Translate this page into:
Ferulic acid as anticarcinogenic agent against 1,2-dimethylhydrazine induced colon cancer in rats
⁎Corresponding author at: Department of Zoology, College of Science, King Saud University, Saudi Arabia. mdkhil@ksu.edu.sa (Mohamed A. Dkhil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The aim of the study was to assess the beneficial effect of ferulic acid (FA) compared to 5-flurouracil (5FU) on colon cancer caused by 1,2-dimethylhydrazine (DMH).
Method
Rats were divided into four groups (Control, FA, 5FU and DMH groups). The DMH group was injected subcutaneously once a week for 15 weeks. FA group was orally administered three times per week for 4 weeks with dose of 50 mg/kg. The 5FU group was intraperitoneally injected once per week with dose of 50 mg/kg for 4 weeks.
Results
Histopathological, immunohistochemical and molecular studies have been undertaken. The mucosa of the induced colon cancer was significant infiltrated by mononuclear cells and hemorrhage. Also, there was improper shape of the crypts with partial loss of polarity. The muscularis mucosa was infiltrated by pleomorphic cells. The mucosa and submucosa have indeed been invaded by a gland-like set of cells, congested blood vessels with extravagance of mononuclear cells, which are concentrated in the mucosa that fills the corium of the connective tissue. Moreover, colon sections of the DMH treated rats showed intensive immunoreactivity to Ki67, CK20 and weak immunoreactivity to P53 and Caspase-3. Moreover, FA could significantly (p < 0.01) upregulate P53 by approximately 5 fold.
Conclusions
Findings of the present study indicated a significant therapeutic effect of ferulic acid against colon cancer by inhibiting proliferation and promoting apoptosis.
Keywords
Colon cancer
Ferulic acid
Histopathology
P53
Rats
1 Introduction
Colorectal cancer is a malignant tumor of the digestive system. It comes third in the estimated mortality among all cancer types in the prediction data of cancers in 2020 (Zhang et al., 2020). Colorectal cancer is a heterogenous disease which involves inactivation of tumor suppressor genes and activation of oncogenes (Han, 2020). In recent decades, while massive improvement has been achieved in the treatment of colorectal cancer through surgery, different chemotherapy methods and combined chemotherapy, the prognosis and survival rate are still not adequate and patients still face numerous challenges. Thus, there is urgent need for new and effective therapeutic targets for colorectal cancer (Dai, 2020; Zhang et al., 2020).
The early example of rational drug design is 5-Fluorouracil (5FU) because it originated from the observation that tumor cells incorporate radiolabelled uracil more effectively into DNA than normal cells, especially intestinal cells. It is documented that 5FU is metabolized in cells to 5′FdUMP, which inhibits thymidylate synthase. In addition, 5FU is metabolized by dihydropyrimidine dehydrogenase, and the deficiency of this enzyme can cause an increase 5FU toxicity (Jameson, 2018).
Administration of 5FU contributes to bone marrow depletion after short infusions. It also induces CNS dysfunction, with conspicuous cerebellar signs, and thrombosis-related endothelial toxicity, including pulmonary and myocardial injuries (Jameson, 2018).
Ferulic acid (FA) has been separated from Ferula foetida, an abundant lignin-bound phenol in plant cell walls, and is a source to many aromatic compounds. FA is commonly present in rice, wheat, oat, grains, flowers, leaves, fruits, bean, seeds of coffee, peanut, nuts, tomato, spinach, broccoli, carrot, avocado and pineapple (Kumar and Pruthi, 2014; Mancuso and Santangelo, 2014). It is easily available compound, cheap and exerts minimal side effect. FA possesses a wide range of biological effects as an antioxidant, anti-inflammatory, antifungal, antibacterial, antiviral, antithrombosis, antitumor, hepatoprotective and cardioprotective (Ghosh et al., 2018; Peng et al., 2012).
FA's anti-carcinogenic activity is due to its ability to scavenge reactive oxygen species and promote cytoprotective enzyme function. FA decreased lipid peroxidation, single-strand DNA breakdown, inhibition of some proteins and rupture of biological membranes (Barone et al., 2009; Kumar and Pruthi, 2014). FA also has the ability to suppress tumors in the cell lines of breast cancer and has been believed to reduce the side effects of carcinoma's chemotherapy by increasing natural immune defense. (Kumar and Pruthi, 2014; Palani Swamy and Govindaswamy, 2015). In addition, FA has significant impacts on different cancer cell lines, particularly cervical carcinoma cells (Karthikeyan et al., 2011), prostate cancer cells (Eroğlu et al., 2015) and pancreatic cancer cells (Eitsuka et al., 2014).
The aim of the research was to explore the therapeutic activity of ferulic acid in rats with colon cancer.
2 Materials and methods
2.1 Animals
Adult male Wistar-albino rats weighing 180 ± 30 g were used and purchased from the Ain Shams Research Institute, (MASRI, Cairo, Egypt). Animals were supplemented with a standard pellet diet and water ad libitum. The study was approved by the ethical committee for animal care at Faculty of Science, Helwan University, Egypt.
2.2 Experimental design
Forty adult rats were divided into two main groups, Group one being a normal control group (n = 10) that acted as a negative control group and injected normal saline subcutaneously (0.9% NaCl). Group two was the induced colon cancer group that was subcutaneously injected with 50 mg/kg/week 1,2-dimethylhydrazine dihydrochloride (DMH) (Sigma Aldrich, Germany) for 15 weeks (Jucá et al., 2014). Animals of group three were orally treated with Ferulic acid (FA) (Sigma Aldrich, Germany) three times per week for 4 weeks with dose of 50 mg/kg (Ghosh et al., 2018). Rats of the fourth group were treated with 5-Flurouracil (5FU) (Sigma Aldrich, Germany). Rats of this group received intraperitoneal injection (once per week) with 50 mg/kg of 5FU for 4 weeks (Muthu et al., 2013).
2.3 Colon Histopathology
Colon was fixed in 10% neutral buffered formalin, dehydrated in ethanol, cleared in xylene then embedded in paraffin and 5 µm sections were obtained, deparaffinized and stained with hematoxylin and eosin then examined by the light microscope (Suvarna et al., 2018).
2.4 Immunohistochemical study
Using anti-Ki67, anti-Cytokeratin-20, anti-Caspase3 and anti-P53 (Thermo Fisher Science, Waltham, Ma, USA), colon sections of 5 μm thickness from paraffin-embedded tissues were immune-stained with primary antibodies for 90 min, followed by secondary antibody using the immunoperoxidase technique (Krajewska et al., 1997).
2.5 Morphometric study
Morphometric measurements were performed using “Leica Qwin 500C” image analyzer computer system (Cambridge, England). The area percent for positive Ki67, CK20, Caspase 3 and P53 immunoreaction was measured in 10 non overlapping fields for every specimen at magnification ×400 for all groups.
2.6 Quantitative real-time PCR
Total RNA was isolated from the colon tissue using an RNeasy Plus Minikit (Qiagen, Valencia, California, USA). Complementary DNA (cDNA) was synthesized using the RevertAid™ H Minus Reverse Transcriptase (Fermentas, Thermo Fisher Scientific Inc., Canada). The primer set of P53 was, forward, 5′-ATGTTTTGCCAACTGGCCAAG-3′ and reverse, 5′- TGAGCAGCGCTCATGGTG-3′ compared to the reference gene β-actin, forward, 5′-GTGACATCCACACCCAGAGG-3′ and reverse, 5′-ACAGGATGTCAAAACTGCCC-3′. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR Green RT-PCR kit (Life Technologies, Carlsbad, California, USA) and was conducted on the Applied Biosystems 7500 instrument (Waltham, Massachusetts, USA). The typical thermal profile is 95 °C for 4 min, followed by 40 cycles of 94 °C for 60 s and 55 °C for 60 s. The fold changes in gene expression between treated and the control were analyzed using delta-delta cycle threshold (Ct) method through the calculation of 2−ΔΔCt (Livak and Schmittgen, 2001).
2.7 Statistical analysis
The results were presented as the mean and standard deviation (SD). Using the statistical program SigmaPlot 2011 (Systat Software, Inc., Chicago, IL, USA), data was analyzed by one-way analysis of variance (ANOVA). Statistical comparisons between the groups were completed using Duncan’s test. P value ≤ 0.05 was considered statistically significant.
3 Results
In the colon sections of the control group, the typical histological characteristics of the mucosa, submucosa and muscularis layers with an intact mucosal glands with lined epithelial and goblet cells were shown (Fig. 1A). The mucosa of DMH treated animals was infiltrated by mononuclear cells and hemorrhage and there was improper shape of the crypts with partial loss of polarity. Also, the mucosa and submucosa were invaded by gland-like collection of cells, congested blood vessels with extravasation of mononuclear cells which were abundant in the mucosa filling the corium of the connective tissue (Fig. 1B).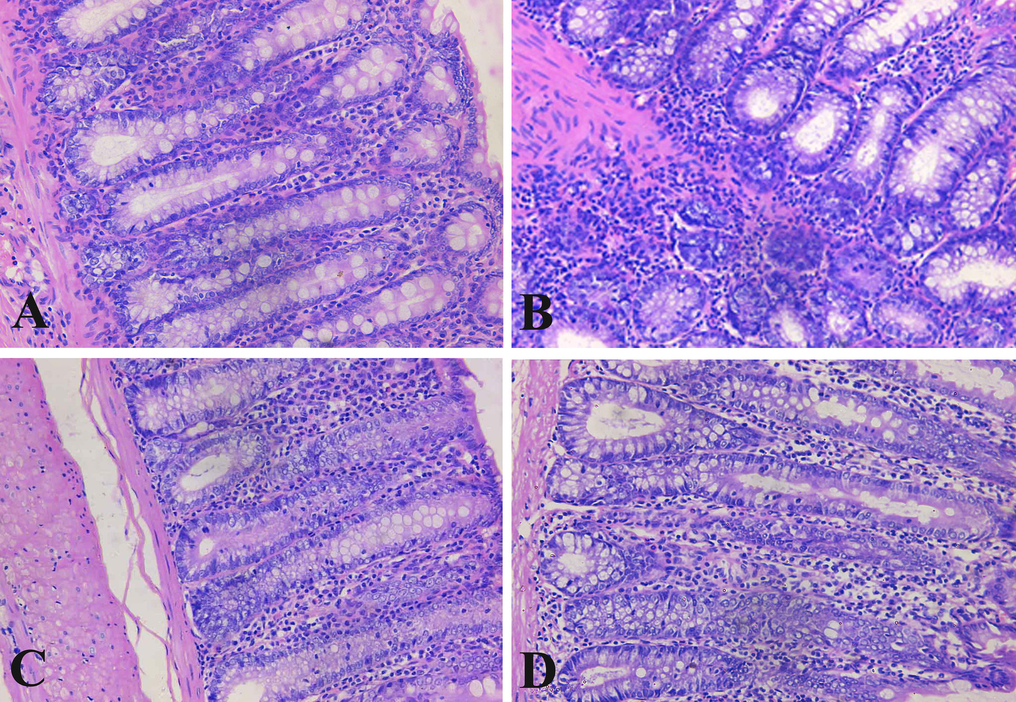
Longitudinal sections of the rat colons. (A): Section of control rat with well-organized intestinal glands appearing with normal lining and covering epithelial cells. (B): Rat section of DMH induced colon cancer. Intestinal gland cells with dysplasia, hyperchromasia, some glands contained necrosis, inflammation and hemorrhage. (C): Ferulic acid treated rat with improvement of glandular intestinal lining and covering cells. (D): Sections of 5-flurouracil treated rat with normal glands. Sections were stained with hematoxylin and eosin. (×400).
The ferulic acid treated rats showed approximately normal architecture of the surface columnar cells. The mucosa appeared intact with infiltrated mononuclear cells with compression on the columnar cells lining the crypts. The submucosa and musculosa showed normal architecture (Fig. 1C). The colon of the 5-flurouracil treated rats appeared with normal structure of the superficial and lining columnar cells of the crypts with infiltration of mononuclear cells. In addition, musculosa and serosa showed normal architecture (Fig. 1D).
The basal glandular cells of the crypts of the control group showed moderate nuclear expression of Ki67, while the apex of the mucosa showed weak immunoreactivity (Fig. 2A). The cells of the DMH treated rats showed clear nuclear Ki67 expression with homogenous intensity in the mucosa layer and irregular distribution of Ki67 in the glandular portion (Fig. 2B). The cells of the ferulic acid treated rats showed moderate nuclear Ki67 expression in the basal portion of the mucosa which approximately similar to the normal rats, while the superficial portion showed weak immunoreactivity (Fig. 2C). The cells of the 5-flurouracil treated rats showed mild nuclear Ki67 expression in the basal portion of the mucosa compared with the DMH group, while the superficial portion showed negative immunoreactivity (Fig. 2D).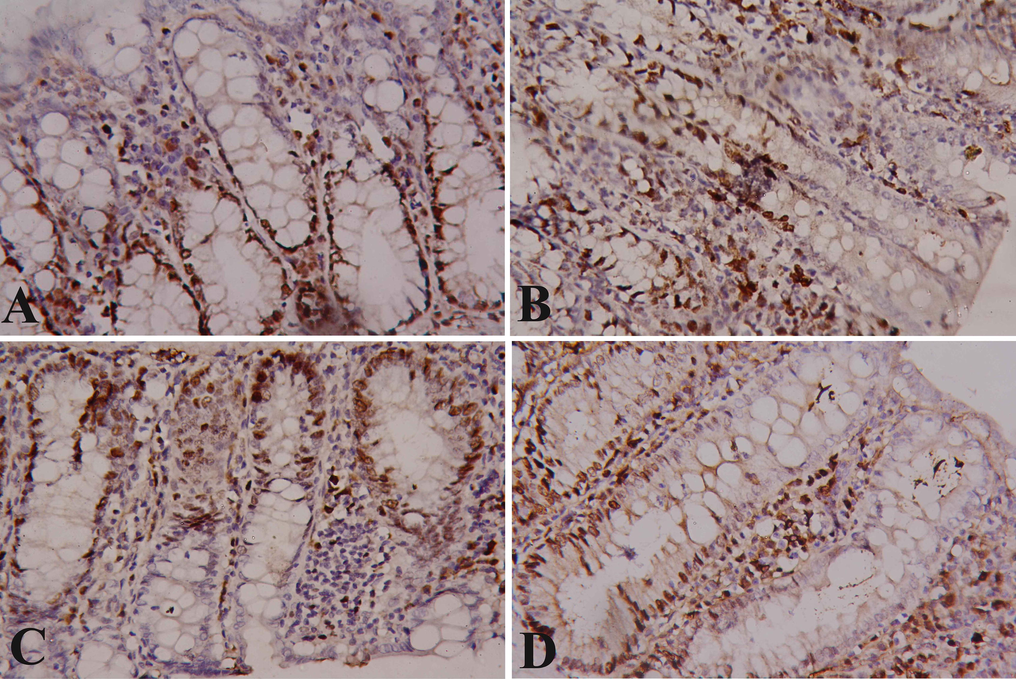
Immunostained colon sections with Ki67. (A): Section of control rat with normal nuclear Ki67 IHC positivity. (B): Rat section of DMH induced colon cancer with intensive increase in anti-Ki67 reaction in the cells of mucosal glands. (C): Ferulic acid treated rat with normal anti-Ki67 reaction in the cells of mucosal glands. (D) Sections of 5-flurouracil treated rats with clear improvement in anti-Ki67 reaction in the cells of mucosal glands. (×400).
The cells of the control colon showed normal cytokeratin 20 filaments immunoreactivity as brown colour filaments stained with avidin–biotin immunoperoxidase technique and using monoclonal antibody against cytokeratin 20. The cytokeratin 20 filaments are more evident at the apical part and lateral borders of the cells. The basal glandular cells showed weak immunoreactivity (Fig. 3A). The cells of the DMH treated rats showed severe cytoplasmic CK20 expression with high intensity in the superficial cells of the mucosa layer and irregular distribution of the expression in the glandular portion (Fig. 3B). The cells of the ferulic acid treated rats showed mild cytoplasmic CK20 expression in the mucosa which approximately similar to the normal rats (Fig. 3C). The cells of the 5-flurouracil treated rats showed mild cytoplasmic CK20 expression in the mucosa compared with the DMH group, while the basal portion showed negative immunoreactivity (Fig. 3D).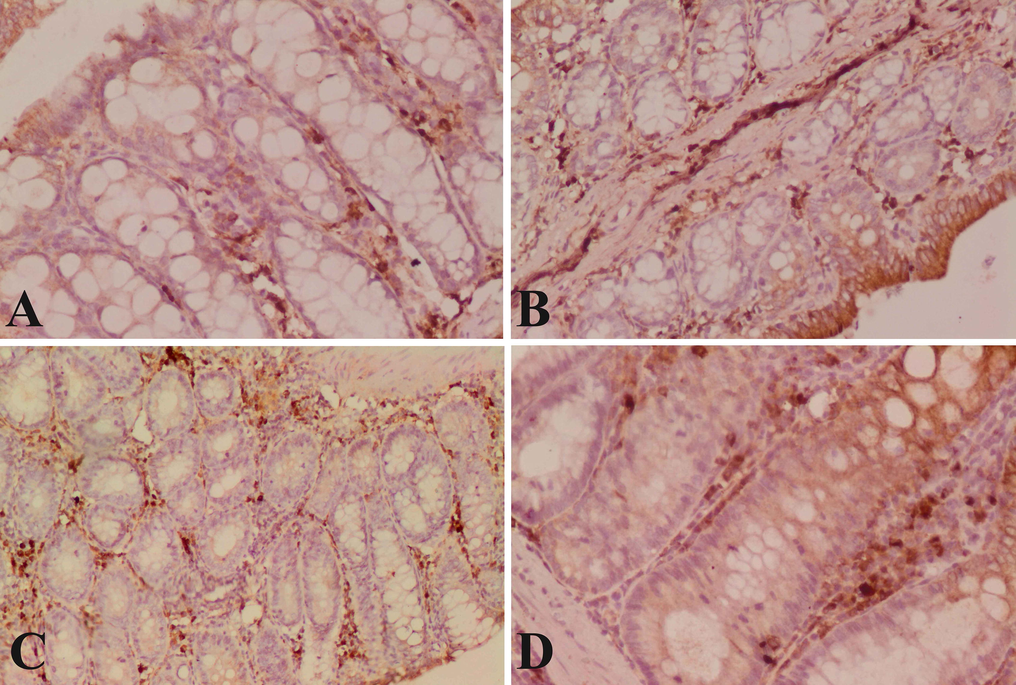
Immunostained colon sections with CK20. (A): Section of control rat with normal cytoplasmic CK20 immunopositivity. (B): Rat section of DMH induced colon cancer with an intensive increase in anti-CK20 reaction in the superficial cells of mucosal glands. (C): Ferulic acid treated rat with normal anti-CK20 reaction in the cells of mucosal glands. (D) Sections of 5-flurouracil treated rats with clear improvement in anti-CK20 reaction in the cells of mucosal glands. (×400).
The glandular cells of the control colon showed normal cytoplasmic moderate Caspase-3 expression (Fig. 4A). The cells of the dimethylhydrazine-induced rats showed weak cytoplasmic Caspase-3 expression with homogenous intensity in the mucosa layer and irregular distribution of the expression in the glandular portion (Fig. 4B). The cells of the ferulic acid treated rats showed moderate cytoplasmic Caspase 3 expression in the mucosa which is higher compared with the DMH group (Fig. 4C). The cells of the 5-flurouracil treated rats showed severe cytoplasmic Caspase-3 expression in the mucosa which is higher than ferulic acid treated rats and with intense increase in Caspase-3 compared with the DMH group (Fig. 4D).
Immunostained colon section with Caspase-3. (A): Section of control rat with normal nuclear Caspase-3 immunopositivity. (B): Rat section of DMH induced colon cancer with weak cytoplasmic Caspase-3 immunopositivity. (C): Ferulic acid treated rat with normal moderate anti-Caspase-3 reaction in the cells of mucosal glands. (D): Sections of 5-flurouracil treated rats with severe cytoplasmic Caspase-3 immunopositivity. (×400).
The healthy colon displayed a strong positive reaction in the glandular cells of the mucosal layer with P53 immunostaining (Fig. 5A). The cells of the dimethylhydrazine-induced rats showed mild P53 expression in the mucosa layer and irregular distribution of the expression in the glandular portion (Fig. 5B). The cells of the ferulic acid treated rats showed severe nuclear P53 expression in the mucosa which is higher compared with the DMH group (Fig. 5C). The cells of the 5-flurouracil treated rats showed severe nuclear P53 expression in the mucosa which is higher than ferulic acid treated rats and much higher compared with the DMH group (Fig. 5D).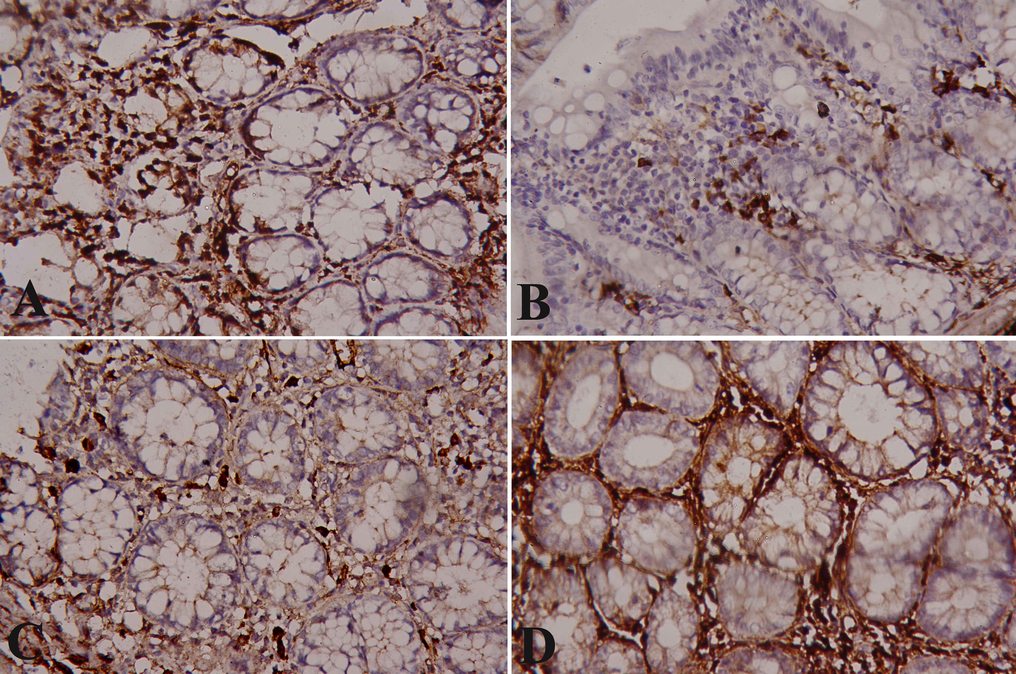
Immunostained colon sections with P53. (A): Normal colon of normal rat showing normal severe nuclear P53 immunopositivity. (B): DMH-induced colon cancer showing mild nuclear P53 immunopositivity. (C): Ferulic acid treated rat showing severe nuclear P53 IHC positivity. (D): 5-Flurouracil treated rat showing severe nuclear P53 IHC positivity. (×400).
Fig. 6 showed the quantitative measurement for positive Ki67, CK20, Caspase 3 and P53 immune reactions. Rats of DMH group appeared with a significant increase in Ki67 and CK20 compared to the control group while there were a significant decrease (p < 0.01) in the intensity of Caspase 3 and P53 compared to the control group. Ferulic acid was able to significantly (p < 0.01) improve the altered change in the immunoreactivity induced by DMH (Fig. 6).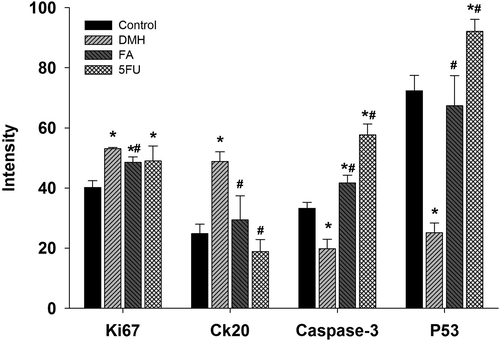
Intensity percentage quantitative measurement for positive Ki67, CK20, Caspase 3 and P53 immune reactions. Values are mean ± SEM at p ≤ 0.05. *, significance against control. #, significance against DMH group.
The qRT-PCR results for P53-mRNA expression was showed in Fig. 7 where a significant (p < 0.01) downregulation in the expression of P53 in DMH administered group was shown. FA could significantly (p < 0.01) upregulate P53 by approximately 5 fold (Fig. 7).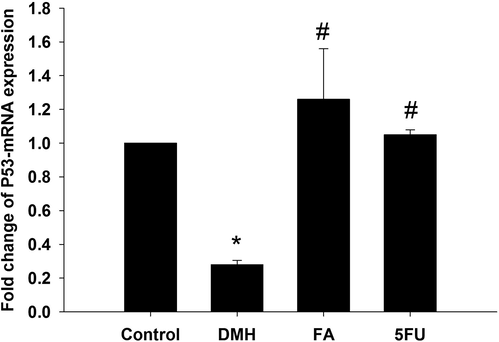
Ferulic acid treatment regulated the gene expression in colon of rat. Expression of hepatic P53 was analyzed by quantitative Real-time PCR in all groups under investigation. Values are mean ± SEM at p ≤ 0.05. *, significance against control. #, significance against DMH group.
4 Discussion
Colorectal cancer (CRC) is one of the most common cancers in the world and is confirmed to be a deadly and multi-factorial malignant tumor involving environmental and dietary agents. With many advancements in our knowledge of carcinogenesis mechanisms, treatments such as surgery, radiation and chemotherapy agents are mostly confined to the treatment of advanced CRC phases (Zhang et al., 2020).
The carcinogenesis and progression of CRC are complicated and are regulated by genetic pathways, characterized by changes in upregulation and formation of proteins controlling proliferation, differentiation and apoptosis (Jucá et al., 2014). The most frequently used chemical-induced colon cancer in animal models is DMH. It is a pro-carcinogenic agent for colon cancer, activated in the liver and passed by bile and blood into the intestine. Its use also enhances the production of free radicals, which cause oxidative damage to the colon and liver cell DNA (De-Souza and Costa-Casagrande, 2018; El-Khadragy et al., 2018). DMH belongs to the indirect inducer group and is capable of promoting DNA hypermethylation of colorectal epithelial cells (El-Khadragy et al., 2018; Jucá et al., 2014).
The rats received a weekly dose of DMH for 15 weeks, and after histopathological examination showed improper shape of the crypts with partial loss of polarity. The muscularis mucosa infiltrated by pleomorphic cells. Dysplasia and anaplasia associated with pleomorphism and hyperchromasia in the lining of epithelial cells of glandular cells were seen in the mucosa and submucosa. These findings are compatible with Abdel-Rahman et al. (2013) and Jucá et al. (2014). The DMH-induced colon cancer rats showed intensive CK20 and Ki67 immunoreactivity and in contrast a weak P53 and Caspase-3 immunoreactivity, these results revealed the malignant nature of cancer cells with increases of proliferation and avoiding apoptosis. The histopathological analysis of FA and 5FU groups showed clear improvements in the mucosa and the glandular lining cells.
The immunohistochemical analysis of FA showed normal protein expression of Ki67 giving evidence for regulation of proliferation. Severe protein expression of Caspase-3, P53 in FA and 5FU treated groups suggests improvement in cell cycle and promoting apoptosis. The DMH treated group showed down-regulated P53 gene expression compared to normal group. While, FA and 5FA groups showed significant elevated regulation of P53 compared to DMH treated.
Ki67 expression is intimately correlated with tumorigenesis and development, and is commonly used as a proliferation marker in routine pathological research. The nuclear protein Ki67 is a proven predictive and prognostic marker for the diagnosis of biopsies from patients (Niotis et al., 2018).
Cytokeratins (CKs) are the epithelial form of intermediate-sized cytoskeleton filaments and mong cytokeratins 20 are the most appropriate cytokeratins (CK20). Due to its highly specific expression, the CK20 protein has recently gained increased interest in both colorectal and gastric cancers (Bayrak et al., 2012; El-Khadragy et al., 2018; Tunca et al., 2013). In the current study, intense CK20 immunostaining was observed in the colorectal tissue of DMH-treated rats compared to mild expression in the control, FA and 5FU treated groups.
Eroglu et al., investigated the molecular mechanism of ferulic acid and evaluated the expression of P53, ATM and ATR which are playing vital role in viability and stability of cell cycle and DNA damage, and reported a significant increase in the gene expression of these genes in FA treated prostate cancer cells compared with control group and suggested that FA enhanced the cellular response by upregulation of P53, ATM and ATR showing antiproliferative effect in prostate cancer cell line (Eroğlu et al., 2015). In addition, FA significantly suppressed the proliferation of J82 bladder cancer cell line (Cherng et al., 2007).
Another study showed that FA in the ECV304 endothelial cells induced cell cycle arrest in the G0/G1 process. In addition, FA prevented the progression of the cell cycle by decreasing CCND1 and phosphorylated RB protein expression and increasing CDKN1A protein expression (Hou et al., 2004).
Nikoleishvili et al. (2008) reported a marked decline in the expressions of the genes CCND1, CCND2, and CCND3 with an increase in RB1 gene expression in the cells of the FA treatment group relative to the cells of the control group in the PC-3 prostate cancer cell line. These findings indicate that because of the down-regulation of genes that played a vital role in cell cycle arrest in the G1/S phase of prostate cancer cell line, FA could cause the progression of the cell cycle to be stopped (Eroğlu et al., 2015; Nikoleishvili et al., 2008).
Peng et al. (2012) assessed the gene expression of Bcl-2, Caspase-3, and Caspase-9 in 2D and 3D cultures of bladder cancers cells and reported that FA showed cytotoxic effect by increasing apoptotic rate in T24 bladder cancer cells. The antiapoptotic signal Bcl-2 was downregulated, while all the apoptotic signals Bax, cleaved Caspase-3, and cleaved Caspase-9 were upregulated by FA, despite in the 2D or the 3D cultures (Peng et al., 2012). In another study, the apoptotic effects of FA were assessed as 18.4 and 22.3%, respectively, in ME-180 and HeLa human cervical carcinoma cells (Karthikeyan et al., 2011). FA also had a significant influence on the proteins of the apoptotic cascade, cyclooxygenase and caspases, decreased cell growth and moderately suppressed colon tumor cells (Palani Swamy and Govindaswamy, 2015). In different cancer cell lines, FA affects cell invasion, migration, and colony formation and could prevent metastasis (Fang et al., 2013).
Collectively, we could be concluded that ferulic acid may have a promising therapeutic role against colon cancer induced by 1,2 dimthylhydrazine as indicated by the observed improvement in the histological structure and measured immunohistochemical and gene expression. These allowed access reasonable treatment strategies for intervention against progressive of colon cancer with special regard to the inflammation, proliferation and apoptosis. Further studies are required, however, regarding the potential hazards of ferulic acid versus the benefits of colon cancer, the delivery mechanism and mode of action.
Acknowledgment
This study was supported by Research Supporting Project (RSP-2020/23), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdel-Rahman, M., Ahmed, H.H., Salem, F.E.-Z.H., Shalby, A.B., Lokman, M.S., 2013. Curcuma longa and Colon Cancer: Evidence and Mechanisms 17.
- Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology. 2009;10:97-108.
- [CrossRef] [Google Scholar]
- The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: cytokeratin 7-/20+ phenotype is more specific than CDX2 antibody. Diagn. Pathol.. 2012;7:9.
- [CrossRef] [Google Scholar]
- Chemopreventive Effects of Minor Dietary Constituents in Common Foods on Human Cancer Cells. Biosci. Biotechnol. Biochem.. 2007;71:1500-1504.
- [CrossRef] [Google Scholar]
- A novel epigenetic signature to predict recurrence-free survival in patients with colon cancer. Acta: Clin. Chim; 2020. p. :7.
- Animal models for colorectal cancer. ABCD Arq. Bras. Cir. Dig. São Paulo. 2018;31
- [CrossRef] [Google Scholar]
- Synergistic inhibition of cancer cell proliferation with a combination of δ-tocotrienol and ferulic acid. Biochem. Biophys. Res. Commun.. 2014;453:606-611.
- [CrossRef] [Google Scholar]
- Bone Marrow Cell Therapy on 1,2-Dimethylhydrazine (DMH)-Induced Colon Cancer in Rats. Cell. Physiol. Biochem.. 2018;45:1072-1083.
- [CrossRef] [Google Scholar]
- Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med.. 2015;36:9437-9446.
- [CrossRef] [Google Scholar]
- Feruloyl-l-arabinose attenuates migration, invasion and production of reactive oxygen species in H1299 lung cancer cells. Food Chem. Toxicol.. 2013;58:459-466.
- [CrossRef] [Google Scholar]
- Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage. Food Chem. Toxicol.. 2018;118:272-286.
- [CrossRef] [Google Scholar]
- Long non-coding RNA MIR503HG inhibits the proliferation, migration and invasion of colon cancer cells via miR-107/Par4 axis. Exp. Cell Res.. 2020;12
- [CrossRef] [Google Scholar]
- Ferulic acid inhibits endothelial cell proliferation through NO down-regulating ERK1/2 pathway. J. Cell. Biochem.. 2004;93:1203-1209.
- [CrossRef] [Google Scholar]
- Jameson J.L., ed. Harrison’s principles of internal medicine (Twentieth ed.). New York: McGraw-Hill Education; 2018.
- Comparative study of 1,2-dimethylhydrazine and azoxymethane on the induction of colorectal cancer in rats. J. Coloproctol.. 2014;34:167-173.
- [CrossRef] [Google Scholar]
- Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA. 2011;25:1366-1375.
- [CrossRef] [Google Scholar]
- Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res.. 1997;57:1605-1613.
- [Google Scholar]
- Potential applications of ferulic acid from natural sources. Biotechnol. Rep.. 2014;4:86-93.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402-408.
- [Google Scholar]
- Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol.. 2014;65:185-195.
- [CrossRef] [Google Scholar]
- Synergistic and individual effects of umbelliferone with 5-flurouracil on the status of lipid peroxidation and antioxidant defense against 1, 2-dimethylhydrazine induced rat colon carcinogenesis. Biomed. Prev. Nutr.. 2013;3:74-82.
- [CrossRef] [Google Scholar]
- Expression of p27(Kip1), cyclin D3 and Ki67 in BPH, prostate cancer and hormone-treated prostate cancer cells. Int. Urol. Nephrol.. 2008;40:953-959.
- [CrossRef] [Google Scholar]
- ki-67 and Topoisomerase IIa proliferation markers in colon adenocarcinoma. J. Buon Off. J. Balk. Union Oncol.. 2018;23:24-27.
- [Google Scholar]
- Palani Swamy, S. kumaran, Govindaswamy, V., 2015. Therapeutical properties of ferulic acid and bioavailability enhancement through feruloyl esterase. J. Funct. Foods 17, 657–666. 10.1016/j.jff.2015.06.013
- Ferulic acid is nephrodamaging while gallic acid is renal protective in long term treatment of chronic kidney disease. Clin. Nutr.. 2012;31:405-414.
- [CrossRef] [Google Scholar]
- Suvarna S.K., Layton C., Bancroft J.D., eds. Bancroft’s theory and practice of histological techniques, Eighth (edition. ed.). Amsterdam: Elsevier; 2018.
- Overexpression of CK20, MAP3K8 and EIF5A correlates with poor prognosis in early-onset colorectal cancer patients. J. Cancer Res. Clin. Oncol.. 2013;139:691-702.
- [CrossRef] [Google Scholar]
- Zhang, Z., Feng, Q., Jia, C., Zheng, P., Lv, Y., Mao, Y., Xu, Y., He, G., Xu, J., 2020. a prognostic signature predicting relapse in I–III colon cancer 9.







