Translate this page into:
First molecular data and morphological re-description of two copepod species, Hatschekia sargi and Hatschekia leptoscari, as parasites on Parupeneus rubescens in the Arabian Gulf
⁎Corresponding author. squraishy@ksu.edu.sa (Saleh Al-Quraishy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Little information is available about the biodiversity of parasitic copepods in the Arabian Gulf. The present study aimed to provide new information about different parasitic copepods gathered from Parupeneus rubescens caught in the Arabian Gulf (Saudi Arabia). Copepods collected from the infected fish were studied using light microscopy and scanning electron microscopy and then examined using standard staining and measuring techniques. Phylogenetic analyses were conducted based on the partial 28S rRNA gene sequences from other copepod species retrieved from GenBank. Two copepod species, Hatschekia sargi Brian, 1902 and Hatschekia leptoscari Yamaguti, 1939, were identified as naturally infected the gills of fish. Here we present a phylogenetic analysis of the recovered copepod species to confirm their taxonomic position in the Hatschekiidae family within Siphonostomatoida and suggest the monophyletic origin this family. Sequencing the 28S rRNA gene query identified a close relationship of the recovered Hatschekia species to Hatschekia japonica (gb| KR048858.1). This study identifies a new host for Hatschekia species isolated from Saudi Arabia and performs the first molecular analysis of their 28S rRNA genes.
Keywords
28S rRNA gene
Arabian Gulf
Hatschekiidae
Marine fish
Parasitic copepods
1 Introduction
Copepods, or “oar-footed”, are a group of very numerous and polymorphic crustaceans that are distributed widely in almost all aquatic habitats; they have different interactions with their hosts (Mariniello, 2010) and can cause pathogenic effects resulting in significant economic damage (Ramdane, 2009). Parasitic copepods are usually found on the exterior surfaces of their hosts and are embedded often in microhabitats such as gills, nostrils, mantle cavities, and genital folds (Rosim et al., 2013); conversely, endoparasites copepods are present in the muscles, digestive tracts, and body cavities of their hosts (Huys and Boxshall, 1991; Huys et al., 2002; Ho et al., 2003). Copepoda was erected in 1840 by Milne Edwards as a subclass of the Hexanauplia class in the Crustacea subphylum. Khodami et al. (2019) described ten copepod orders with three infraclasses (Progymnoplea Lang, 1948, Gymnoplea Giesbrecht, 1882, and Podoplea Giesbrecht, 1882). The first infraclass includes the order of Platycopioida Fosshagen and Iliffe, 1985, and the second comprises the order of Calanoida Sars, 1903, and the latter consists of the other eight orders.
Siphonostomatoida Burmeister, 1835 is a Podoplea order containing approximately 75% of the parasitic copepods in fish within 39 families (Boxshall, 2013). Hatschekiidae Kabata, 1979 is a copepod family of Siphonostomatoida and is most commonly distributed in tropical and subtropical waters (Bakhrebah, 2006). Because these small copepods do not typically produce many eggs, they are remarkably effective, infecting several fish species and sometimes aggregating in large numbers in their hosts (Jones, 1998). According to Walter and Boxshall (2020), this family includes nine genera Congericola van Beneden, 1851, Hatschekia Poche, 1902, Bassettithia Wilson, 1922, Pseudocongericola Yu, 1933, Prohatschekia Nunes-Ruivo, 1954, Wynnowenia Boxshall, 1987, Laminohatschekia Boxshall, 1989, Brachihatschekia Castro-Romero and Baeza-Kuroki, 1989, and Mihbaicola Uyeno, 2013. Both male and female hatschekiids lack maxillipeds, have a second maxillae with bifid claws, and display a specific leg structure (the first and second legs are biramous, and the third and fourth legs are reduced to setae). The genus Hatschekia is the largest genus of the Hatschekiidae family and contains parasites found in marine teleosts' gill filaments (Ho and Kim, 2001; Boxshall and Halsey, 2004a, 2004b; Bakhrebah, 2006). This genus includes approximately 152 species (Walter and Boxshall, 2020) that display a moderate degree of variability in their external morphology (Scott-Holland et al., 2006).
The relatively low number of copepodologists is the main reason why limited information is available about ectoparasitic copepods (Ho, 2001). Consequently, there is a lack of knowledge of the essential morphological and anatomical features that can classify copepods (Hamza et al., 2007; Ramdane, 2009). Several molecular methods were recently used to test the taxonomic status of copepod species independently of their morphology (Fls et al., 2006; Ferrari and von Vaupel Klein, 2019). DNA barcoding was used recently in siphonostomatoid copepod taxonomy to verify intra- and interspecific morphological distinctions to determine their phylogenetic relationship (Yazawa et al., 2008). However, few studies were performed using molecular data to restore inter-ordinal associations within Copepoda, including insufficient taxon sampling or sequencing uncertainty (Kim and Kim, 2000; Ferrari et al., 2010). Huys et al. (2007) found that the most useful genetic markers are the nuclear ribosomal genes (18S and 28S rRNA) form semi-conserved domains interspersed with divergent regions, thus allowing a phylogenetic reconstruction via a wide range of taxonomic levels.
Therefore, the present research intended to identify Hatschekia species infecting Parupeneus rubescens, which was performed using light and scanning electron microscopic analyses. Additionally, the nuclear large subunit rRNA (28S rRNA) gene's partial nucleotide sequences were used to assess these species' phylogenetic position within Siphonostomatoida.
2 Materials and methods
2.1 Collection of fish and parasitic examination
Eighty specimens of rosy goatfish, Parupeneus rubescens, were collected from fishermen in the Arabian Gulf coast at Dammam City, Saudi Arabia. Fish were transported to the Laboratory of Parasitology Research at the Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia. The collected fish samples were identified according to the guidelines on the website fishbase.org. All fish were dissected within 48 hrs of sampling. Macro- and microscopic examinations of the collected fish samples were performed externally, and the gills were examined for the presence of any parasitic infection according to standard parasitological techniques. The sites and number of parasite species were recorded per fish.
2.2 Light microscopy examination of copepods
Copepods were recovered from the host fish's gills by careful scraping under a dissecting microscope (Nikon SMZ18, NIS ELEMENTS software) and then fixed and preserved in ethanol (70%). After preservation, the parasitic copepods were cleared and stained in lactic acid to identify morphological characteristics under a Leica DM 2500 microscope (NIS ELEMENTS software, Leica Microsystems, Morrisville, USA).
2.3 Scanning electron microscopy examination of copepods
The copepods were also prepared for scanning electron microscopy (SEM). The specimens were post-fixed overnight in OsO4 before being dehydrated in increasing ethanol grades. The specimens then reached the critical CO2 drying stage using the BOMER–900 dryer “Leica Microsystems, Morrisville, USA”, were placed on SEM stubs with double adhesive tape, were sputtered with gold at 15 mA using a TECHNICS HUMMER V, and were tested under JEOL JSM-6060LV (Tokyo, Japan). Copepod identification was based on Kabata (1979) guidelines and Huys and Boxshall (1991) for morphological features.
2.4 Molecular analysis
2.4.1 DNA extraction and PCR amplification
Total DNA was extracted from ethanol-preserved samples using the QIAGEN® DNeasy® tissue kit© (Hilden, Germany) following the manufacturer’s instructions. The 28S rRNA partial fragments were amplified using the 28S universal primer pair, 28SF (5′-ACA ACT GTG ATG CCC TTA G-3′) and 28SR (5′-TGG TCC GTG TTT CAA GAC G-3′) designed by Song et al. (2008). Thermal cycler (Biometra, Göttingen, Germany) was used to conduct PCR amplification under the following parameters: 5 min at 94⁰C, accompanied by 30 cycles of 1 min at 94⁰C, 30 sec at 59⁰C, 2 min at 72⁰C, and 7 min at 72⁰C. The PCR amplification reaction volume (20 µl) consisted of 2 μl of genomic DNA extract, 4 μl of 5 × FIREPol® Master Mix (Solis BioDyne), 0.6 μl of each forward and reverse PCR primer, and nuclease-free water to reach the desired volume. PCR amplicons were tested by electrophoresis and purified using the QIAquickTM PCR purification kit (QIAGEN, Hilden, Germany).
2.4.2 Sequencing and phylogenetic analysis
All nucleotide sequences were determined through direct sequencing of the same set of primers used for PCR amplification with the ABI Prism BigDye Terminator Cycle Sequencing Kit (Perkin Elmer) using ABI 3130 × 1 DNA Analyzer (Applied Biosystems®, Thermo Fisher Scientific, USA). The sequences were blasted with the GenBankTM nucleotide databases. Subsequently, all sequences were matched using CLUSTAL-XTM (Thompson et al., 1997). Each sequence was manually edited for accuracy using ABI Editview (Perkin-Elmer). The aligned sequences were trimmed in BIOEDIT 4.8.9 (Hall, 1999). Maximum likelihood, Neighbor-Joining, UPGMA methods were applied to construct the best fitting trees. The software for Molecular Evolutionary Genetics Analysis (MEGA 7.0) was used to perform this phylogenetic analysis (Kumar et al., 2016).
3 Results
Forty-three of the 80 (53.75%) rosy goatfish, Parupeneus rubescens were infected naturally with two different copepod parasites recovered from the infected fish samples' gills, both of which belonged to the genus Hatschekia within the family Hatschekiidae. These copepod species were Hatschekia sargi Brian, 1902 and Hatschekia leptoscari Yamaguti, 1939.
3.1 Hatschekia sargi Brian, 1902
Cephalothorax oval with circular margins and a distinctly conical posterolateral process, and wider than long (length: width ratio 0.85). Trunk sub-cylindrical and approximately seven times the cephalothorax's length; separated anteriorly from the cephalothorax by a deep constriction and only slightly narrower posteriorly. Posterolateral margins of the trunk taper to the abdomen without lobes or processes. Abdomen small, conical, and not delimited by distinct borders from the trunk, with caudal rami present.
3.2 Description (Figs. 1, 2)
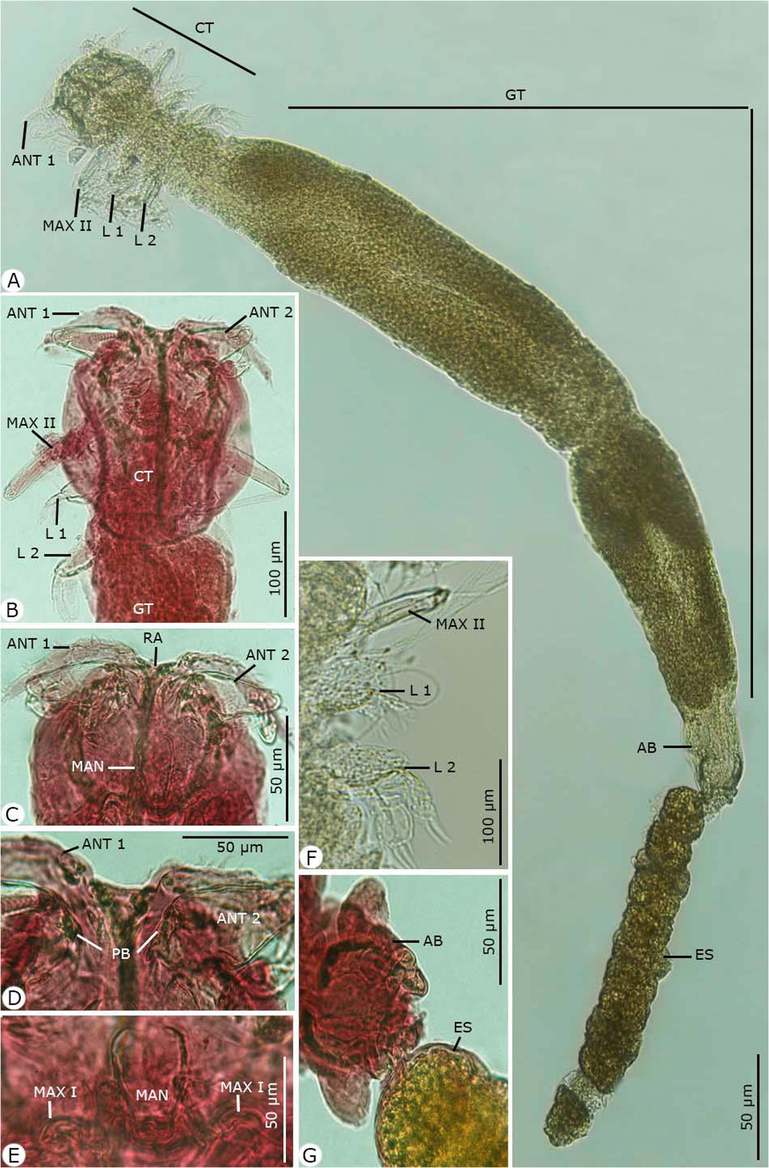
Photomicrographs of the adult female Hatshekia sargi infecting Parupeneus rubescens. (A) Whole-mount preparation. (B-O) High magnifications for different body parts showing: (B, C) Anterior region of cephalothorax with thoracic appendages. (D) The rostral region with the first and second antenna. (E) Postoral appendages of the mandible and maxilla I. (F) Thoracic zone with maxilla II and two pairs of legs. (G) Abdomen bearing egg strings. Note: AB, abdomen; ANT 1, antenna 1; ANT 2, antenna 2; CT, cephalothorax; ES, egg string; GT, genital trunk; L 1, leg 1; L 2, leg 2; MAN, mandible; MAX I, maxilla I; MAX II, maxilla II; PB, parabasal body; RA, rostral area.
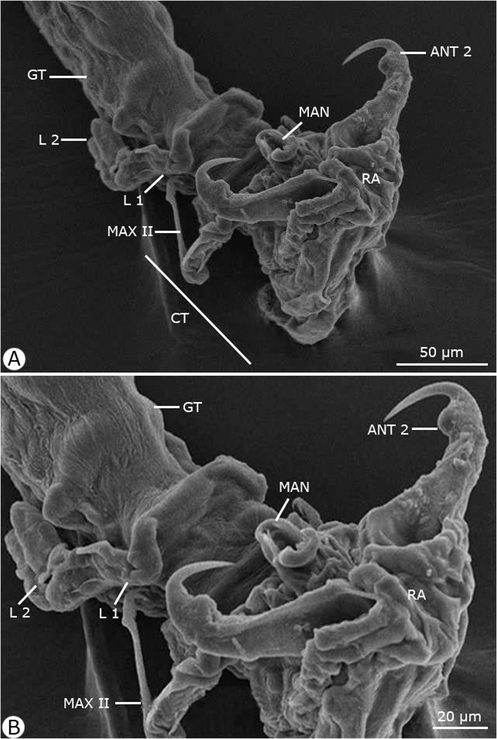
Scanning electron micrographs of the anterior region of the adult female Hatshekia sargi infecting Parupeneus rubescens. Note: ANT 2, antenna 2; CT, cephalothorax; GT, genital trunk; L 1, leg 1; L 2, leg 2; MAN, mandible; MAX II, maxilla II; RA, rostral area.
Rostrum concealed beneath frontal projections of a dorsal cephalic shield and comprised two pairs of humplike processes. Antennule (antenna I) obscurely segmented, slender, protruding beyond the lateral margin of the cephalothorax; the anterior margin contained 11 stout setae, the posterior margin contained one slender seta on the second half and one relatively small seta near the apex; and the apical armature contained 11 setae and one aesthete. Antenna (antenna II) two-segmented with a long second segment and relatively short, robust, and sharply hooked claw; the parabasal body was small, soft, and suboval. Maxillule (maxilla I) bilobate, both lobes armed with two sharp tapering processes. Maxilla (maxilla II) contained four segments: a proximal unarmed segment; the second segment (lacertus) the largest, with short seta on the inner margin near the base; the third segment (brachium) slender, with small distal seta on the inner margin; and the distal segment tipped with slender seta and bifid claw. No maxilliped was present. Mandible slender and tapered distally. Two pairs of biramous legs present (first and second thoracopods), with two indistinctly segmented exopods. The first leg's endopod much smaller than the exopod, and the second leg had rami of approximately equal size. Third and fourth legs were not observed. Caudal rami digitiform, with six setae each.
Dimensions: body length excluding caudal rami 1.60 (1.56–1.79); cephalothorax length 0.15 (0.12–0.19), width 0.19 (0.17–0.21); trunk length 1.30 (1.28–1.34), width 0.32 (0.30–0.36); egg string length 0.75 (0.70–1.15), diameter 0.10 (0.08–0.12).
H. sargi was first observed by Brian (1902) as specimens that were isolated from the gills of Sargus (=Diplodus) rondeletii, D. salvani, and D. annularis (Sparidae) from Geneva, followed by Bakhrebah (2008), who recovered the same species from Carcharhinus melanopterus (Quoy and Gaimard, 1824) (Carcharhinigae) from the Arabian Gulf of Jeddah, Saudi Arabia. The morphological features re-described here agree with the original description of H. sargi, particularly with the presence of a linear trunk and smoothly convex lateral margins to the cephalothorax, but except for the body proportions and the occurrence of different host species. Bakhrebah (2008) claimed that in order to determine the validity of the Hatschekia species, it was appropriate to compare it with its congeners possessing cephalothoraxes of approximately similar length and width, with distinctly posterolateral conical protuberances and a long slender trunk not fitted with posterolateral lobes or processes which were compatible with the findings presented herein. Thus, H. sargi shows a close resemblance to Hatschekia pagellibogneravei Hesse, 1879, which infects Diplodus annularis from the Mediterranean, except for the latter having postero-lateral margins of the trunk tapered to the abdomen and a trunk approximately eight times the length of the cephalothorax.
3.3 Hatschekia leptoscari Yamaguti, 1939
Cephalothorax relatively large and comprised one-fourth or more of the total length; it was heart-shaped, with the anterior margin formed small lobes on either side of the median line, and each lateral curved in crescent to meet the convex posterior margin. Leg bearing segments fused to the trunk. Genital trunk ovoid, broadened in the anterior fourth and almost as wide as the cephalothorax. Abdomen contained one sub-rectangular segment and buried with the anterior half into the trunk's distal end, and the portion posterior to the dorsolateral gonopores free, with caudal rami present.
3.4 Description (Figs. 3 and 4)
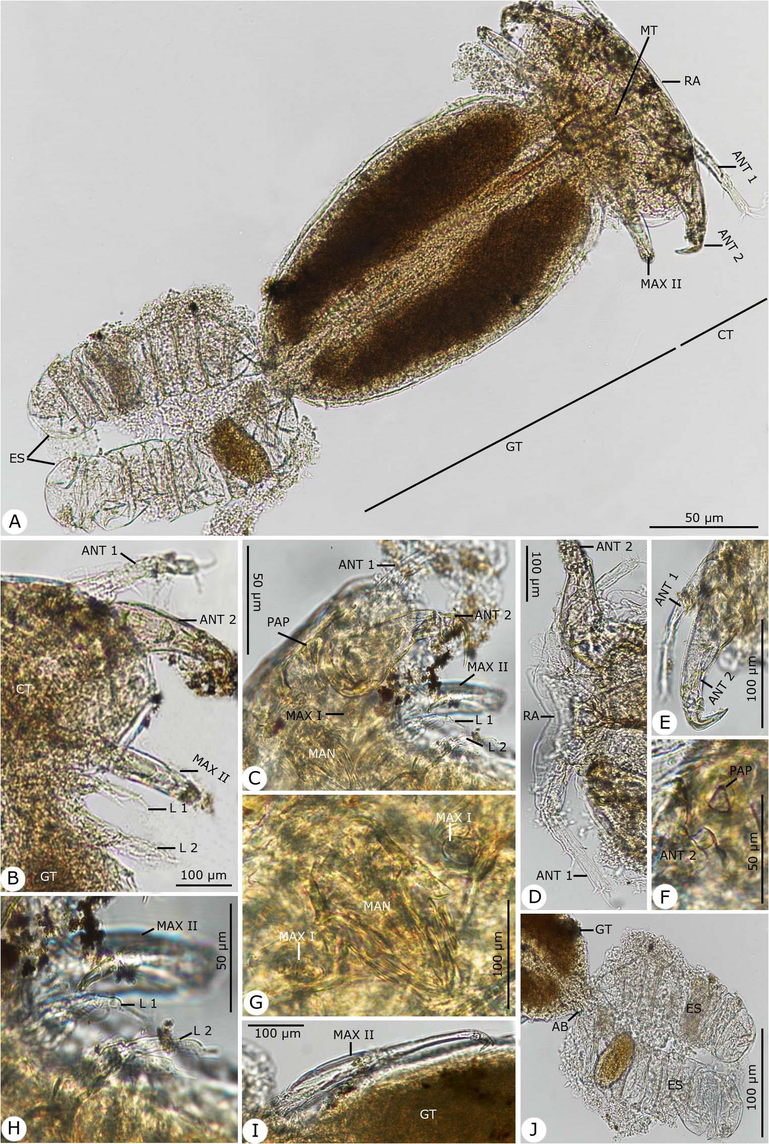
Photomicrographs of the adult female Hatschekia leptoscari infecting Parupeneus rubescens. (A) Whole-mount preparation. (B-O) High magnifications for different body parts showing: (B, C) Anterior region of cephalothorax. (D) The rostral region with the first antenna. (E) First and second antenna. (F) Second antenna with the post-antennary process. (G) Postoral appendages of the mandible and maxilla I. (H) Maxilla II with first and second thoracic legs. (I) Maxilla II. (J) Abdomen bearing egg strings. Note: AB, abdomen; ANT 1, antenna 1; ANT 2, antenna 2; CT, cephalothorax; ES, egg string; GT, genital trunk; L 1, leg 1; L 2, leg 2; MAN, mandible; MAX I, maxilla I; MAX II, maxilla II, MT, mouth tube; PAP, post-antennary process; RA, rostral area.
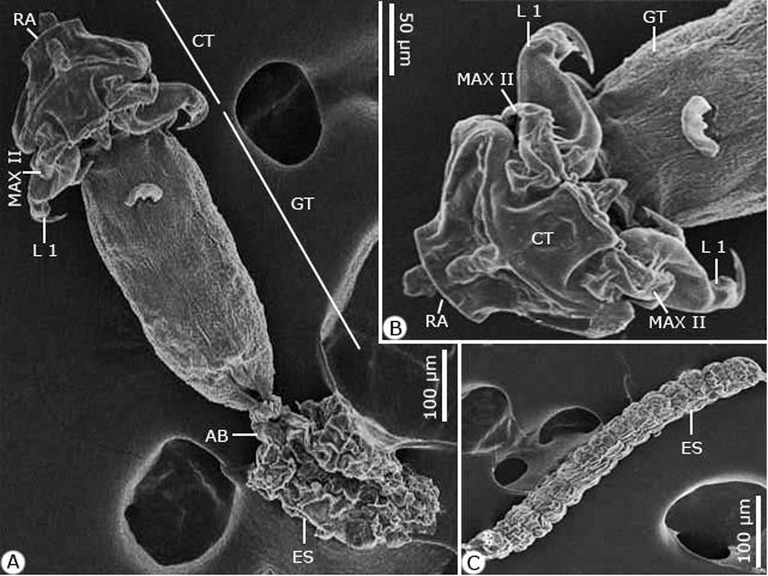
Scanning electron micrographs of the adult female Hatschekia leptoscari infecting Parupeneus rubescens. (A) Whole-mount preparation. (B, C) High magnifications for (B) Cephalothorax. (C) Egg string. Note: AB, abdomen; CT, cephalothorax; ES, egg string; GT, genital trunk; L 1, leg 1; MAX II, maxilla II; RA, rostral area.
Rostral area contained a pair of ventral swellings. Antennule (antenna I) five-segmented diminished in size distally and armed with squat, blunt spines, and short setae. Antenna (antenna II) accompanied by the postantennary process laterally and contained three segments; the second segment constructed at approximately the middle, with anterodistal expansion, and the third segment formed a claw and inner seta on the shaft. Mouthparts and maxilla arranged side by side on the posteroventral margin of the cephalothorax. Oral cone present with a distal membrane on the labium. Mandible composed of a broad base and styliform gnathobase. Maxillule (maxilla I) bilobate, with the outer lobe tipped with two setae and the inner lobe with one seta, each distally and ventrally. Maxilla (maxilla II) four-segmented; the second segment had proximoventral seta, the third segment had distal seta, and the fourth segment had a bifid claw and distal seta. No maxilliped present. Leg I biramous; the exopodite two-segmented, with the basal segment bearing seta at the apex and the terminal segment containing five apical setae; the endopodite one-segmented, bearing five apical setae. Leg 2 biramous; the exopodite two-segmented, with the basal segment bearing one seta and the terminal segment containing three setae; the endopodite one-segmented with four apical setae. Legs 3 and 4 represent the lateral seta situated at the anterior 50 and 80% of the trunk, respectively.
Dimensions: body length excluding caudal rami 0.83 (0.70–0.91); cephalothorax length 0.27 (0.20–0.32), width 0.31 (0.28–0.35); trunk length 0.56 (0.49–0.64), width 0.36 (0.30–0.43); egg string length 0.47 (0.40–0.51), diameter 0.12 (0.10–0.17), containing a single egg.
H. leptoscari was initially described by Yamaguti (1939) as specimens recovered from Leptoscarus japonicas in Japanese waters, followed by Bakhrebah (2006), was isolated the same species from the rusty parrotfish Scarus ferrugineus (Scaridae) from the Red Sea coast of Jeddah, Saudi Arabia. Izawa (2016) recovered the same species from the Japanese parrotfish Calotomus japonicus (Scaridae) in Seto, Wakayama Prefecture. The morphological features re-described here agree with the original description from the other related studies, except for variations in size and body proportions, which is explained by the intraspecific variation and not interspecies differences as mentioned by Jones (1985). Additionally, H. leptoscari shows a close resemblance to Hatschekia elongate recovered by Redkar et al. (1950) from Lutjanus johnii in Bombay, but it can be distinguished by the armature of the second leg and the shape of the claw on the second antenna. It also bears a resemblance to Hatschekia novaculopsi Izawa (2016) from Calotomus japonicus in Wakayama Prefecture. However, it can be differentiated by two posterolateral processes on the trunk and a cuticular frame between the intercoxal bars of the first two legs. Similarly, it bears a resemblance to Hatschekia pseudolabri Yamaguti, 1953, which infects Pseudolabrus japonicus and Bodianus vulpinus from Japan latter that having three setae and one-minute seta on the terminal segment of the first exopod.
3.5 Molecular analysis
To study the marine recovered copepod species' taxonomy and classification, phylogenetic analyses focused on the 28S rRNA gene sequences. For H. sargi, the amplified and sequenced 28S rRNA gene regions (GC content) included 630 bp (46.5%) and were deposited in GenBank under accession number MT677851.1. The sequence of H. leptoscari included 621 bp (45.6% for GC content) and deposited in GenBank under accession number MT677850.1. The gene sequences obtained were correlated with one another and with other gene sequences accessible from GenBank. BLAST searches found that these sequences are closely related to those of other copepods that were previously sequenced but were not similar to either of these species. Sequence variation in 28S rRNA among all specimen sequences was 0.24. The present cladograms were constructed from Podoplean species within the Copepoda and described by three orders: Cyclopoida, Harpacticoida, and Siphonostomatoida (Figs. 5–7). These phylogenetic trees consist of two clades; the major clade is comprised of members belonging to the three orders mentioned above with different families, as follows: Harpacticoida (Ameiridae, Cylindropsyllidae, Canthocamptidae, Thompsonulidae, Idyanthidae, and Aegisthidae); Cyclopoida (Cyclopidae); and Siphonostomatoida (Asterocheridae, Pandaridae, Pennellidae, Megapontiidae, Nicothoidae, Caligidae, and Hatschekiidae). The minor clade includes the remaining Siphonostomatoida members represented by the closely allied Sphyriidae and Lernaeopodidae families. Harpacticoida was shown to be closely related to Cyclopoida. Siphonostomatoida was more similar to Cyclopoida than Harpacticoida. Phylogenetic trees endorsed a paraphyly of Siphonostomatoida with monophyletic assemblages from its families. Hatschekiidae is forming a sister clade to Caligidae. There was a strong relationship between Sphyriidae and Lernaeopodidae. The ME tree showed a well-resolved distinct clade with other Siphonostomatoida species members and the recovered Copepoda species, particularly those belonging to the Hatschekiidae family and deeply embedded within the Hatschekia genus, with close relationships to the previously mentioned Hatschekia japonica (gb| KR048858.1) in the same taxon.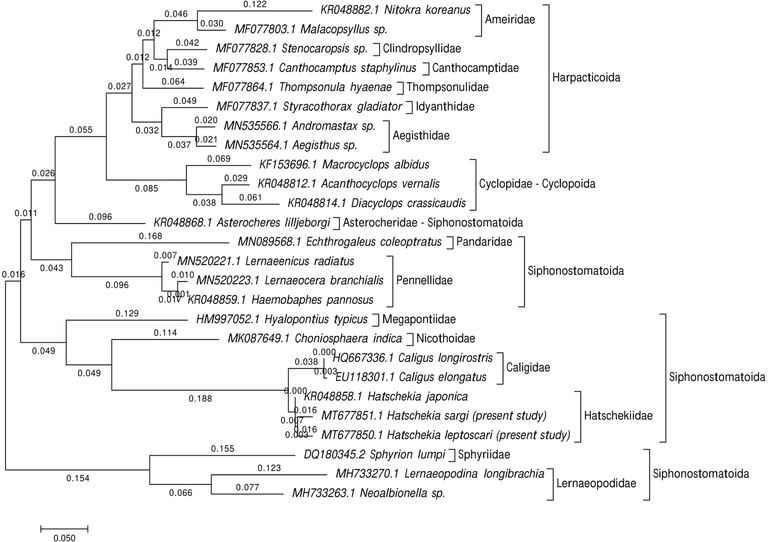
The phylogenetic analysis focused on a partial 28S rRNA sequence indicating the recovered Hatschekia species' position with other species of copepods. The tree has been constructed using the Maximum Likelihood approach based on the K2P model. The tree with the highest log likelihood (−4558.32) is shown.
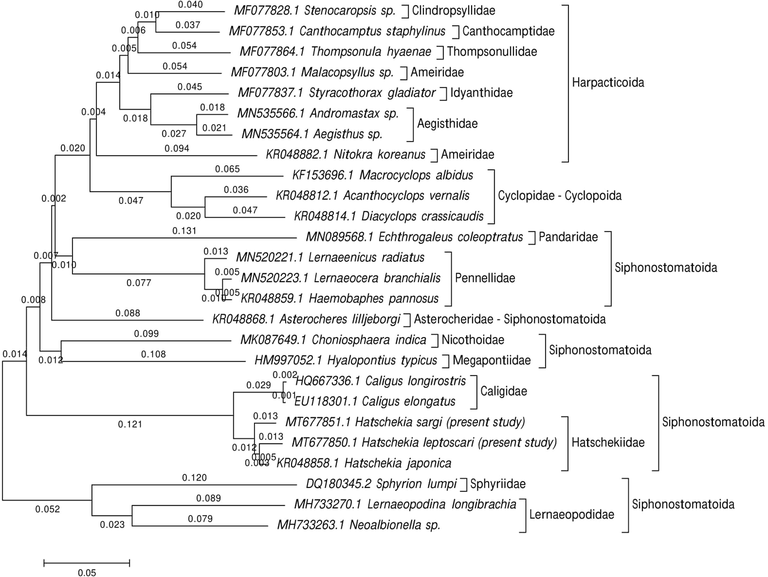
Evolutionary relationships of taxa were inferred using the Neighbor-Joining method and computed with the p-distance method.
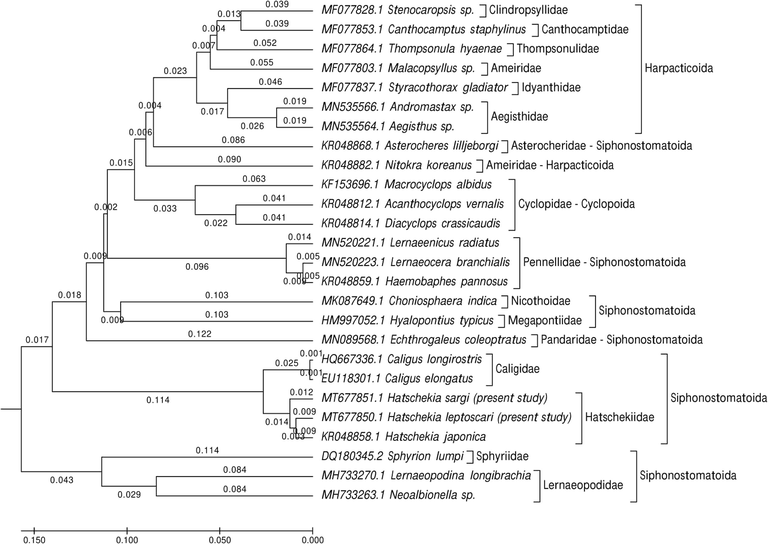
Evolutionary taxa relationships were inferred using the UPGMA method and computed using the p-distance method and are in the units of the number of base differences per site.
4 Discussion
Goatfish are considered a commercially important fish species, both locally and regionally; however, knowledge regarding the parasite fauna is limited (Abdel-Gaber et al., 2020). In this study, two copepod species were found that had naturally infected the gill region of the rosy goatfish, which is a perciform belonging to the family Mullidae that inhabits the coasts of Jeddah, Saudi Arabia. Since most species of Hatschekia are parasitic on fish of the same family, species that utilize fish hosts from more than two families are relatively rare (El-Rashidy and Boxshall, 2011). This finding may also imply that host specificity in the Hatschekia species is usually high (Uyeno and Ali, 2013). The Hatschekia genus is among the most successful genera of siphonostomatoid copepods within the Hatschekiidae family.
Fish gills are considered the most suitable attachment site for all Hatschekia genus members except, as stated by Wierzbicka (1989), of Hatschekia reinhardtii found on the ventral fin of the Greenkand halibut Reinhardtius hippoglossoides. Ectoparasites attached to the gills commonly display a preference for certain holobranches (Lo and Morand, 2001). In the present study, the recovered Hatschekia species did not display a preference for the left or right side of the fish, which is consistent with previous findings for most other species of Hatschekia and many other ectoparasites (Geets et al., 1997; Muñoz and Cribb, 2005; Scott-Holland et al., 2006). Conversely, Collins (1984) found significant differences in the abundance of Hatschekia oblonga Wilson, 1913 between the left and right gill of the host, Ocyurus chrysurus (Bloch); however, this was concluded likely due to chance given the low number of individuals involved.
In the present study, Hatschekia species were recovered from the gills of 53.75% of the rosy goatfish specimens, indicating that it is a moderately prevalent parasite. This was similar to the rate of infection (57.1%, Wierzbicka, 1989) of H. reinhardtii in Reinhardtius hippoglossoides from the Gulf of Bering Sea; Hatschekia sp. in Scolopsis dubuosus (53.33%; Purivirojkul and Areechon, 2008) from Thailand; H. conifer in Thyrsites atun (54.6%, Nunkoo et al., 2016) from the west coast of South Africa; and H. longiabdominalis in Arothron hispidus (53.3%, Soler-Jiménez et al., 2019) from Japan. However, this prevalence rate was higher than that of H. mulli inhabited the Tunisan coasts (29.03%, Boualleg et al., 2010); H. gracilis in Nemipterus japonicus (20%; Tadros et al., 2014) from Al-Ghardaqah; Japan (11.1%, Nagasawa, 2017) of H. pagrosomi from Evynnis tumifrons; and H. bicaudata from Chaetodon aureofasciatus (23.1%, Soler-Jiménez et al., 2019) in Australia. However, this prevalence rate was lower than that of H. caudate in Lutianus vitta from the Gulf of Thailand (82.35%; Purivirojkul and Areechon, 2008).
Very little is known about the feeding mechanisms of Hatschekiidae on their host tissues (Scott-Holland et al., 2006). For example, there is a lack of information on the characteristics of the mouthparts because they are not deemed necessary for taxonomic identification (Jones, 1985). Based on the diagnostic characteristics of species of the genus Hatschekia Poche, 1902 as described by Kabata (1979) and Jones (1985), the recovered species are related to the Hatschekia genus in that they possess the same body size (rarely exceeding a length of 2 mm), the cephalothorax is usually separated from the trunk by a neck-like constriction, the trunk ranges in shape from sub-cylindrical to sub-spherical, the abdomen is one-segmented (partly or entirely incorporated in the trunk), the second antenna is prehensile, the maxilliped is absent, the presence of two pairs of biramous legs while other pairs are vestigial or absent, and the females produce a small number of eggs.
Globally, high levels of diversity for Copepoda species have been reported (Brancelj, 2002). DNA sequencing was used to estimate the phylogenetic relationships between siphonostomatoid families infecting elasmobranchs (Kress et al., 2015; Baek et al., 2016). Previous studies have used rRNA genes (18S rRNA and 28S rRNA) to propose phylogenetic relationships among copepod families and genera (Huys et al., 2012; Brown et al., 2010; Blanco-Bercial et al., 2011; Hayward et al., 2011; Yeom et al., 2018). The present study demonstrated the partial 28S rRNA gene sequence variation as a taxonomic marker for the species identification of the ecologically important yet understudied siphonostomatoid copepod genus Hatschekia from the Arabian Gulf fish. González et al. (2016) reported that the 28S rRNA gene has a low mutation rate and is limited in species-level identification beyond the basal branch in phylogenetic trees. The 28S rRNA sequence alone cannot be used for species identification; however, combined with a morphological description, it can provide a basis for species identification (Weigand et al., 2010; Morales-Serna et al., 2019).
The present ME tree is constructed of two clades representing three orders within the Podoplea infraclasses. It is consistent with the phylogeny of Huys and Boxshall (1991) based on the nuclear rRNA sequences using maximum parsimony and Bayesian tree reconstruction except for the presence of Calanoida. This analysis demonstrated that Siphonostomatoida is paraphyletic, consistent with results reported by Huys et al. (2007). Siphonostomatoida in the present phylogeny was placed as a sister-group to Cyclopoida, which agrees with the Boxshall scheme (1986) who stated that this relationship was based on the synapomorphies’ possession of, at most, a one-segmented antennary exopod, and the loss of the entire exopod in the last order. Eyun (2017) reported that Harpacticoida is a basal-branch group of Podoplea and is strongly linked to Cyclopoida, which agrees with the results presented herein.
The present 28S dataset for nine families within Siphonostomatoida and the present ML tree strongly supported these families' monophyly. This is consistent with the Kakui (2016) hypothesis reporting the monophyletic origin of Siphonostomatoida families but did not recover Nicothoidae (represented by Choniosphaera indica) monophyletic, which is not observed herein. According, Huys and Boxshall (1991) remarked that Nicothoidae is the only invertebrate-associated family with a cephalic frontal filament attaching the infective copepod to its host.
Herein, the basal position of the two lernaeopodid taxa (Lernaeopodina longibrachia and Neoalbionella sp.) in this clade is of particular significance since the Lernaeopodidae form a close relationship with Sphyriidae in regards to the morphology of the dwarf males as stated by Kabata (1981), and the known copepod stage of the Sphyriidae that closely resemble those of the Lernaeopodidae as reported by Wilson (1932). Both families form a plesiomorphic group of the vertebrate associates in the estimated topology, have retained the antennary exopod, and don’t have the apomorphic uniseriate type of egg sac in which discoid eggs are tightly packed to form a cylindrical egg string as mentioned by Huys et al. (2007).
The current phylogeny demonstrates a relationship between caligids, lernaeopodids, and hatschekids, which agrees with previous studies by Boxshall and Halsey (2004a), Boxshall and Halsey (2004b), Ho and Lin (2004), Johnson et al. (2004), Dippenaar (2009), and Moon and Kim (2012) stated the hypothesis for this relationship is that they are dominant among the parasitic copepods of fish. Additionally, there was a close relationship between Pandaridae and Pennellidae, according to the hypotheses of Kabata (1972), Kabata (1979), Boxshall (1986), Huys et al. (2007), Muñoz et al. (2015), and Yasuike et al. (2012), reported that both species have linear and coiled egg sacs. Herein, Hatschekiidae is described by the one genus Hatschekia. The species analyzed here, H. sargi and H. leptoscari, have been confirmed by molecular analyses to be distinct species with a close relationship to the previously described H. japonica (KR048858.1), as mentioned in the morphological taxonomic analyses.
5 Conclusion
In this study, the host reported Parupeneus rubescens, a new host fish recorded for Hatschekia sargi and Hatschekia leptoscari in Saudi Arabia. This study was considered a re-description of those Hatschekiidae species based on distinctive morphological characters. However, it is the first report on using partial 28S rRNA gene sequences to help determine the taxonomic position within the Hatschekiidae family.
Acknowledgements
This study was supported by the Researchers Supporting Project (RSP-2020/25), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Light and scanning electron microscopic studies of Lepeophtheirus salmonis (Siphonostomatoida: Caligidae) infecting the rosy goatfish Parupeneus rubescens. J. Asia Pac. Biodivers.. 2020;13:282-287.
- [Google Scholar]
- DNA barcoding of metazoan zooplankton copepods from South Korea. PLoS ONE. 2016;11(7):e0157307.
- [Google Scholar]
- New records for Hatshekia sargi (Copepoda: Hatschekidae) from carcharhinid fish in red sea of Saudi Arabia. Egypt. J. Aquat. Res.. 2008;34:181-186.
- [Google Scholar]
- A brief revision of the genus Hatschekia Poche, 1902 and redescription of Hatschekia leptoscari, Yamaguti, 1939 parasitic on the Red Sea fish “Scarus ferrugineus” in Saudi Arabia. Alex. J. Agric. Res.. 2006;51(3):41-44.
- [Google Scholar]
- Note sur un Crustace parasite noveau, avec I 'enumeration des especes de cette classe qu'on observe sur les poissons du littoral de Belgique. Bull. Acad. R. Sci.. 1851;18:286-290.
- [Google Scholar]
- Molecular phylogeny of the Calanoida (Crustacea: Copepoda) Mol. Phylogenet. Evol.. 2011;59:103-113.
- [Google Scholar]
- Les copepods parasites des poissons Téléostéens du littoral Est Algérien. Bulletin de l’institut Scientifique, Rabat, Section, Sciences de la Vie. 2010;32(2):65-72.
- [Google Scholar]
- Boxshall, G.A., Halsey, S.H., 2004. An Introduction to Copepod Diversity. Part II. The Ray Society, London, 966 p.
- Boxshall, G., 2013. Siphonostomatoida (http://www.Marinespecies.org/aphia.php?p=taxdetails&id= 1104). WoRMS. World Register of Marine Species. Retrieved October 12, 2013.
- A new genus and species of parasitic copepod (Siphonostomatoida: Hatschekiidae) from an Australian conger eel. J. Nat. Hist.. 1987;21:191-197.
- [Google Scholar]
- Parasitic copepods of fishes: a new genus of the Hatschekiidae from New Caledonia, and new records of the Pennellidae, Sphyriidae and Lernanthropidae from the South Atlantic and South Pacific. Syst. Parasitol.. 1989;13:201-222.
- [Google Scholar]
- Boxshall, G.A., Halsey, S.H., 2004. An Introduction to Copepod Diversity. London: The Ray Society.
- Microdistribution and high diversity of Copepoda (Crustacea) in a small cave in central Slovenia. Hydrobiologia. 2002;477(1):59-72.
- [Google Scholar]
- Note su alcuni crostacei parassiti dei pesci del Meditterraneo. Atti della Societa ligustica di Scienze Naturalie geografiche. 1902;13:30-45.
- [Google Scholar]
- Distribution, diversity and toxin composition of the genus Alexandrium (Dinophyceae) in Scottish waters. Eur. J. Phycol.. 2010;45:375-393.
- [Google Scholar]
- Beschreibung einiger neuen oder weniger bekannten Schmarotzerkrebse, nebst allgemeinen Betrachtungen über die Gruppe, welcher sie angehören. Nova Acta Physico-Medica Academiae Caesareae Leopoldino-Carolinae Naturae Curiosorum (Acta der Kaiserlichen Leopoldinisch-Carolinischen Deutschen Akademie der Naturforscher) Halle. 1835;17(1):269-336.
- [Google Scholar]
- A new genus of Copepoda: Hatschekiidae parasitic on Dicrolene nigra off the Chilean coast. J. Nat. Hist.. 1989;23:129-135.
- [Google Scholar]
- Hatschekia oblonga (Copepoda, Caligoida) from yellowtail snapper (Ocyurus chrysurus) in the Florida Keys. J. Wildl. Dis.. 1984;20(1):63-64.
- [Google Scholar]
- Estimated molecular phylogenetic relationships of six siphonostomatoid (Copepoda) families symbiotic on elasmobranchs. Crustaceana. 2009;82
- [Google Scholar]
- Two new species of parasitic copepods (Crustacea) on two immigrant fishes from the Red Sea of family Siganidae. Syst. Parasitol.. 2011;79:175-193.
- [Google Scholar]
- Phylogenomic analysis of Copepoda (Arthropoda, Crustacea) reveals unexpected similarities with earlier proposed morphological phylogenies. BMC Evol. Biol.. 2017;17:23.
- [Google Scholar]
- Body architecture and relationships among basal copepods. J. Crust. Biol.. 2010;30:465-477.
- [Google Scholar]
- Rhabdomoplea, a new superorder for the thaumatopsylloid copepods: the consequence of an alternative hypothesis of copepod phylogeny. Crustaceana. 2019;92:177-188.
- [Google Scholar]
- Small subunit rDNA and Bayesian inference reveal Pectenophilus ornatus (Copepoda incertae sedis) as highly transformed Mytilicolidae, and support assignment of Chondracanthidae and Xarifiidae to Lichomolgoidea (Cyclopoida) Biol. J. Linn. Soc.. 2006;87:403-425.
- [Google Scholar]
- Fosshagen, A., Iliffe, T.M., 1985. Two new genera of Calanoida and a new order of Copepoda, Platycopioida, from marine caves on Bermuda Sarsia 70(4), 345–58, figs. 1–8.
- Ectoparasites of the whitespotted rabbitfish, Siganus sutor (Valenciennes, 1835) off the Kenyan coast: distribution within the host population and site selection on the gills. Parasitology. 1997;115:69-79.
- [Google Scholar]
- Giesbrecht, W., 1882. Die freilebenden Copepoden der Kieler Foehrde. VI. Ber. Comm. wiss. Untersuch. dt. Meere 1877-1881, Abt. 1: 87–168, pls. 1–12.
- Sea lice (Siphonostomatoida: Caligidae) diversity on littoral fishes from the south-eastern Pacific coast determined from morphology and molecular analysis, with description of a new species (Lepeophtheirus confusum) Parasitol. Inter.. 2016;65:685-695.
- [Google Scholar]
- BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser.. 1999;41:95-98.
- [Google Scholar]
- A new species of Prohatschekia Nune-Ruivo, 1954 (Copepoda: Hatschekiidae) parasitic on Scorpaena elongata (Cadenat) of Algeria. Syst. Parasitol.. 2007;67:119-124.
- [Google Scholar]
- Sea lice infections of wild fishes near ranched southern bluefin tuna (Thunnus maccoyii) in South Australia. Aquaculture. 2011;320:178-182.
- [Google Scholar]
- Hesse, M., 1879. Description de 10 nouveaux Crustaces dout 7 appartiennent au genre Cycnus de Kreyer et 3 au genre Kreyer de van Beneden. Ann. Sci. Nat., Zool. Biol. Anim. series 6. vol. 8(11). 34p.
- Ho, J.S., Lin, C.L., 2004. Sea Lice of Taiwan (Copepoda: Siphonostomatoida: Caligidae). Keelung, Taiwan: The Sueichan Press.
- A new species of Copepoda (Thaumatopsyllidae) symbiotic with a brittle star from California, USA, and designation of a new order Thaumatopsylloida. J. Crustac. Biol.. 2003;23:582-594.
- [Google Scholar]
- New species of Hatschekia Poche, 1902 (Copepoda: Hatschekiidae) parasitic on marine fishes of Kuwait. Syst. Parasitol.. 2001;49:73-79.
- [Google Scholar]
- Brooding in cocculiniform limpets (Gastropoda) and familial distinctiveness of the Nucellicolidae (Copepoda): misconceptions reviewed from a chitonophilid perspective. Biol. J. Linn. Soc.. 2002;75(2):187-217.
- [Google Scholar]
- Evolution of the bomolochiform superfamily complex (Copepoda: Cyclopoida): new insights from ssrDNA and morphology, and origin of umazuracolids from polychaete-infesting ancestors rejected. Inter. J. Parasitol.. 2012;42:71-92.
- [Google Scholar]
- Huys, R., Boxshall, G.A., 1991. Copepod Evolution. Ray Society, London, pp. 159, 1–468.
- Extraordinary host switching in siphonostomatoid copepods and the demise of the Monstrilloida: integrating molecular data, ontogeny and antennulary morphology. Mol. Phylogenet. Evol.. 2007;43:368-378.
- [Google Scholar]
- Some new and known species of Hatschekia Poche, 1902 (Copepoda, Siphonostomatoida, Hatschekiidae) parasitic on the branchial lamellae of Japanese actinopterygian fishes belonging to perciformes, with revision of the known species of the genus. Crustaceana. 2016;89:209-238.
- [Google Scholar]
- A review of the impacts of parasitic copepods on marine aquaculture. Zool. Stud.. 2004;43:8-19.
- [Google Scholar]
- A revison of Hatschekia Poche, 1902 (Copepoda: Hatschekiidae), parasitic on marine fishes. N. Z. J. Zool.. 1985;12:213-271.
- [Google Scholar]
- Kabata, Z., 1972. Developmental stages of Caligus clemensi: (Copepoda: Caligidae). 1. Fish. Res. Bd. Can. 29, 1571–1593.
- Copepoda (Crustacea) parasitic on fishes: problems and perspectives. Adv. Parasitol.. 1981;19:1-71.
- [Google Scholar]
- Kabata, Z., 1979. Parasitic Copepoda of British fishes. The Ray Society, London, pp. 1–486.
- Descriptions of two new species of Rhizorhina Hansen, 1892 (Copepoda: Siphonostomatoida: Nicothoidae) parasitic on tanaidacean crustaceans, with a note on their phylogenetic position. Syst. Parasitol.. 2016;93:57-68.
- [Google Scholar]
- Molecular evidence for the retention of the Thaumatopsyllidae in the order Cycloppoida (Copepoda) and establishment of four suborders and two families within the Cyclopoida. Mol. Phylogenet. Evol.. 2019;138:43-52.
- [Google Scholar]
- Molecular phylogeny of poecilostome copepods based on the 18S rDNA sequences. Korean J. Biol. Sci.. 2000;4:257-261.
- [Google Scholar]
- DNA barcodes for ecology, evolution, and conservation. Trends Ecol. Evol.. 2015;30:25-35.
- [Google Scholar]
- MEGA7: molecular evolutionary genetics analysis version 7.0. Mol. Biol. Evol.. 2016;33:1870-1874.
- [Google Scholar]
- Lang, K., 1948. Copepoda 'Notodelphyoida' from the Swedish west-coast with an outline on the systematics of the Copepoda. Arkiv för Zoologi (A), 40(14), 1–36, figs. 1–17, pl. 1.
- Gill parasites of Cephalopholis argus (Teleostei: Serranidae) from Moorea (French Polynesia): site selection and coexistence. Folia Parasitol.. 2001;48:30-36.
- [Google Scholar]
- Mariniello, L., 2010. Copepodi parassiti ed associate parasitic and associated copepods. Biol. Mar. Mediterr. 17 (suppl. 1), 438–451.
- Milne Edwards, H., 1840. Ordre des Copepodes. In: Histoire naturelle des Crustaces, comprenant l'anatomie, la physiologie et la classification de ces animaux 3, 411–529.
- Sea lice (Copepoda, Siphonostomatoida, Caligidae) new to Korea, including three new species. J. Species Res.. 2012;1:175-217.
- [Google Scholar]
- Description of Echthrogaleus spinulus n. sp. (Copepoda: Pandaridae) parasitic on a torpedo ray from the central Pacific Ocean utilising a morphological and molecular approach. Syst. Parasitol.. 2019;96:777-788.
- [Google Scholar]
- Infracommunity structure of parasites of Hemigymnus melapterus (Pisces: Labridae) from lizard island, Australia: the importance of Habitat and parasite body size. J. Parasitol.. 2005;91(1):38-44.
- [Google Scholar]
- Muñoz, G., Landaeta, M.F., Palacios-Fuentes, P., López, Z., González, M.T., 2015. Parasite richness in fish larvae from the nearshore waters of central and northern Chile. Folia Parasitol. 62, 1–12.
- Two species of copepods, Lernanthropus atrox and Hatschekia pagrosomi, parasitic on crimson seabream, Evynnis tumifrons, in Hiroshima Bay, western Japan. Biosphere Sci.. 2017;56:13-21.
- [Google Scholar]
- Parasites de poissons de mer ouest-africains récoltés par M. J. Cadenat III. Copépodes (2e note). Genres Prohatschekia n. gen. et Hatschekia Poche. Bulletin de l’Institute Français d’Afrique noire (A). 1954;16:479-505.
- [Google Scholar]
- Community ecology of the metazoan parasites of snoek Thyrsites atun (Euphrasen, 1791) (Perciformes: Gempylidae) off South Africa. Afr. J. Mar. Sci.. 2016;38:363-371.
- [Google Scholar]
- Poche, F., 1902. 3. Bemerkungen zu der Arbeit des Herrn Bassett-Smith: 'A systematic description of parasitic Copepoda found on fishes, with an enumeration of the known species'. Zool. Anz. 26(685), 8–20.
- A survey of parasitic copepods in marine fishes from the Gulf of Thailand, Chon Buri Province. Kasetsart J. (Nat. Sci.). 2008;42:40-48.
- [Google Scholar]
- Quoy, J.R.C., Gaimard, J.P., 1824. Description des Poissons. Chapître IX. In: Freycinet, L. de, Voyage autour du Monde...exécuté sur les corvettes de L. M. “L'Uranie” et “La Physicienne,” pendant les années 1817, 1818, 1819 et 1820. Paris. Description des Poissons. Description des Poissons. Chapter IX.: 192–401 [1–328 in 1824; 329–616 in 1825], Atlas pls. 43–65.
- Ramdane, Z., 2009. Identification et écologie des ectoparasites Crustacés des poissons Téléostéens de la côte Est algérienne. Thèse de doctorat de l’Université Badji Moktar Annaba p. 235.
- Two new species of Hatschekia parasitic on Bombay fishes. Bombay Univ. J., N.s.. 1950;196(3):35-42.
- [Google Scholar]
- A novel microhabitat for parasitic copepods: a new genus of Ergasilidae (Copepoda: Cyclopoida) from the urinary bladder of a freshwater fish. Parasitol. Int.. 2013;62:347-354.
- [Google Scholar]
- Sars, G.O., 1903. An account of the Crustacea of Norway, with short descriptions and figures of all the species: IV. Copepoda Calanoida. Bergens Museum: Bergen, 171, Plates I-CII & suppl. I-VI pp.
- Distribution of an asymmetrical copepod, Hatschekia plectropomi, on the gills of Plectropomus leopardus. J. Fish Biol.. 2006;68:222-235.
- [Google Scholar]
- Parasitic copepods (Crustacea, Hexanauplia) on fishes from the lagoon flats of Palmyra Atoll, Central Pacific. ZooKeys. 2019;833:85-106.
- [Google Scholar]
- Phylogeny of freshwater parasitic copepods in the Ergasilidae (Copepoda: Poecilostomatoida) based on 18S and 28S rDNA sequences. Parasitol. Res.. 2008;102:299-306.
- [Google Scholar]
- Light and scanning electron microscopy on monogenean parasite from Red sea fish Nemipterus japonicus (Trieadin braens) in Egypt. Assiut Vet. Med. J.. 2014;60(141):54-62.
- [Google Scholar]
- The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nuc. Acids Res.. 1997;25:4876-4882.
- [Google Scholar]
- Parasitic copepods from two species of commercial fishes collected off Iraq, with description of Hatschekia shari n. sp. Syst. Parasitol.. 2013;86:301-312.
- [Google Scholar]
- A new genus and species of hatschekiid copepod (Siphonostomatoida) from groupers (Actinopterygii: Serranidae) collected off the Ryukyu Archipelago, Japan. Syst. Parasitol.. 2013;84:89-95.
- [Google Scholar]
- Walter, T.C., Boxshall, G., 2020. World of Copepods database. Progymnoplea. Accessed through: World Register of Marine Species at: http://www.marinespecies.org/aphia.php?p=taxdetails&id= 155875 on 2020-06-12.
- A new approach to an old conundrum - DNA barcoding sheds new light on phenotypic plasticity and morphological stasis in microsnails (Gastropoda, Pulmonata, Carychiidae) Mol. Ecol. Resour.. 2010;11:255-265.
- [Google Scholar]
- Hatschekia reinhardtii sp. nov. (Copepoda, Hatschekidae), a parasite of Greenland halibut, Reinhardtius hippoglossoides (Walbaum, 1792). Acta Ichthyol. Piscat. Vol. XIX, Fasc.. 1989;2:107-116.
- [Google Scholar]
- Wilson, C.B., 1922. North American parasitic copepods belonging to the family Dichelestiidae. Proc. U. S. Natl. Mus. 60(5) (2400), 1–100, pls. 1–13.
- Wilson, C.B., 1913. Crustacean parasites of West Indian fishes and land crabs, with descriptions of new genera and species. Proc. U. S. Natl. Mus.44, 189–277, PIs. 18 53.
- Yamaguti, S., 1939. Parasitic copepods from fishes of Japan. Part 5. Caligoida, III. In: Volume Jubilare pro Prof. Sadao Yoshida, vol. 2; 1939. pp. 443e487.
- Yamaguti, S., 1953. Parasitic copepods from fishes of Japan. Pt. 7, Cyclopoida, III and Caligoida. IV. Publ. Seto Mar. Biol. Lab. 3, 221–231, PIs. 1–5.
- Genomic resources for sea lice: analysis of ESTs and mitochondrial genomes. Mar. Biotechnol.. 2012;14:155-166.
- [Google Scholar]
- EST and mitochondrial DNA sequences support a distinct pacific form of salmon louse, Lepeophtheirus salmonis. Mar. Biotechnol.. 2008;10:741-749.
- [Google Scholar]
- A new minute ectosymbiotic harpacticoid copepod living on the sea cucumber Eupentacta fraudatrix in the East/Japan Sea. PeerJ. 2018;6:e4979.
- [Google Scholar]
- Yu, S.C., 1933. Chinese parasitic copepods collected by H. W. Wu, with descriptions of new genera and species. Bull. Fan Mem. Inst. Biol. 4, 117–139, pis. 1–8.







