Fenvalerate induced toxicity in Zebra fish, Danio rerio and analysis of biochemical changes and insights of digestive enzymes as important markers in risk assessment
⁎Corresponding authors. ahmahmoud@ksu.edu.sa (Ahmed Hossam Mahmoud), hak3962@sch.ac.kr (Hak-Jae Kim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Fenvalerate is widely used insecticide that severely causes toxicological effects in various aquatic organisms especially in fish. Physiochemical factors of water influenced fenvalerate toxicity. This study investigated physiochemical content of water, the effects of fenvalerate on mortality, changes in protein profile in the gut of Zebra fish and digestive enzymatic changes after 28 days of exposure. Zebra fish was exposed to ranges of fenvalerate and LC50 value was determined. Alkaline pH of the water and high temperature critically influenced mortality. The sub lethal concentration of fenvalerate decreased total protein content in various organs and induced elevated level of free aminoacids. Fenvalerate affected major digestive enzymes activity in the gut of Zebra fish. Protease and amylase assay revealed 39 ± 2.4% protease activity loss and 24.5 ± 5.5% amylase activity loss due to fenvalerate toxicity. Fenvalerate induced disintegration of enzymes from the gut at sub lethal concentration. Fenvalerate reduce the enzyme activity of proteases and amylases significantly. The present finding suggests that accumulation of fenvalerate critically alter not only the biochemical changes in the intestine, but also the specificity of protease and amylase towards various substrates.
Keywords
Freshwater
Danio rerio
Fenvalerate
Sub lethal
Mortality
Protease
Amylase
1 Introduction
Fish and various wildlife populations have been seriously affected by environmental pollutants. The frequent use of industrial chemicals and pesticides affect water bodies and soil. Also, pesticide affect mammals, fishes and birds have been reported previously by various research groups. The utilization of these pesticides has increased yield of crop and significantly reduced post harvest losses (Ravichandran et al., 2017; Arasu et al., 2016, 2017a, 2017b; Ravichandran et al., 2016). Moreover, the continuous use of these pesticides causes adverse effect on human health. More than 1400 pesticides are currently in use worldwide, as herbicides, insecticides, rodenticide and fungicides (Turinek et al., 2009). All pesticides are toxic to animals and humans through directly or indirectly by exposure or dietary intake. These organophosphate compounds were also frequently involved in occupational and accidental poisoning. It was reported that in 2013, the cumulative insecticide usage was about 3120 tonnes against 2889 tonnes in the previous year (2012). Among the synthetic pesticides, organophosphate ranked fist than other pesticide consumption (Al-Sharbati et al., 1998).
In recent years, increase in the application of various pesticides to preserve the crops from the pests and to enhance crop production cause severe pollution (Gupta et al., 2014; Chaurasia et al., 2016a; Kumaresan et al., 2016; Chaurasia et al., 2016b; Kumaresan et al., 2015a; Arockiaraj et al., 2015a). Due to heavy use of pesticides, the natural resources like ponds, streams, reservoirs and low-lying areas are severely polluted all over the world (Ngidlo, 2013). Pesticides affect the entire ecosystem, leading to mortality of aquatic organisms, in general and fish in particular as reported previously (Gupta et al., 2013). Numerous xenobiotic compounds have been detected in groundwater, surface water, agricultural activities and environmental contamination. Aquatic pollution due to pesticides is one of the major problem and mass mortality and changes in fauna due to sublethal effects of various pollutants have been described (Lazartigues et al., 2013). The pollution of lakes and rivers with various chemicals of anthropogenic origin has severe consequences. Continuous and unrestricted application of pesticides first in agricultural sector ultimately leads to the severe contamination of aquatic ecosystem and becomes toxic to the non target aquatic organisms. The widespread use of these chemical pesticides has attracted much more attention of environmentalists and ecologists to understand the impacts of these xenobiotics on aquatic biota (Relyea, 2005). Fenvalerate also cause poisoning in human beings and has been reported throughout the world. About two hundred cases of acute pyrethroid poisoning as occupational hazard due to poor handling were first identified in China (He et al., 1989). The most of the cases involved exposure of pesticides such as, cypermethrin, deltamethrin, fenvalerate and other pyrethroid pesticides (Chen et al., 1991). He et al. (1989) also reported fatal poisoning due to oral ingestion of fenvalerate at 2 g/kg body weight dosage. In Sweden, fenvalerate has been banned due to side effects from workers due to occupational poisoning (Hadnagy et al., 2003; Muller-Mohnssen and Hahn, 1995). Also, indoor application of pyrethroids and chronic applications affect labours. Absorption of synthetic pyrethroids by dust particles causes side effects and pesticide-related illness has been reported earlier in Oregon (Soderlund et al., 2002).
In Brazil, organophosphates such as, methyl parathion has been extensively applied in food storage shelters, agriculture, pest control programs, aquaculture ponds to eliminate various insects to protect from predators (De Aguiar et al., 2004). The effective control measure of various insect pests involves the consistent application of synthetic insecticides, but the wide-spread application of these chemicals has led to very serious concerns including toxic residue on plant and toxicity to various non-targeted organisms such as fishes, birds and mammals (Sathyamoorthi et al., 2017, 2018, 2019; Kumaresan et al., 2019; Ravichandran et al., 2018). These synthetic substances interfere with numerous vital physiological functions by altering the levels of many biochemical constituents in fishes (Sharma and Ansari, 2011). Synthetic pyrethroids were recently developed as the good alternative to carbonate and organophosphorus pesticides, however, these insecticides also affect non target organisms such as, fishes and proved to be detrimental to domestic organism, fishes and human beings (Adhikari et al., 2006).
The synthetic pyrethroids are very much toxic in nature than naturally available pyrethrins to various organisms, including insects, and fishes (Coats et al., 1989). Many reports show that insects from aquatic ecosystem are very much sensitive to pyrethroid toxicity, even at very low concentrations (<1 μg L−1) that produce severe toxic effects (Mokry and Hoagland, 1990; Mian and Mulla, 1992). The sensitive to pesticides are the immuno-competent organs of fishes especially the kidney and liver. To evaluate the significant role of contaminants such as presently used pesticides like fenvalerate, it is very important to understand their impact to the environment, mainly on aquatic organisms such as fishes in terms of their immunosuppressant ability, prophylactic measures, amelioration and mitigation. There is an increasing research effort to explore toxicity of pyrethroids to aquatic organisms (Cengiz and Unlu, 2006; Pimpao et al., 2001).
The significance of various synthetic pesticides such as, organophosphates, carbonates and organochlorides damage the complex food-web, food web energetic and population dynamics (Chandra et al., 2001). Among the freshwater organisms, fishes are highly sensitive to pesticide contamination (Palanisamy et al., 2015; Arockiaraj et al., 2015b; Chaurasia et al., 2015; Kumaresan et al., 2015b; Rao et al., 2015). Also, pollutants such as insecticides and herbicides may potentially affect physiological and biochemical processes when they enter into the organs of freshwater fishes (Barbee et al., 2020). Liver is the most important part susceptible in fish due to toxicity (Gijare et al., 2011). Also, most of the synthetic pesticides severely cause changes in protein level in the body. Earlier, Grant and Mehrle (1970) exposed endrin at chronic level and reported decreased total protein content in the goldfish (Carassius auratus). Xenobiotic agents are considered as environment stress, immunological stress, and growth retardation causing agents in various animals (Slaninova et al., 2009; Chebbi and David, 2010). Xenobiotic substances critically affect digestive enzyme activity in the gut of fish. Enzymes such as, amylase and protease are the important enzymes which are directly related to carbohydrate and protein metabolism. Disorders of various functional characteristics of the digestive tract of the fish were noted upon exposure to various xenobiotics (Golovanova and Talikina, 2006). Analysis of the effects of pollutants on digestive enzyme activities are useful to highlight the risk of xenobiotic substances at very low concentrations which affect the activity of digestive enzymes and biochemical features of fish.
2 Materials and methods
2.1 Culture and maintenance of Danio rerio in aquarium tank
In the present study, freshwater Zebra fish (Danio rerio) was collected from the aquarium and stocked under laboratory conditions. Fish was cultured in an aquarium tank with light and dark cycle (with 14 h/10 h). The fish was fed with pellet feed and tubefex worm daily (two times). Brine shrimp was also fed in alternate days. Zebra fish was maintained in a glass aquarium (60 × 30 × 40 cm). The aquarium tank was continuously aerated using a mechanical air compressor. In this study, matured adult fish was subjected for all experiments.
2.2 Physio-chemical properties of water
The parameters such as, pH, electrical conductivity, potassium, sodium, magnesium, calcium, chloride and bicarbonate were analysed. pH is one of the important factors and measured using a digital pH meter, electrical conductivity (EC) was measured using a conductivity meter. Flame photometer was used for the determination of sodium and potassium. Magnesium and calcium was assayed using EDTA titration method. Chloride and bicarbonates were determined by titration method. All other parameters were analyzed by standard method.
2.3 Fenvalerate induced toxicity in D. Rerio
The Zebra fish was divided into six experimental groups (20 fish each) and a control group. Fenvalerate was added with the experimental groups at various concentrations (0.1, 25, 50, 75, and 100 µg/L). To the control group of Zebra fish pesticide was not administered. Experiment was performed for 96 h and mortality of fish was registered every 24 h and percentage mortality was calculated. In this study, mortality rate of Zebra fish was observed and Probit analysis was performed as suggested by Abbott (1992) and Finney (1971) to determine LC50 value. Two way analysis of variance (ANOVA) was carried out to study the impact of independent factor. The p-value (<0.05) was considered as significant at 95% confidence level.
2.4 Effect of temperature on fenvalerate toxicity
In this study, Zebra fish (0.25 ± 0.1 gm) was exposed to the fenvalerate in an aquarium tank at sub lethal concentrations. Mortality rate was recorded at room temperature. In an another tank, same sized Zebra fish was maintained in a same sized tank and proper feeding was provided like control. The temperature of the fenvalerate tank was increased and decreased at 2 °C level. The fenvalerate concentration was maintained as 25 µg/L and incubated for 96 h. Further, mortality rate was registered in lower and high temperature than room temperature.
2.5 Effect of pH on fenvalerate toxicity
The experimental Zebra fish was exposed to various pHs and survival time was registered. Initially, the pH of water was tested and increased or decreased. Then survival rate of the fish was evaluated. The selected pH of aquarium water was 6.0, 7.0, 8.0, and 9.0, respectively. To the aquarium water 25 µg/L fenvalerate was added.
2.6 Sample preparation
The control and experimental fish was used for sample preparation. The tissues, liver, gut and gills were carefully dissected from the control and experimental groups. 0.2 gm of gill, liver and body tissue was homogenized using a glass homogenizer with Tris-HCl buffer (pH 7.4, 100 mM). Then it was centrifuged for 10 min at 8000 ×g. The supernatant was separated and used as the sample for biochemical assays. All samples were prepared fresh before to perform experiments.
2.7 Determination of total protein and free aminoacids
Total protein estimation in gill and muscle sample was performed as suggested previously (Lowry et al., 1951). Bovine Serum Albumin (BSA) was used as the standard. For the determination of free amino acid, sample was prepared in trichloroacetic acid (10%) and centrifugation of gill and muscle at 6000 g for 15 min at 4 °C. Then, the amount of free amino acids present in the gill and muscle was estimated (Spies, 1957). About 0.2 ml of sample was transferred into test tubes and 3.0 ml of ninhydrin reagent was added. Further, the solution was mixed by shaking using an orbital shaker. Then the reaction was extended for 20 min in a boiling water bath. Then 6 ml alcohol solution was added in all test tubes and incubated for 30 ± 2 °C for 20 min. The optical density of the sample was finally measured using a UV–Visible spectrophotometer at 580 nm against reagent blank. Glycine was used as the standard to determine free amino acids from the gill and muscle.
2.8 Determination of digestive enzymes from the gut of Zebra fish
Protease and amylase are the two important groups of enzymes involved in digestion process. To analyze the proteolytic activity of gut, agar plate was made with 1% (v/w) casein. To determine amylase activity, soluble starch (1%, v/w) was included with agar plates (Bhowmik et al., 2015). About 20 µl sample was loaded into the well and incubated at room temperature in sterile condition. After 12 h of incubation, 5 ml trichloro acetic acid (10%) was flooded to visualize hydrolytic zone. In starch – agar plate, Gram’s Iodine was flooded and a clear zone around the sample well indicated enzymatic activity.
2.9 Protease and amylase assay
Zebra fish gut was used as the sample for both protease and amylase assay. Protease assay was performed using casein as a substrate as described previously (Anson, 1938). One unit of enzyme activity is defined as the amount of enzyme required to liberate 1 μg tyrosine per ml per minute under the standard assay conditions. Amylase assay was performed as suggested by Rick and Stegbauer (1984) using 3, 5-dinitrosalicylic acid method. Maltose was used as the standard curve. One unit of amylase activity was defined as the number of micromoles of maltose released per min per mg of protein at 30 ± 2 °C.
2.10 Analysis of fenvalerate induced protein changes in gut of Zebra fish using sodium dodecyl suphate polyacrylamide gel electrophoresis
SDS-PAGE analysis was performed to evaluate protein profile of control and fenvalerate exposed Zebra fish. The specific organs were dissected out and sample was prepared as described previously. 12% separating gel and 5% stacking gel was used to resolve proteins.
2.11 Zymogram analysis of protease from the gut of Zebra fish
In this study SDS-PAGE zymography was carried out to determine proteolytic activity. A casein based protease activity determination was performed according to the method of Garcia-Carreno et al. (1993). Briefly, casein was prepared at 1% (w/v) level and incorporated into the separating gel. The enzyme sample was not denatured and heated. The gel was run at 4 °C and constant power supply (50 V), after separation, it was incubated in buffer (Tris buffer, pH 8.0, 0.05 M) containing 2.5% (v/v) Triton X – 100 for 30 min. Further, the gel was incubated in buffer for 5 h and stained with coomassie brilliant blue R-250.
2.12 Zymogram analysis of amylase from the gut of Zebra fish
After SDS-PAGE the gel was washed carefully twice with 0.5% (v/v) Triton X-100 for 30 min to remove SDS completely. Then the gel was incubated in sodium phosphate buffer (pH 7.4, 100 mM) at 50 °C for 20 min. The gel was further incubated in this buffer for 10 min at room temperature and then stained with (0.3% I2 in 3% KI) iodine solution for 4 min. Amylase activity was visible as a halo zone in blue background.
3 Results
3.1 Physio-chemical parameters of water
Temperature is an important physio-chemical parameter to analyze water quality because the metabolic process of all organisms based on temperature of the ecosystem. Temperature of water was recorded as 34 ± 2 °C and the pH was determined as 7.6 ± 2. Total alkalinity of water was 112 ± 21 mg/L and EC of water was found to be very high (1800 ± 54 microScm−1). Potassium level was 4.3 ± 0.18 mg/L and increased sodium level was reported (218 ± 13.2 mg/L). Calcium content of water was 180 ± 13.1 mg/L and magnesium level was found to be 219 ± 36.3 mg/L. Chloride level was also found to be very high (712 ± 3.4 mg/L) and total bicarbonate content was 183.2 ± 14 mg/L and fluoride was also detected in water (0.13 ± 0.07 mg/L).
3.2 Fenvalerate induced toxicity in Zebra fish
In this study, Zebra fish was subjected to analyze fenvalerate induced toxicity at various concentrations (25 µg/L to 100 µg/L). After 24 h of treatment, mortality rate was 27 ± 0% in 25 µg/L fenvalerate. However at 100 µg/L concentration, 66 ± 4% mortality was registered. Likewise, after 48 h of treatment mortality rate was 35 ± 3.1% and it increased as 71 ± 5.0% at 100 µg/L. Mortality rate was increased as 33 ± 3.0% at 25 µg/L after 96 h. After 72 h exposure, 100 µg/L showed 100% mortality on tested Zebra fish. Two way ANOVA was applied to reveal the influence of fenvalerate concentrations and exposure time on Zebra fish. Probit analysis was performed to evaluate LC50 value. The log10 of fenvalerate concentration (µg/L) was 1.397, 1.698, 1.875 and 2.0. Probit values were ranged from 4.39 to 5.41 after 24 h of exposure and R2 value was 0.862 (Fig. 1a). After 48 h of exposure, the Probit values ranged from 4.48 to 5.58 and R2 value was 0.891 (Fig. 1b). After 72 h of exposure, the calculated Probit values varied between 4.56 and 5.74 and R2 value was 0.932 after 72 h of fenvalerate exposure (Fig. 1c). The observed Probit value was ranged from 4.97 to 7.33 and R2 value was 0.923 after 96 h of fenvalerate treatment (Fig. 1d).
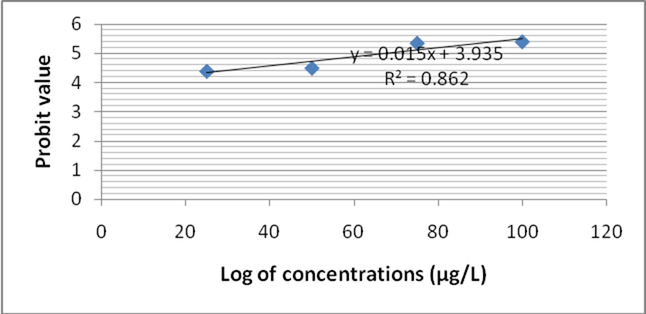
- LC 50 values for fenvalerate toxicity to Zebra fish after 24 h of treatment.
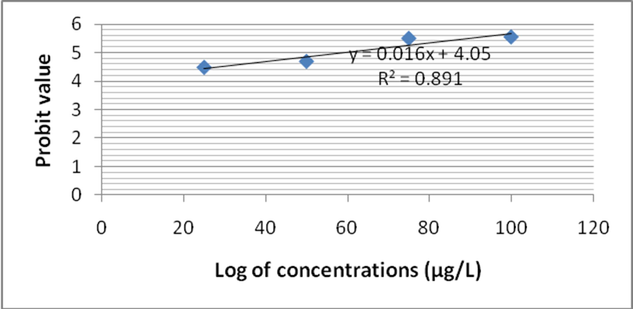
- LC 50 values for fenvalerate toxicity to Zebra fish after 48 h of treatment.
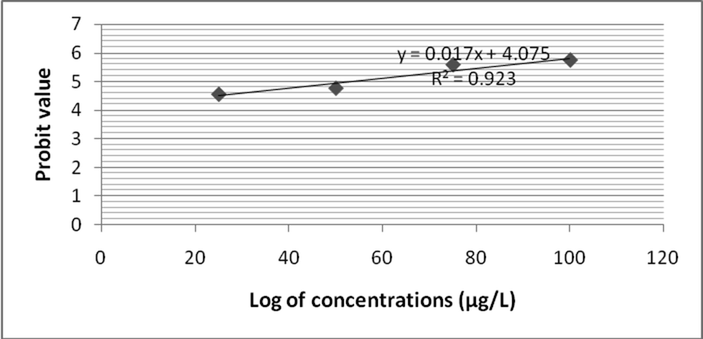
- LC 50 values for fenvalerate toxicity to Zebra fish after 72 h of treatment.
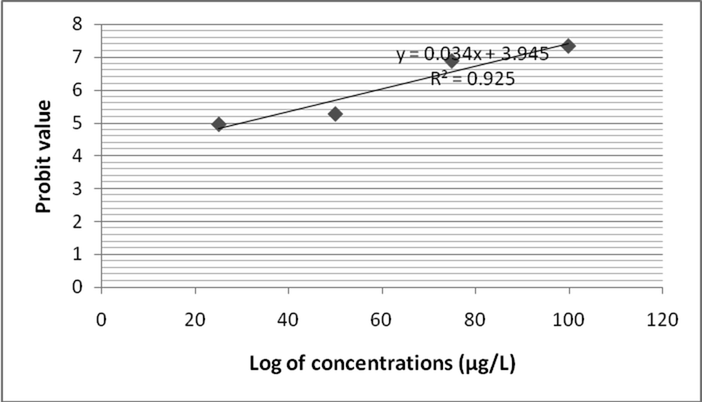
- LC 50 values for fenvalerate toxicity to Zebra fish after 96 h of treatment.
3.3 Influence of temperature on fenvalerate toxicity
In the present study fenvalerate induced toxicity at various temperatures. At 25 °C, 15 ± 1.2% mortality was observed and this increased as 20 ± 2.3% at 30 °C. At 40 °C, 55 ± 4.5% mortality was registered. However, 100 ± 3.8% mortality was registered at 45 °C after 96 h of incubation (Fig. 2).
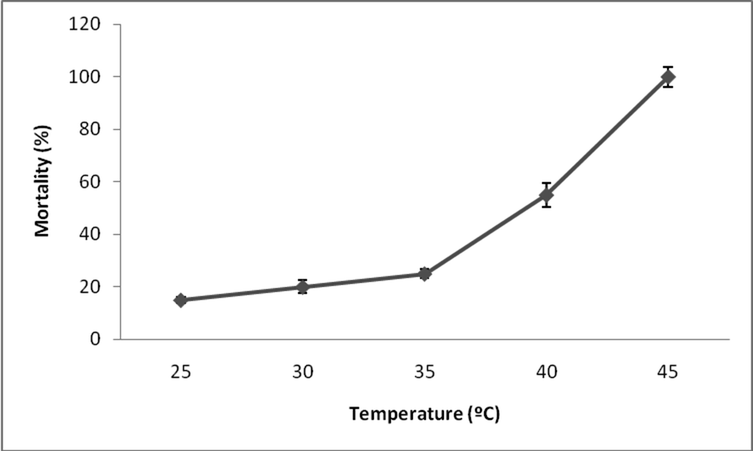
- Effect of temperature on fenvalerate toxicity in freshwater Zebra fish at sublethal concentrations.
3.4 Influence of pH on fenvalerate toxicity
In this study, the influence of fenvalerate towards various pHs was analyzed. At low pH value mortality was very low and at higher pHs, mortality increased. These clearly implied the role of hydrogen ions on fenvalerate toxicity. In this study, 25 µg/L fenvalerate was incorporated and mortality was registered after 96 h. At pH 6.0, 25% fish mortality was observed and this increased as 40% at pH 7.0. In alkaline range of pH, for example at pH 8.0, 85% mortality was registered and this raise as 95% at pH 9.0 (Fig. 3).
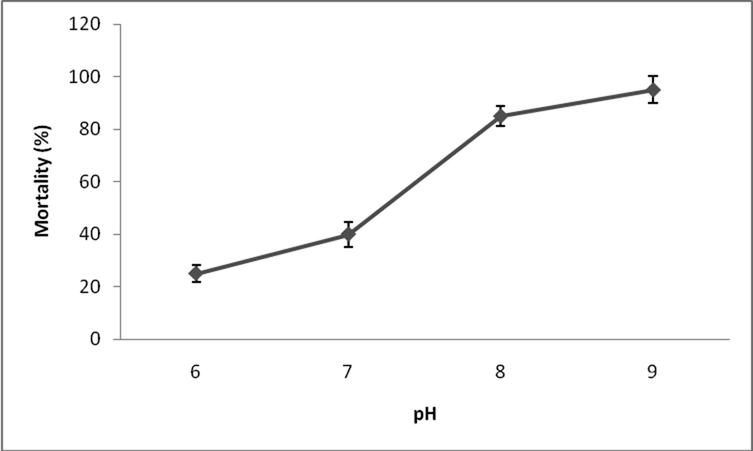
- Effect of pH on fenvalerate toxicity in freshwater Zebra fish at sublethal concentrations.
3.5 Fenvalerate induced changes of total protein content at sub lethal concentrations in Zebra fish
Total protein content of the normal gill of control fish was 75.4 ± 1.3 mg/g and it increased as 118.1 ± 1.3 mg/g (Fig. 4a). The total protein level of the muscle of the control fish was 123.1 mg/g and it decreased marginally as 121.4 ± 2.4 mg/g after 24 h of treatment (Fig. 4b). Two way ANOVA revealed that depletion of protein content between muscle and gill were found to be statistically significant (P < 0.05).
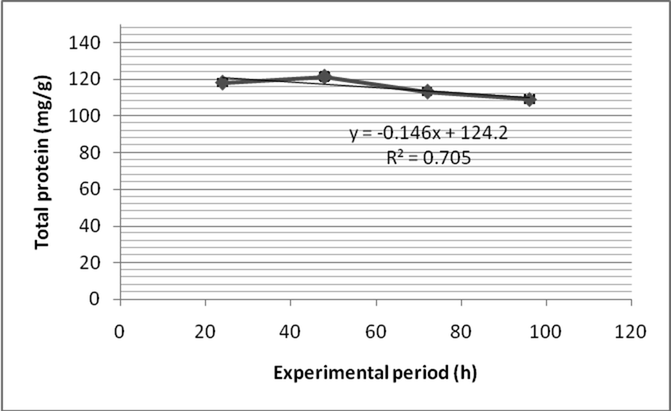
- Fenvalerate induced changes in total protein content in the muscle of Zebra fish.
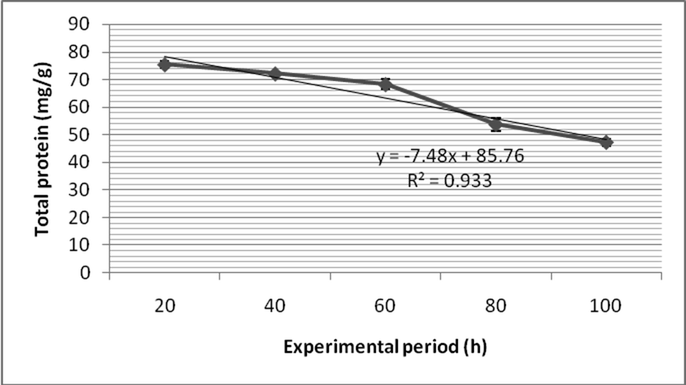
- Fenvalerate induced changes in total protein content in the gill of Zebra fish.
3.6 Free amino acid content of gill and muscle of Zebra fish exposed to fenvalerate at sub lethal concentration
Fenvalerate induced elevated level of free amino acid in Zebra fish. After 24 h of exposure, the free amino acid level in the gill was 25.76 ± 1.7 mg/g and decreased as 24.8 ± 2.3 mg/g after 48 h. After 72 h, free amino acid content was 29.7 ± 1.3 mg/g and increased continuously (30.3 ± 1.8 mg/g) after 96 h (Fig. 5a). The free amino acid content was 28.1 ± 3.1 after 24 h of treatment of fenvalerate at sub lethal concentration in the muscle. Also, it increased as 29.7 ± 1.8 mg/g after 48 h of exposure. After 96 h exposure, the free amino acid level was marginally decreased (23.1 ± 2.7 mg/g) in the gill of Zebra fish (Fig. 5b). Two – way ANOVA as a function of free amino acid level in gill and tissue and pesticide exposure at various intervals were found to be statistically significant (p > 0.05). The increase of free amino acid in gill and muscle indicated that it undergoes proteolysis due to sub lethal exposure of fenvalerate. Also, increase of free amino acid was highly correlated with decrease of total protein content.
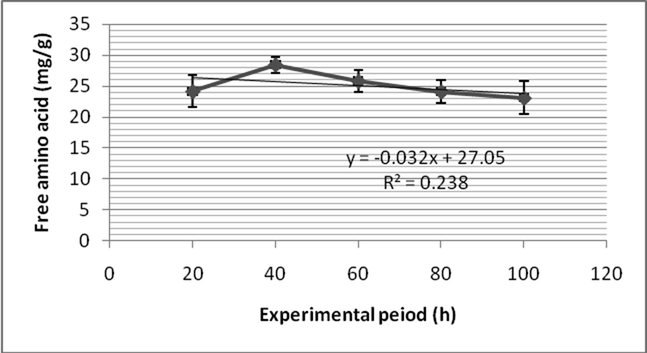
- Free amino acid content of the gill of Zebra fish exposed to fenvalerate at sub lethal concentration.
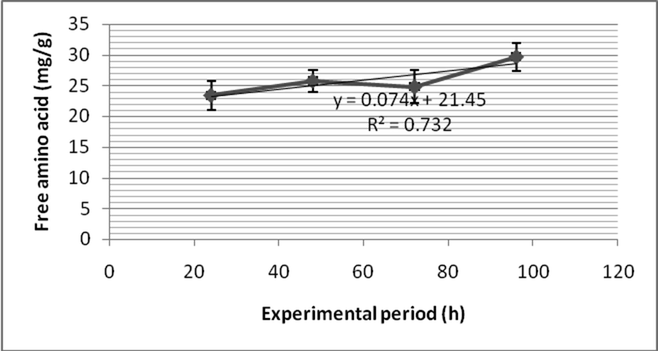
- Free amino acid content of the muscle of Zebra fish exposed to fenvalerate at sub lethal concentration.
3.7 Qualitative and quantitative analysis of digestive enzymes
In this study, qualitative analysis of protease and amylase was performed to investigate the impact of fenvalerate on digestive enzymes. Analysis of protease revealed significant inactivation of protease activity by fenvalerate (Fig. 6a). The control fish gut showed 13 mm zone of hydrolysis on casein agar plates, whereas about 9 mm zone was registered in fenvalerate exposed gut. Likewise, amylolytic activity was inhibited by sub lethal concentration of fenvalerate. The fenvalerate exposed gut showed 7 mm zone of hydrolysis whereas, 17 mm in control fish (Fig. 6b). The gut of control Zebra fish showed 263 ± 12.5 U protease activity per mg protein, and lost 39 ± 2.4% enzyme activity in the fenvalerate exposed fish. Also, the control gut of fish showed 164 ± 3.1 U amylase activity and inactivated 24.5 ± 5.5% enzyme activity.
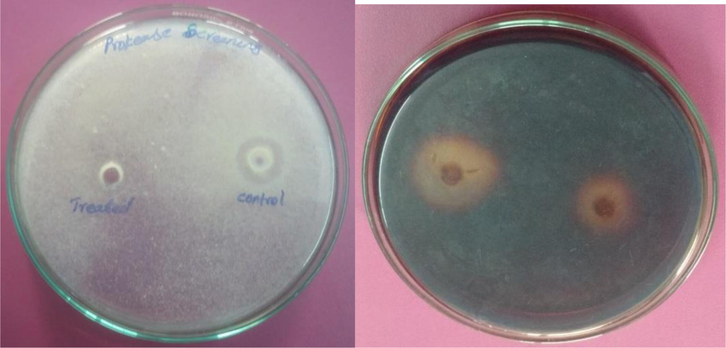
- (a-b) Proteolytic and amylolytic activity of the gut sample from Zebra fish (fenvalerate treated and control). Zebra fish was exposed to fenvalerate for 28 days at sub-lethal concentrations. To the control group, fenvalerate was not added.
3.8 Fenvalerate induced protein destruction in the gut of Zebra fish at sub lethal concentration
In this study, differences in protein profile were observed in the gut of fenvalerate exposed Zebra fish. Gut is most susceptible to degeneration of proteins due to fenvalerate toxicity at sub lethal concentrations. In the control fish, protein subunits corresponding to 35 kDa and 57 kDa were predominant, whereas, these two subunits were not detected in fenvalerate exposed fish. Also, various protein subunits between 35 kDa and 150 kDa were high intensity in the gut of control fish, and these subunits were very low intensity in fenvalerate exposed fish (Fig. 7). In SDS-PAGE zymography protease appeared as colourless band (Fig. 8a). SDS-PAGE zymography also proved the inhibition of protease activity about 40% due to fenvalerate toxicity. In this result, protease activity appeared as colourless band, whereas, in the experimental gut, protease bind on the substrate of the gel and the enzyme band is not very clear. Likewise, SDS-PAGE zymography revealed that amylase activity was inhibited due to the exposure of fenvalerate (Fig. 8b).
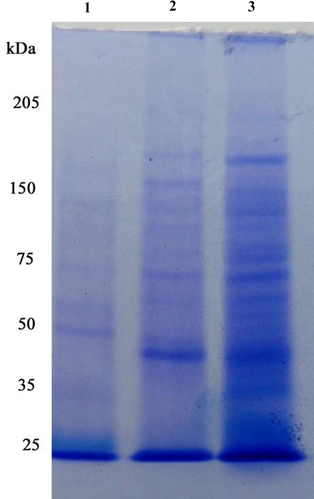
- SDS-PAGE analysis of fenvalerate induced protein disintegration in Zebra fish at sub-lethal concentrations (1- protein molecular weight marker, 2- fenvalerate exposed Zebra fish and 3- control).
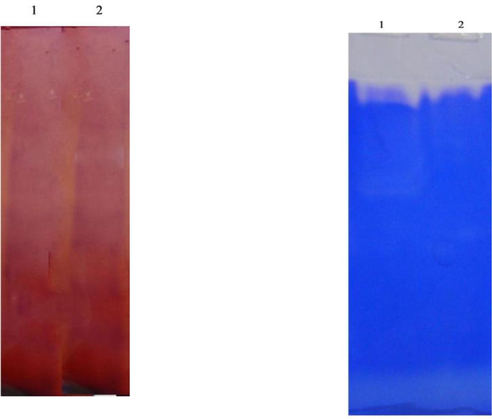
- a and b. Zymography analysis of amylase (a) and protease (b) activity from the gut of Zebra fish. a) Lane 1- control , Lane 2- fenvalerate exposed fish.
4 Discussion
Water is the important medium where fish live in this ecosystem. Many physicochemical parameters of soil and water can critically affect the toxicity of chemicals in aquatic environment. In this study water quality was analyzed and some tested parameters were not within permissible limit. pH of water was within standards given by WHO. Narmada River, the physiochemical parameters tested were 7.4 to 9.7 (Sharma et al., 2008) and this was almost similar to the present study. Different abiotic factors of soil and water affect toxicity of chemicals in freshwater ecosystem (Datta et al., 2002c). Water quality deterioration can be very much explained by the pH of the ecosystem and in this study the pH of water was 7.6 ± 2. The EC is a measurement of capacity of water medium to transmit electric current in a water ecosystem. EC represents the total concentration of mineral salts or soluble salts in water (Goyal, 1986). EC was also termed as salinity, is the important parameter that is very much used to evaluate concentration of dissolved solids. In this study EC was registered as 1800 ± 150 microScm−1 which clearly indicated high concentrations of various ions. Fish size and water quality affect toxicity of endosulfan to rainbow trout, mainly temperature, alkalinity and total hardness (Capkina et al., 2006). In aquatic ecosystem ammonia level significantly influenced on elevated pH level. Also, low oxygen concentration in water ecosystem also facilitates ammonia toxicity. Industrial effluent discharges without any treatment leads to chlorine contamination in freshwater ecosystem. Physiological factors such as temperature critically affect toxicity of heavy metals. Temperature and pH variations affect blood parameters, also high heart beat rate due to high temperature, and disturbances in the ion regulatory mechanism at high temperature was reported in common shore crab Carcinus maena (Jones, 1975).
Toxicity analysis is a significant component in evaluating the impact of pesticides on aquatic environment because they indicate toxic properties of chemicals in organism by altering their behaviour and morphology initially and survival rate at later stage (Kaushal and Misha, 2013). It gives base line data on the effect of pesticides on aquatic organisms, especially fishes and ecosystem as a whole. These analyses are important in creating awareness about potential harmful effects of pesticide in the environment (Adedeji et al., 2008). Also, study of behavioural changes to assess the toxicant affects are the most successful and sensitive indicator of toxicology (Drummond and Russom, 1990). Many studies revealed that fish exposed to various pesticides exhibited morphological, biochemical and behavioural changes (Devi and Misha, 2013).
In this study, fenvalerate induced toxicity was assayed using Zebra fish as a model organism at various concentrations (25 µg/L to 100 µg/L). After 24 h of treatment, mortality rate was 27 ± 0% in 25 µg/L fenvalerate and mortality rate was increased as 33 ± 3.0% at 25 µg/L after 96 h. Two ways ANOVA revealed the influence of fenvalerate concentrations and exposure time on the mortality of Zebra fish. In this study probit analysis was performed to evaluate LC50 value. The analyzed Probit value was ranged from 4.97 to 7.33 and R2 value was 0.923 after 96 h of fenvalerate treatment. Generally, administration of pyrethroids are very safe, when administered orally, however it is highly toxic to animals once it reach central nervous system. This cause behaviour changes and mortality. These observations are in good agreement with previous reports (Bradbury et al., 1985; Tripathi, 1992). Statistical analysis showed that both exposure time and fenvalerate concentration significantly influenced on Zebra fish mortality (p < 0.0001).
In fishes, toxicity of fenvalerate is mainly due to accumulation and metabolism. It was previously reported that even at sub lethal concentration of fenvalerate can leads to heavy accumulation of fenvalerate residues (Susan et al., 1999). The LC50 values of fenvalerate to various fishes were reported by Chapman et al. (1985). These studies showed that fenvalerate is very toxic to fishes than technical grade fenvalerate. In the present study fenvalerate induced toxicity and was temperature dependent. At 25 °C, 15 ± 1.2% mortality was observed and this increased as 20 ± 2.3% at 30 °C. At 40 °C, 55 ± 4.5% mortality was registered. However, 100 ± 3.8% mortality was registered at 45 °C after 96 h of incubation. In this study, the influence of fenvalerate towards various pHs was analyzed. At low pH value mortality was very low and at higher pHs, mortality increased. These clearly implied the role of hydrogen ions on fenvalerate toxicity. In this study, 25 µg/L fenvalerate was incorporated and mortality was registered. At pH 6.0, 25% fish mortality was observed and this increased as 40% at pH 7.0. In alkaline range of pH, for example at pH 8.0, 85% mortality was registered and this raise as 95% at pH 9.0. Fenvalerate is considered to have very low toxicity to most of the mammals, however, it causes various complications in fishes. Temperature is one of the important factors greatly influence the toxicity of various xenobiotic chemicals. However mortality rate of fish species decrease or increase based on the selected pesticide and dosage. Generally, at high temperature of pesticide in the water, decreased the oxygen content. Hence the experimental fishes may die due to low oxygen level in the water. The cat fish, Hystus gulio (Ham) was exposed to various heavy metals and decreased oxygen content was reported (Sultana and Umadevi, 1995). The chemical reactions are increased as the temperature increases whereas respiratory and cardiac functions are slowed down immediately resulting in hypoxia in fishes at very low temperature. Also, in Heteropneustes fossilis (Bloch) copper and cadmium toxicity decreased the availability of oxygen in the water results mortality (Gupta and Rajbanshi, 1991).
The pH of the aquarium water played an important role in fenvalerate toxicity. At low pH value, decreased mortality was observed (6.0). At neutral pH value, 40 ± 4.5% mortality was observed and it increased as 95 ± 5.2% mortality at pH 9.0. pH of the water severely alters fenvalerate toxicity through physiological responses, as pH changes critically causes chemical stress to the aquatic organisms. At low pH anoxia and sodium loss was reported in trout due to acidosis (Packer and Dinson, 1972). In Salvelinus fontinalis, metal accumulation was increased at high pH in aquarium water (Drummond et al., 1974). The degree of toxicity caused by the pesticide residues is dose dependent upon various environmental conditions such as pH, temperature, oxygen content and presence of other residual substances (Capkin et al., 2006). It was previously stated that lipid, carbohydrate and protein play a significant role as precursors of energy under stress. Many enzymes play significant role in detoxification and metabolism of pollutants and accumulation of pesticides in the body of fish create various metabolic changes at cellular level by influencing enzyme systems. Hence, decreased oxygen content in aquarium water is critical as reduced availability of oxygen to the organism (Meister, 1992). Rise in temperature in the water beyond the optimum range of fish species critically affects its enzyme system, causing very less resistance to pesticides resulting in very high mortality.
Fenvalerate induced significant changes of total protein content at sub lethal concentrations in the experimental Zebra fish model. Two way ANOVA revealed that significant difference in protein content between muscles and was found to be statistically significant. The decreased total protein content in various tissues was reported previously. In another study, Oreochromis niloticus (L.) was exposed to waterborne pyrethroid neobiputrin for 24 h at half of LC50 concentration resulted in changes in intestinal epithelical cells, decrease overall protein and enzymes and also alter permeability of the intestinal mucosa. The decrease of total protein content in the muscle and gill of Zebra fish clearly shows that it was under stress due to fenvalerate toxicity. In Cyprinus carpio, fenvalerate decreased total protein content in liver, muscle tissue and brain. The biochemical changes were increased at increased exposure time (Reddy et al., 1991). In Channa punctatus, total protein content was depleted heavily after the exposure of nuvacron in liver and muscle. The present result also highly agreement to the previous studies (Sastry and Dasgupta, 1991). Kalita et al. (2003) also reported decreased total protein content in the muscle of Heteropneustes fossilis due to the exposure of toxic chemicals.
In Zebra fish, fenvalerate induced changes in free amino acid level at sub lethal dose in gill and muscle, respectively. Total protein content decreased in the treated fish, whereas, free amino acids level increased. Two – way ANOVA as a function of free aminoacid level in organs (gill and tissue) and pesticide exposure at various intervals were found to be statistically significant. The increase of free aminoacids in gills and muscle indicated that it undergoes proteolysis due to sub lethal exposure of fenvalerate. Also, increase of free aminoacid was negatively correlated with the availability of total protein content. In Oreochromis mossambicus, biochemical changes have been reported due to pesticide stress in various tissues (Palanichamy et al., 1986). In Tilapia mossambica, exposure of endosulfan mediated elevated level of free amino acids (Muley et al., 1996). Also, exposure of cypermethrin in Cirrhinus mrigala induced elevated level of free amino acid (Prashanth et al., 2011). The decreased level of total protein content and increased level of free amino acid in Labeo rohita was reported by Tripathi et al. (2003) due to proteolytic activity under stress induced by pesticides.
Fenvalerate affected protease activity and amylase activity in the gut of Zebra fish after 28 days of exposure. Initially, protease and amylase activity was screened and confirmed inhibition of enzymes by the action fenvalerate. The zone of hydrolysis for protease activity was about 45% high in the control fish than fenvalerate exposed fish. In gut, control fish showed more than 40% proteolytic activity upon quantitative analysis. Zebra fish lost about 25% amylase activity after it was exposed to fevalerate at sub lethal concentration after 28 days. In fishes, gut is most susceptible to degeneration of proteins due to fenvalerate toxicity at prolonged exposure at sub lethal concentrations. Fenvalerate affected some protein expressions and induced expression of various stress proteins. The expressed and suppressed protein subunits were determined using SDS-PAGE. These studies clearly implied the interference of fenvalerate in protein synthesis pathway. In Labeo rohita, fenvalerate induced changes in protein profile at sub lethal concentration (Suneetha et al., 2010). SDS-PAGE zymography also proved the inhibition of protease activity about 40% due to fenvalerate toxicity. In this result, protease activity appeared as colourless band, whereas, in the experimental gut, protease bind on the substrate of the gel and the enzyme band is not very clear. Also, SDS-PAGE zymography revealed that amylase activity was severely inhibited due to the exposure of fenvalerate. This may due to the alteration of enzyme binding sites by fenvalerate. In fishes, pesticides enter into the body through water, bottom sediments and food. Thus pollutants may accumulate in tissues and organs and affect growth, development and reproduction (Tietge et al., 1998). In fishes intestine plays very important role in the initial absorption and metabolism of various organic pollutants (Yuen et al., 2007). Absorption of zenobiotic substances in intestine results impaired function of absorption of various energy sources (Sastry and Siddiqui, 1982). In Dicentrarchus labrax (L.), exposure of polycyclic aromatic hydrocarbon, benzo(a)pyrene severely caused changes in the intestine epithelium, the destruction of intercellular components, hyperplasia of enterocytes and the formation of crypts (Yuen et al., 2007). Organic pollutants from various sources may alter enzyme activity in the digestive tract of fish. In fishes, exposure of chlorophos affects affinity of amylase with substrate (Golovanova and Talikina, 2006). In carp, exposure of mercury in the alimentary canal reduced protease activity by 23.5 to 27% (Kuzmina et al., 2011). In Roach (Rutilus rutilus (L.) mercury accumulation resulted inhibition of amylase activity in the experimental fish (Golovanova et al., 2008).
5 Conclusion
The present finding revealed that analysis of digestive enzymes such as, protease and amylase may be highly useful to measure the toxicity index in freshwater fishes. These kinds of study provide more insights than monitoring of biochemical changes in gill and other organs. The determinations of enzyme activity from the digestive tract using SDS-PAGE provide first hand information on the impact of fenvalerate. The present finding suggests that accumulation of fenvalerate critically alter not only the structural changes in the intestine, but also alter the specificity of protease and amylase towards substrate specificity. The finding on the effect of fenvalerate and other pesticides pollution, on the activity of major digestive enzymes may be used to insight the risk of pesticide to the fishes on the efficiency of biochemical status of fishes.
Acknowledgment
The authors extend their appreciation to The Researchers Supporting Project number (RSP-2019/108) King Saud University, Riyadh, Saudi Arabia. The authors Hak-Jae Kim thank the support received from Soonchunhyang University research fund for this research work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A method of computing the effectiveness of an insecticide. J. Econ. Entomol.. 1992;18:265-267.
- [Google Scholar]
- Acute toxicity of diazinon to the African catfish (Clarias gariepinus) Afr. J. Biol.. 2008;7:651-654.
- [Google Scholar]
- Effect of cypermethrin on breeding performances of a freshwater fish, Labeo rohita (Hamilton) Chem. Ecol.. 2006;22:211-218.
- [Google Scholar]
- The estimation of pepsin, trypsin, papain, and cathepsin with haemoglobin. J. Genetic Physiol.. 1938;22:79-89.
- [Google Scholar]
- Bactericidal activity of fish galectin 4 derived membrane-binding peptide tagged with oligotryptophan. Develop. Comp. Immunol.. 2017;71:37-48.
- [Google Scholar]
- Bacterial membrane binding and pore formation abilities of carbohydrate recognition domain of fish lectin. Develop. Comp. Immunol.. 2017;67:202-212.
- [Google Scholar]
- Coagulation profile, gene expression and bioinformatics characterization of coagulation factor X of striped murrelChannastriatus. Fish Shellfish Immunol.. 2016;55:149-158.
- [Google Scholar]
- Molecular and functional roles of 6C CC chemokine 19 in defense system of striped murrelChannastriatus. Fish Shellfish Immunol.. 2015;45:817-827.
- [Google Scholar]
- Fish chemokines 14, 20 and 25: a comparative statement on computational analysis and mRNA regulation upon pathogenic infection. Fish Shellfish Immunol.. 2015;47:221-230.
- [Google Scholar]
- Acute toxicity of chlorantraniliprole to non-target crayfish (Procambarus clarkii) associated with rice-crayfish cropping systems. Pest Manage.. 2010;66:996-1001.
- [Google Scholar]
- Protease producing bacteria and activity in gut of tiger shrimp (Penaeus monodon) J. Fish. Aqua. Sci.. 2015;10:489-500.
- [Google Scholar]
- Different toxicity and uptake of two fenvalerate formulations in fathead minnows (Pimephales promelas) Environ. Toxicol. Chem.. 1985;4:533-541.
- [Google Scholar]
- Histopathological changes in brown trout(Oncorhynchus mykiss) after exposure to sublethal composite nitrogen fertilizers. Ecotoxicol. Environ. Safe.. 2006;72:1999-2004.
- [Google Scholar]
- Water quality and fish size affect toxicity of endosulfan, an organochlorine pesticide, to rainbow trout. Chemosphere. 2006;64:1793-1800.
- [Google Scholar]
- Sublethal effects of commercial deltamethrin on the structure of the gill, liver and gut tissues of mosquitofish, Gambusia affinis: A microscopic study. Environ. Toxicol. Pharmacol.. 2006;21:246-253.
- [Google Scholar]
- Toxic effect of carbofuran on certain hematological parameters in yearlings of Cyprinus carpio. Aquaculure. 2001;2:37-140.
- [Google Scholar]
- Toxicity of fenvalerate to developing steelhead trout following continuous or intermittent exposure. J. Toxicol. Environ. Health.. 1985;15:447-457.
- [Google Scholar]
- Molecular importance of prawn large heat shock proteins 60, 70 and 90. Fish Shellfish Immunol.. 2016;48:228-238.
- [Google Scholar]
- In-silico analysis and mRNA modulation of detoxification enzymes GST delta and kappa against various biotic and abiotic oxidative stressors. Fish Shellfish Immunol.. 2016;54:353-363.
- [Google Scholar]
- Molecular profiles and pathogen-induced transcriptional responses of prawn B cell lymphoma-2 related ovarian killer protein (BOK) Fish Shellfish Immunol.. 2015;45:598-607.
- [Google Scholar]
- Quinalphos induced alterations in the levels of ions and whole animal oxygen consumption of freshwater fish, Cyprinus carpio (Linnaeus, 1758) J. Veterinar. Sci. Technol.. 2010;1:102.
- [Google Scholar]
- An epidemiological study on occupational acute pyrethroid poisoning in cotton farmers. Occupat. Environ. Med.. 1991;48:77-81.
- [Google Scholar]
- Toxicology of synthetic pyrethroids in aquatic organisms. An overview. Env. Toxi. Chem.. 1989;8:671-679.
- [Google Scholar]
- Soil sediment and hardness of water reduce acute toxicity of deltamethrin to scale carp. Pesticide Res. J.. 2002;14:327-336.
- [Google Scholar]
- Metabolical effects of Folidol 600 on the neotropical freshwater fish matrinxã, Brycon cephalus. Environ. Res.. 2004;95:224-230.
- [Google Scholar]
- Study of behavioural and morphological anomalies of fry fish of fresh water teleost, Channa punctatus under chlorpyrifos intoxication. Int. J. Pharm. Biosci.. 2013;4:865-874.
- [Google Scholar]
- Behavioral toxicity syndromes, a promising tool for assessing toxicity mechanisms in juvenile fat-head minnows. Environ. Toxicol. Chem.. 1990;9:37-46.
- [Google Scholar]
- Recent advances in the use of ixodicides to control ticks affecting livestock. Bull. Int. Epiz.. 1974;81:47-63.
- [Google Scholar]
- Finney, 1971. Probit Analysis, A Statistical Treatment of the Sigmoid Curve. DJ Cambridge University Press, London.
- Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal. Biochem.. 1993;214:65-69.
- [Google Scholar]
- Influence of cypermethrin on liver of the freshwater fish Ophiocephalus punctatus. Poll. Res.. 2011;31:37-40.
- [Google Scholar]
- Hydrolysis of carbohydrates in roach (Rutilus rutilus (L.) at different levels of mercury accumulation. Inland Water Biol.. 2008;1:296-302.
- [Google Scholar]
- On the impact of low concentrations of chlorophos in the period of early ontogenesis on digestive carbohydrases of underyear lings of roach Rutilus rutilus. J. Ichthyol.. 2006;46:404-408.
- [Google Scholar]
- Goyal, T., 1986. Chemical and Biological Methods for Water Pollution Studies. Env. Publications, Karad, India.
- Chronic endrin poisoning in goldfish, Carassius auratus. J. Fish. Board Cana.. 1970;27:2225-2232.
- [Google Scholar]
- Toxicity of copper and cadmium to Heteropneustes fossilis (Bloch) Acta Hydrochim. Hydrobiol.. 1991;19(3):331-340.
- [Google Scholar]
- Supplementation of microbial levan in the diet of Cyprinus carpio fry (Linnaeus, 1758) exposed to sublethal toxicity of fipronil: effect on growth and metabolic responses. Fish. Physiol. Biochem.. 2013;39(6):1513-1524.
- [Google Scholar]
- Chandraprakash Akhtar, M.S.; Prusty, A.K. Dietary microbial levan ameliorates stress and augments immunity in Cyprinus carpio fry (Linnaeus, 1758) exposed to sub-lethal toxicity of fipronil. Aquacul. Res.. 2014;45(5):893-906.
- [Google Scholar]
- Pyrethroids used indoors–immune status of humans exposed to pyrethroids following a pest control operation a one year follow-up study. Inter. J. Hygie. Environ. Heal.. 2003;206(2):93-102.
- [Google Scholar]
- Clinical manifestations and diagnosis of acute pyrethroid poisoning. Arch. Toxicol.. 1989;63:54-58.
- [Google Scholar]
- Synergistic effects of salinity, temperature and heavy metals on mortality and osmoregulation in marine and estuarine isopods (Crustacea) Mar. Biol.. 1975;30:13-20.
- [Google Scholar]
- Study on the effect of water pollutants on carbohydrate profile in fish, Heteropneustes fossilis. Aquaculture. 2003;4:237-340.
- [Google Scholar]
- Investigation of acute toxicity of cadmium on snakehead fish Channa punctatus- a comparative toxicity analysis on median lethal concentration. Int. J. Adv. Biol. Res.. 2013;3:289-294.
- [Google Scholar]
- Arockiaraj J.A novel antimicrobial peptide derived from fish goose type lysozyme disrupts the membrane of Salmonella enterica. Mol. Immunol.. 2015;68:421-433.
- [Google Scholar]
- Comparative analysis of CsCu/ZnSOD defense role by molecular characterization: gene expression-enzyme activity-protein level. Gene. 2015;564:53-62.
- [Google Scholar]
- A comparative transcriptome approach for identification of molecular changes in Aphanomycesinvadans infected Channastriatus. Mol. Biol. Reports. 2019;45:2511-2523.
- [Google Scholar]
- Multifunctional murrel caspase 1, 2, 3, 8 and 9: Conservation, uniqueness and their pathogen-induced expression pattern. Fish Shellfish Immunol.. 2016;49:493-504.
- [Google Scholar]
- The influence of elevated mercury levels in fish diet on the processes of exotrophy in carp, in Mater.VI Vseros. konf. po vodnoy ekotoksikologii (Proc. VI All Russia Conf. on Aquatic Ecotoxicology) Borok. 2011;1:146-150.
- [Google Scholar]
- Pesticide pressure and fish farming in barrage pond in Northeastern France. Part II: residues of 13 pesticides in water, sediments, edible fish and their relationships. Environ. Sci. Poll. Res.. 2013;20:117-125.
- [Google Scholar]
- Protein measurement with the Folin-phenol reagent. J. Biol. Chem.. 1951;193:265-275.
- [Google Scholar]
- Farm Chemicals Handbook ‘92. Willoughby: Meister Publishing Company; 1992.
- Effects of pyrethroid insecticides on nontarget invertebrates in aquatic ecosystems. J. Agri. Entomol.. 1992;9:73-98.
- [Google Scholar]
- Acute toxicities of five synthetic pyrethroidinsecticides to Daphnia magna and Ceriodaphnia dubia. Environ. Toxi. Chem.. 1990;9:1045-1051.
- [Google Scholar]
- Endosulfan toxicity in the freshwater fish Tilapia mossambica. Proc. Acad. Environ. Biol.. 1996;5:49-55.
- [Google Scholar]
- A new method for early detection of neurotoxic diseases (exemplified by pyrethroid poisoning) Gesundheitswesen. 1995;57:214-222.
- [Google Scholar]
- Impacts of pesticides and fertilizers on soil, tail water and groundwater in three vegetable producing areas in the Cordillera Region, Northern Philippines. Am. J. Exp. Agric.. 2013;3:780-793.
- [Google Scholar]
- Anoxia and Sodium loss associated with the depth of brook trout low pH. Comp. Biochem. Physiol.. 1972;41:17-26.
- [Google Scholar]
- Sublethal effect of selected pesticides on protein, carbohydrate and lipid content of different tissues of Oreochromis massambicus. Pest Resid. Environ. Poll. 1986:97-102.
- [Google Scholar]
- Functional roles and gene regulation of tumor necrosis factor receptor 1 in freshwater striped murrel. Mol. Immunol.. 2015;66:240-252.
- [Google Scholar]
- Effects of deltamethrin on haematological parameters and enzymatic activity in Ancistrus mutispinis (Pisces, Teleistoi) Pest. Biochem. Physiol.. 2001;88:122-127.
- [Google Scholar]
- Free cyanide induced physiological changes in the freshwater fish, Poecilia reticulate. J. Exp. Sci.. 2011;2:27-31.
- [Google Scholar]
- Defense properties in the epidermal mucus of different freshwater fish species. Aquaculture Aquarium Conserv. Legislation. 2015;8:184-194.
- [Google Scholar]
- Pellino-1 derived cationic antimicrobial prawn peptide: bactericidal activity, toxicity and mode of action. Mol. Immunol.. 2016;78:171-182.
- [Google Scholar]
- A cumulative strategy to predict and characterize antimicrobial peptides (AMPs) from protein database. Int. J. Peptide Res. Therapeutics. 2017;23:281-290.
- [Google Scholar]
- Bactericidal and fungistatic activity of peptide derived from GH18 domain of prawn chitinase 3 and its immunological functions during biological stress. Int. J. Biol. Macromol.. 2018;106:1014-1022.
- [Google Scholar]
- Fenvalerate induced biochemical changes in the selected tissues of freshwater fish, Cyprinus carpio. Biochem. Int.. 1991;23:1087-1096.
- [Google Scholar]
- The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol. Appl.. 2005;15:618-627.
- [Google Scholar]
- Alfa-amylase: measurement of reducing groups. In: Methods of Enzymatic Analysis (third ed.). Weinhein, Germany: ChemieVerlag; 1984. p. :885-889.
- [Google Scholar]
- Eeffect of nuvacron on the kidney of freshwater fish Channa punctatus. J. Environ. Biol.. 1991;12:243-248.
- [Google Scholar]
- Effect of endosulfan and quinalphos on intestinal absorption of glucose in the freshwater murrel, Channa punctatus. Toxicol. Lett.. 1982;12:289-293.
- [Google Scholar]
- Gene expression and in silico analysis of snakehead murrel interleukin 8 and antimicrobial activity of C-terminal derived peptide WS12. Veterinary Immunol. Immunopathol.. 2017;190:1-9.
- [Google Scholar]
- Therapeutic cationic antimicrobial peptide (CAP) derived from fish aspartic proteinase cathepsin D and its antimicrobial Mechanism. Int. J. Peptide Res. Therapeutics. 2019;25:93-105.
- [Google Scholar]
- Fish heat shock cognate 70 derived AMPs CsHSC70 A1 and CsHSC70 A2. Int. J. Peptide Res. Therapeutics. 2018;24:143-155.
- [Google Scholar]
- Effect of Deltamethrin and a neem-based pesticide Achook on some biochemical parameters in tissues liver, ovary and muscle of zebrafish, Danio rerio (Cyprinidae) Res. J. Chem. Sci.. 2011;1:125-134.
- [Google Scholar]
- Statistical evaluation of hydrobiological parameters of Narmada River water at Hoshangabad City India. Environ. Monit. Assess.. 2008;143:195-202.
- [Google Scholar]
- Review: oxidative stress in fish induced by pesticide. Neuro. Endocrinol. Lett.. 2009;30:2-12.
- [Google Scholar]
- Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171:53-59.
- [Google Scholar]
- Colorimetric procedures for amino acids. In: Colowick S.P., Kaplan N.O., eds. Methods in Enzymology. New York: Academic Press; 1957. p. :467-477.
- [Google Scholar]
- Oxygen consumption in a cat fish Hystus gulio (Ham)exposed to heavy metals. J. Environ. Biol.. 1995;16:207-210.
- [Google Scholar]
- Changes in protein subunits induced by endosulfan and fenvalerate in fresh water fish Labeo rohita through SDS-PAGE. J. Environ. Biol.. 2010;31:759-763.
- [Google Scholar]
- A study on the bioaccumulation of fenvalerate-a synthetic pyrethroid in the body tissue of L. rohita, C. catla, C. mrigala by gas liquid chromatography. Pollut. Res.. 1999;18:57-59.
- [Google Scholar]
- Reproductive toxicity and disposition of 2,3,7,8 tetra chlorodibenzo_p_dioxin in adult brook trout (Salveli nus fontinalis) following a dietary exposure. Environ. Toxicol. Chem.. 1998;17:2394-2407.
- [Google Scholar]
- Relative toxicity of aldrin, fenvalerate, captan and diazinon to the freshwater foodfish, Clarias batrachus. Biomed. Environ. Sci.. 1992;5:33-38.
- [Google Scholar]
- Toxic effect of dimethoate (Organophosphate) on metabolism and enzyme system of fresh water teleost fish Channa punctatusI. Asian J. Fish. Sci.. 2003;16:349-359.
- [Google Scholar]
- Biodynamic agriculture research progress and priorities. Renew. Agri. Food Sys.. 2009;24:146-154.
- [Google Scholar]
- Induction and recovery of morphofunctional changes in the intestine of juvenile carnivorous fish (Epinephelus coioides) upon exposure to foodborne benzo[a]pyrene. Aqua. Toxicol.. 2007;82:181-194.
- [Google Scholar]







