. A study of structure-activity relationship of flavonoids revealed that polyhydroxylated substitutions on rings A and B, a 4-keto moiety, a free 3-hydroxyl substitution and a 2,3-double bond determines lipid peroxidative property of flavonoids.(Barnaba and Medina-Meza, 2019) Further, we observed that fisetin treatment enhanced the phase-II enzymes and subsequently inhibited phase-I enzymes in DMH-induced animals. Recently, (Shrestha et al., 2018) reported the ability of fisetin inhibitory action against eight isoforms of human cytochrome-P450 (CYP) through non-competitive inhibition more than its main metabolite.
The chronic inflammation plays a key factor in promoting tumor progression due to the release of different cytokines, interleukins and most importantly ROS generation (Lin et al., 2019). The prolonged use of anti-inflammatory drugs may reduce the tumor development via delaying the proliferation, angiogenesis, and metastasis (Gurpinar et al., 2013). We observed that DMH-induced animals showed increased expression of inflammatory markers. However, the treatment of fisetin inhibited TNF-α, IL-6 and IL-1β in DMH-induced animals. Previous studies also confirmed the inhibitory effects of fisetin against the expression of TNF-α, IL-6 and IL-1β in different experimental models (Prakash et al., 2013; Yu et al., 2016). Moreover, Gutiérrez-Venegas et al., (2014) (Gutiérrez-Venegas et al., 2014) found that the fisetin mediated anti-inflammatory activity is linked with inhibition of MAPK, NF-κB and COX-2 without affecting cell viability.
Apoptosis is a kind of cell necrosis that helps to eliminate potentially harmful and/or unwanted cells. The defects in apoptosis can evident as cancer or autoimmunity diseases (Hassan et al., 2014). In this study, we noticed that DMH-induced animals showed the altered patterns of apoptotic protein. This substantially increases the tumor burden in DMH-induced animals. Whereas, fisetin treatment induced the apoptotic proteins such as caspases-3, Caspase-9 in DMH induced animals. In this way, fisetin may be reduces the tumor burden in DMH induced animals (Through, 1285) also reported that fisetin stimulates apoptosis in renal carcinoma cells via p53 mediated stimulation of DR5 mechanism. It is documented that fisetin treatment reduces the mitochondrial membrane potential and increases the stimulation of caspases such as caspases-3, -8 and -9 by increasing the pro-apoptotic proteins such as BAK and BAX meanwhile diminished anti-apoptotic protein (BCL-2) in oral cancer cells (Shih et al., 2017). Liu et al., (2017) (Sabarwal et al., 2017) demonstrated the activation of caspase-3 signaling induced by fisetin is necessary to induce apoptosis in the mouse model of liver cancer. It is also supported by Sabarwal et al., (2017) (Kashyap et al., 2018), they found that fisetin induced mitochondria regulated apoptosis in gastric cancer cells. Kashyap et al., (2018) [46] clearly documented that the ability of fisetin to block multiple signaling associated with cancer.
5 Conclusion
The outcomes of current investigation clearly demonstrated that the fisetin treatment considerably reduces the tumor incidence and numbers by restoring the antioxidant status, phase-II enzymes and inhibiting the functions of phase-I enzymes and lipid peroxidative events. In addition, fisetin treatment significantly inhibited the pro-inflammatory cytokine production. Further, fisetin treatment substantially inhibited the anti-apoptotic and induced pro-apoptotic Caspase-9 and Caspase-3 expression, thereby induces apoptosis. Hence, we conclude that the antioxidant and anti-inflammatory effect of fisetin might be responsible for this anti-tumor activity against DMH-induced colon cancer model.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP-2019/5) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2018;68:394-424.
- [Google Scholar]
- Prevalence and risk factors of colorectal cancer in Asia. Intest Res.. 2019;17(3):317-329.
- [Google Scholar]
- Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRFAICR continuous update project. Ann. Oncol.. 2017;28:1788-1802.
- [Google Scholar]
- Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett.. 2013;345(2):164-173.
- [Google Scholar]
- Oral nonsteroidal anti-inflammatory drugs (NSAIDs) for cancer pain in adults. Cochrane Database Syst. Rev. 2017
- [Google Scholar]
- Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett.. 2017;28(387):95-105.
- [Google Scholar]
- Fisetin inhibits liver cancer growth in a mouse model: relation to dopamine receptor. Oncol. Rep.. 2017;38(1):53-62.
- [Google Scholar]
- Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6) 1244 60.e16
- [Google Scholar]
- Fisetin: a dietary antioxidant for health promotion. Antioxid. Redox Signal.. 2013;19(2):151-162.
- [Google Scholar]
- Brajesh Kumar Maurya Surendra Kumar Trigun Fisetin Modulates Antioxidant Enzymes and Inflammatory Factors to Inhibit Aflatoxin-B1 Induced Hepatocellular Carcinoma in Rats Oxidative Medicine and Cellular Longevity 2016 2016 1 9 10.1155/2016/1972793
- Fisetin attenuates hydrogen peroxide-induced cell damage by scavenging reactive oxygen species and activating protective functions of cellular glutathione system. Vitro Cell DevBiol. Anim.. 2014;50(1):66-74.
- [Google Scholar]
- Fisetin suppresses macrophage-mediated inflammatory responses by blockade of Src and Syk. BiomolTher (Seoul).. 2015;23(5):414-420.
- [Google Scholar]
- Fisetin confers cardioprotection against myocardial ischemia reperfusion injury by suppressing mitochondrial oxidative stress and mitochondrial dysfunction and inhibiting glycogen synthase kinase 3β activity. Oxid. Med. Cell. Longevity 2018:9173436.
- [Google Scholar]
- Fisetin and rutin as 3C protease inhibitors of enterovirus A71. J. Virol. Methods. 2012;182(1–2):93-98.
- [Google Scholar]
- Fisetin averts oxidative stress in pancreatic tissues of streptozotocin-induced diabetic rats. Endocrine. 2013;44(2):359-368.
- [Google Scholar]
- A hydroxylatedflavonol, fisetin inhibits the formation of a carcinogenic estrogen metabolite. Steroids. 2017;119:53-56.
- [Google Scholar]
- Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget. 2016;7(2):1227-1241.
- [Google Scholar]
- Using cell nuclei features to detect colon cancer tissue in hematoxylin and eosin stained slides. Cytometry A. 2017;91(8):785-793.
- [Google Scholar]
- Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxid. Med. Cell. Longevity. 2019;2019:1279250.
- [Google Scholar]
- Early incorporation of carbon-labeled carbon tetrachloride into rat liver particulate lipids and proteins. ExpMolPathol. 1969;10(2):219-228.
- [Google Scholar]
- Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991;26(10):853-856.
- [Google Scholar]
- Protein phase diagrams II: nonidealbehavior of biochemical reactions in the presence of osmolytes. Biophys. J.. 2007;92(1):245-256.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Ind. J. BiochemBiophys.. 1984;21(2):130-132.
- [Google Scholar]
- Rapid method for the quantification of reduced and oxidized glutathione in human plasma and saliva. Anal. Chem.. 2017;89(5):2901-2908.
- [Google Scholar]
- High concentrations of TNF-α induce cell death during interactions between human umbilical cord mesenchymal stem cells and peripheral blood mononuclear cells. PLoS One. 2015;10(5):e0128647
- [Google Scholar]
- Fisetin disposition and metabolism in mice: identification of geraldol as an active metabolite. Biochem. Pharmacol.. 2011;82(11):1731-1739.
- [Google Scholar]
- Loizzo M. R. Tundis R. Plant Antioxidant for Application in Food and Nutraceutical Industries. Antioxidants (Basel). 2019; 8 (10): 453. Published 2019 Oct 5. https://doi.org/10.3390/antiox8100453.
- Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem.. 2019;400(5):589-612.
- [Google Scholar]
- Alterations of antioxidant enzymes and biomarkers of nitro-oxidative stress in tissues of bladder cancer. Oxid. Med. Cell. Longevity. 2019;2019:2730896.
- [Google Scholar]
- Timing of antioxidant supplementation is critical in improving anorexia in an experimental model of cancer. Int. J. Food SciNutr.. 2013;64(5):570-574.
- [Google Scholar]
- Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res.. 2019;9(8):1517-1535.
- [Google Scholar]
- Fisetin, a novel flavonol attenuates benzo (a) pyrene-induced lung carcinogenesis in Swiss albino mice. Food Chem. Toxicol.. 2011;49(5):1141-1147.
- [Google Scholar]
- Flavonoids ability to disrupt inflammation mediated by lipid and cholesterol oxidation. Adv. Exp. Med. Biol.. 2019;1161:243-253.
- [Google Scholar]
- Selective inhibition of CYP2C8 by fisetin and its methylated metabolite, geraldol, in human liver microsomes. Drug Metab. Pharmacokinet.. 2018;33(2):111-117.
- [Google Scholar]
- Cancer and ER stress: mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharmacother.. 2019;118:109249
- [Google Scholar]
- Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. Neuromolecular Med.. 2013;15(1):192-208.
- [Google Scholar]
- The effects of fisetin on lipopolysaccharide-induced depressive-like behavior in mice. Metab. Brain Dis.. 2016;31(5):1011-1021.
- [Google Scholar]
- Anti-inflammatory activity of fisetin in human gingival fibroblasts treated with lipopolysaccharide. J. Asian Nat. Prod. Res.. 2014;16(10):1009-1017.
- [Google Scholar]
- Apoptosis and molecular targeting therapy in cancer. Biomed. Res Int.. 2014;2014:150845
- [Google Scholar]
- Min K.J., Kwon T.K., Fisetin Induces Apoptosis Through p53-Mediated Up-Regulation of DR5 Expression in Human Renal Carcinoma Caki Cells Molecules 22 8 1285 10.3390/molecules22081285.
- Shih Y.L., Hung F.M., Lee C.H., Yeh M.Y., Lee M.H., Lu H.F., Chen Y.L., Liu J.Y., Chung J.G. Fisetin Induces Apoptosis of HSC3 Human Oral Cancer Cells Through Endoplasmic Reticulum Stress and Dysfunction of Mitochondria-mediated Signaling Pathways Vivo. Nov-Dec; 31(6) 2017 1103 1114.
- Fisetin inhibits cellular proliferation and induces mitochondria-dependent apoptosis in human gastric cancer cells. Mol. Carcinog.. 2017;56(2):499-514.
- [Google Scholar]
- Fisetin: a bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci.. 2018;1(194):75-87.
- [Google Scholar]







