Translate this page into:
Factors influencing food composition, feeding habits and intensity of Glossogobius giuris caught from the vietnamese mekong delta
⁎Corresponding author. dmquang@ctu.edu.vn (Quang Minh Dinh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Knowledge of diet composition and feeding habits are essential to our understanding of fish ecological adaptation and aquaculture studies. This study aims to gain knowledge on diet composition, feeding intensity and habits for Glossogobius giuris – a commercial goby residing in brackish to freshwater regions of the Vietnamese Mekong Delta.
Methods
A collection of 1,291 samples (632 males and 659 females) were caught monthly from January 2020 to December 2020 at four sites using trawl nets. A combined analysis of prey occurrence and weight was used to calculate the diet composition of this fish.
Results
This fish displayed high feeding intensity, and its diet composition was seen to be comprised of small fish, Acetes spp., Uca spp., molluscs, and others (e.g., detritus and fish scales). Although males and females of two fish size groups, two seasons and at the four sites displayed the same feeding trends as Costello graphical analysis showed, the diet composition was seen to vary with season and site. It suggested that the difference in salinity between seasons and the four sites regulated food composition within this fish.
Conclusion
These findings advance the understanding of fish adaptation capacities and food flexibility.
Keywords
Carnivore
Food composition
Feeding habit and intensity
Glossogobius giuris
1 Introduction
Fish diet and feeding ecology are crucial to understanding the roles of fish in a community (Wootton, 1996). Fish diet composition varies over seasons; e.g., Neogobius melanostomus in Canada ingests mainly ostracods in autumn, but fish eggs in spring (Brush et al., 2012). The fish diet composition is also related to food availability, e.g., Parapocryptes serperaster consumes mainly diatoms in Malaysia (Khaironizam & Norma-Rashid, 2000), but detritus in the Vietnamese Mekong Delta (VMD) (Dinh et al., 2017c). Diet and feeding ecology also were seen to improve fish adaptation to habitat, but limited to gobiid species in the VMD.
Glossogobius is a genus of the family Gobiidae with 29 species in the world documented by Hoese et al. (2015). In the VMD, this genus has three species: G. aureus, G. giuris and G. sparsipapillus (Tran et al., 2013). Glossogobius giuris is widely inhabited from marine to brackish and freshwater from Africa to Oceania (Riede, 2004), including the VMD (Dinh, 2008; Diep et al., 2014; Tran et al., 2020). This fish is a target is a carnivore ingesting mainly small fish and crustaceans in Bangladesh, reported by Hossain et al. (2016). In the VMD, Glossogobius giuris plays essential roles in the local food supply, but knowledge of its biology and ecology is fragmented and patchy at best, with some data on morphology, classification, environmental requirements, reproducing traits and growth patterns existing (Tran et al., 2013; Dinh & Ly, 2014; Nguyen & Dinh, 2021; Phan et al., 2021). Meanwhile, there is no data on its diet composition, feeding intensity and habits within the VMD, where its population is currently overfished (Dinh et al., 2017b). The study aims to provide data on the diet composition, feeding intensity and habits of Glossogobius giuris in order to increase our understanding of fish adaptation, food flexibility and aquaculture.
2 Materials and methods
2.1 Study site
Fish were sampled from two sites in Hau River at Cai Rang, Can Tho (CRCT; 10°00′42.6″N-105°48′42.4″E) and Long Phu, Soc Trang (LPST; 9°37′34.4″N-106°08′25.6″E) and two sites along the coast at Hoa Binh, Bac Lieu (HBBL; 9°12′02.5″N-105°43′01.2″E) and Dam Doi, Ca Mau (DDCM; 8°58′11.3″N-105°22′54.4″E) (Fig. 1). Fish were sampled every month between January 2020 to December 2020. The tide in these sites is semi-diurnal with range of ∼ 1.2 m. The temperature and pH of these regions are ∼ 27 °C and ∼ 8, respectively. The salinity was ∼ 12 ‰ in LPST and 0 ‰ in CRCT. It rains heavily with ∼ 400 mm precipitation in the wet season (June to December) but roughly no rain in the dry season (January to May) (Le et al., 2006).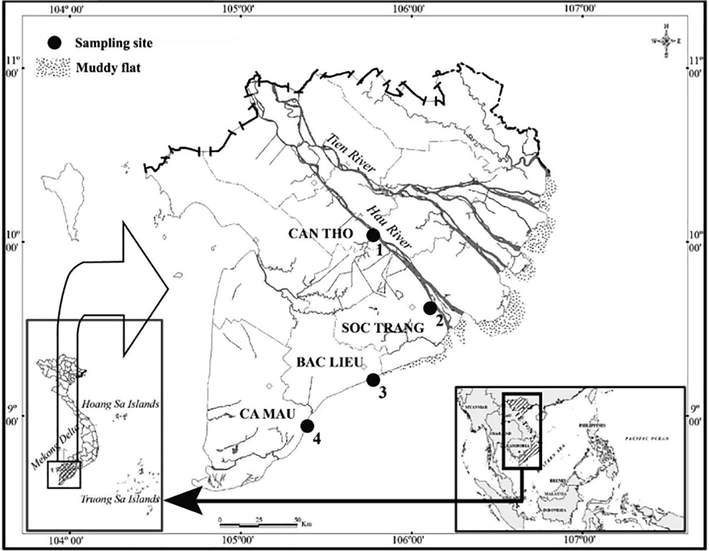
The sampling map modified from Fig. 1 of Dinh (2018) (1: Cai Rang, Can Tho; 2: Long Phu, Soc Trang; 3: Hoa Binh, Bac Lieu; 4: Dam Doi, Ca Mau).
2.2 Fish collection and analysis
Trawl nets (with a cod-end mesh size of 1.5 cm) were used to catch fish. After setting up at high tide, nets were retrieved to collect specimens during the ebb tide at each site. Then fish was identified based on its external morphology (Tran et al., 2013) before sexing using urogenital papilla shape, e.g., oval in females and triangle in males documented by Dinh & Ly (2014). Fish were then anesthetised by Tricaine Methanesulfonate and stored in a 5 % formalin jar before transportation to the laboratory. Before removing the intestinal tract, the fish weight and total length were measured to the nearest 0.01 g and nearest 0.1 cm, respectively.
2.3 Food and feeding habits
A stereomicroscope was used to analyse food items, which were identified up to a suitable taxonomic level using the identification description provided by Nguyen et al. (2013). The stomach contents were then calculated by prey occurrence (%Oi = 100 × Oi/N, where Oi: number of fish consuming prey i and N: all examined fish) (Hynes, 1950) and prey weight (%Wi = 100 × Wi/Wtotal, Wi: weight of prey i, Wtotal: all prey weight) (Hyslop, 1980). The diet composition was quantified using a combined analysis of prey occurrence and weight (e.g., biovolume) as (Vi: i-prey biovolume, Oi: i-prey occurrence, Wi: i-prey weight) (Natarajan & Jhingran, 1961; Hyslop, 1980).
All prey biovolume values were used to test if diet composition changed with sex, fish size, season, and site (Baeck et al., 2013). This test was performed using PRIMER v.6.1.11 with PERMANOVA + v.1.0.1 add-on package (Clarke & Gorley, 2006; Anderson et al., 2008). If diet composition was varied significantly between sexes or seasons or fish sizes, the Mann-Whitney U test was used to verify which prey contributed to the differences. The Kruskal-Wallis test was applied to prove which prey contributed to the spatial variation in diet composition (Dinh et al., 2017c). The difference in the number of fish with empty stomachs and fish with stomach contents between months was tested using the χ2. These tests were performed by SPPS v.21. All tests were set at p < 0.05.
3 Results
3.1 Feeding intensity and habits
A collection of 1,291 fish specimens (659 females and 632 males) were presented in Table 1. Data analysis showed that the proportion of fish with an empty stomach (27.19 %) was significantly higher than that of the fish with stomach contents (∼72.81 %, χ2 = 6.33, p = 0.01). The Costello graphic illustrates that G. giuris was a generalist feeder, consuming mainly Acetes spp., followed by others and small fish (Fig. 2). Male and female G. giuris in different fish groups and seasons (Fig. 3) and sites (Fig. 4) fed predominantly on Acetes spp., others and small fish.
Cai Rang, Can Tho
Long Phu, Soc Trang
Hoa Binh, Bac Lieu
Dam Doi, Ca Mau
Sampling time
Total
No. of fish with empty stomach
Total
No. of fish with empty stomach
Total
No. of fish with empty stomach
Total
No. of fish with empty stomach
M
F
M
F
M
F
M
F
M
F
M
F
M
F
M
F
Jan-20
7
6
4
4
11
19
10
16
14
11
11
7
18
9
11
5
Feb-20
7
13
5
11
19
16
10
7
11
14
11
11
32
33
21
18
Mar-20
19
11
11
6
14
10
8
5
8
19
8
17
13
17
8
9
Apr-20
11
12
9
11
17
15
6
11
23
14
16
12
18
11
6
4
May-20
16
15
10
12
17
15
8
8
18
7
16
6
25
5
18
4
Jun-20
19
11
12
5
10
6
9
5
14
12
8
10
17
10
13
6
Jul-20
9
21
0
8
21
6
20
6
11
18
8
9
10
19
7
17
Aug-20
8
23
8
23
9
12
7
10
9
12
7
10
13
13
13
13
Sep-20
11
19
11
19
7
21
5
19
11
13
9
10
15
15
15
15
Oct-20
9
12
7
10
6
7
6
7
12
8
7
7
11
18
9
17
Nov-20
9
12
7
10
13
14
9
12
12
14
10
12
9
20
3
17
Dec-20
9
21
8
11
4
11
3
10
13
8
6
5
13
11
10
9
Sum
134
176
92
130
148
152
101
116
156
150
117
116
194
181
134
134
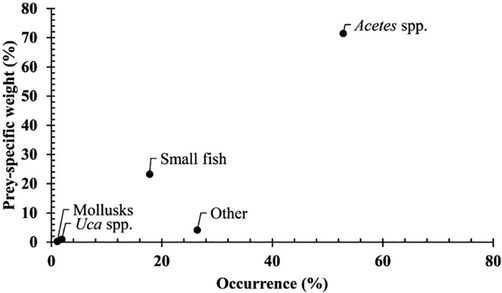
The modified Costello graphic of Glossogobius giuris.
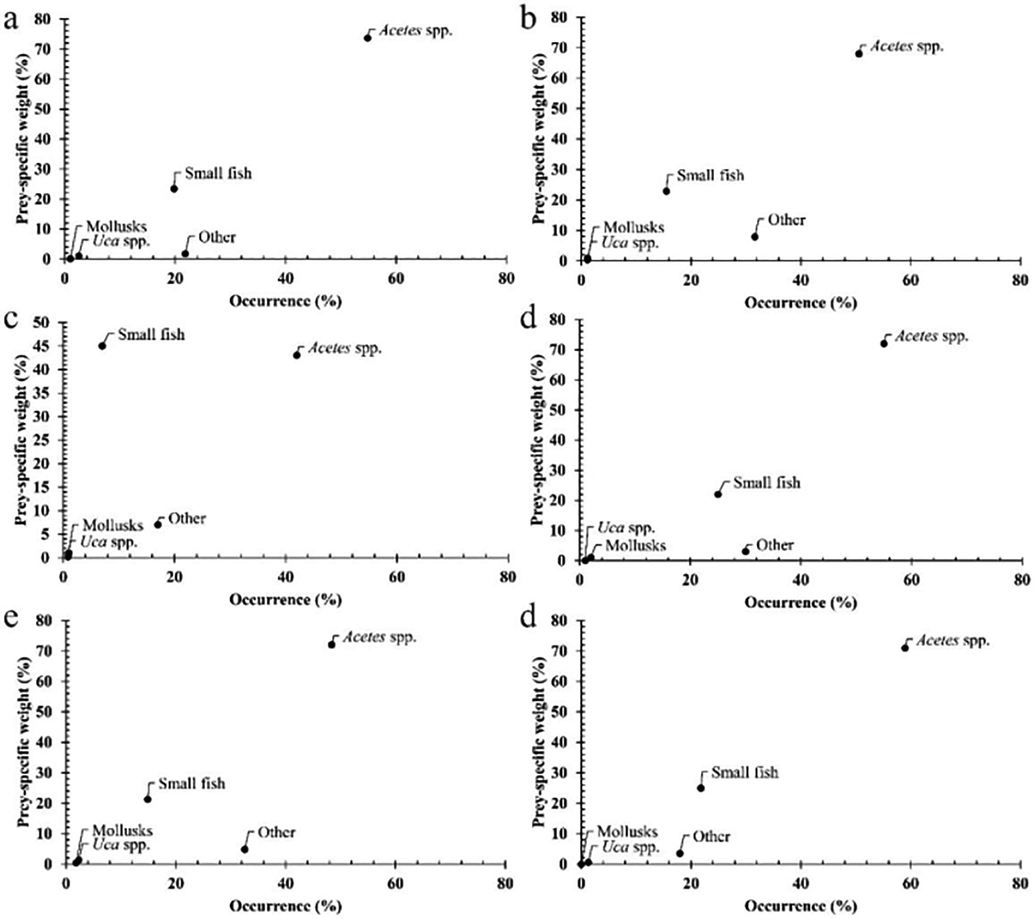
The modified Costello graphics of Glossogobius giuris in sex, fish sizes and seasons (a: male, n = 632; b: female, n = 659; c: immaturity, n = 406; d: maturity, n = 885; e: dry, n = 590 and d: wet, n = 701).
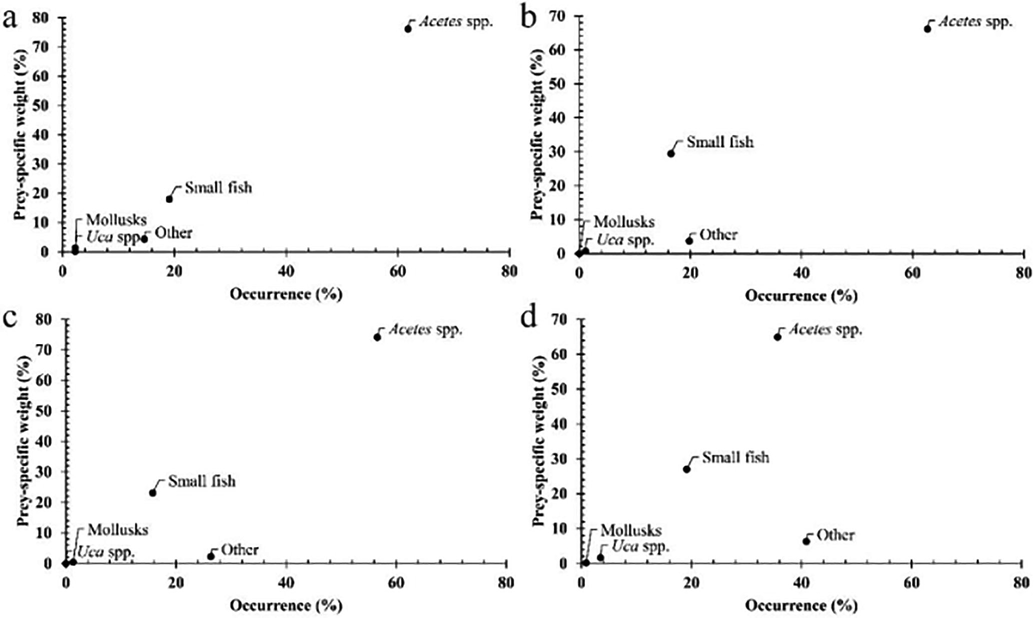
The modified Costello graphics of Glossogobius giuris in four sites (a: CRCT, n = 310; b: LPST, n = 300; c: HBBL, n = 306; d: DDCM, n = 375).
3.2 General food composition of Glossogobius giuris
The diet composition of this goby comprised of small fish, Acetes spp., Uca spp., molluscs and others (e.g., detritus and fish scales). Frequency analysis showed that the goby fed primarily on Acetes spp (52.83 %), followed by others (26.42 %) and small fish (17.79 %), but ingested randomly Uca spp (1.89 %) and molluscs (1.08 %). Meanwhile, the weight and biovolume analyses showed this goby primarily consumed Acetes spp. (71.47 %) and secondarily small fish (23.23 %), but randomly fed on other prey (Table 2). (CRCT: Cai Rang, Can Tho; LPST: Long Phu, Soc Trang; HBBL: Hoa Binh, Bac Lieu; DDCM: Dam Doi, Ca Mau).
Food items
Frequency
Category
Acetes spp.
Small fish
Mollusks
Uca spp.
Others
Occurrence
Male
54.82
19.80
1.02
2.54
21.83
Female
50.57
15.52
1.15
1.15
31.61
Weight
Male
73.68
23.45
0.12
0.99
1.76
Female
67.96
22.88
0.34
0.91
7.91
Biovolume
Male
73.68
23.45
0.12
0.99
1.76
Female
67.96
22.88
0.34
0.91
7.91
Occurrence
Dry season
48.37
14.88
1.86
2.33
32.56
Wet season
58.97
21.79
–
1.28
17.95
Weight
Dry season
72.06
21.28
0.43
1.38
4.86
Wet season
70.95
24.95
–
0.59
3.51
Biovolume
Dry season
72.06
21.28
0.43
1.38
4.86
Wet season
70.95
24.95
–
0.59
3.51
Occurrence
CRCT
50.57
15.52
1.15
1.15
31.61
LPST
62.64
16.48
–
1.10
19.78
HBBL
56.58
15.79
1.32
–
26.32
DDCM
35.65
19.13
0.87
3.48
40.87
Weight
CRCT
67.96
22.88
0.34
0.91
7.91
LPST
66.14
29.44
–
0.72
3.70
HBBL
74.14
23.12
0.45
–
2.29
DDCM
64.33
30.68
0.19
–
4.80
Occurrence
CRCT
67.96
22.88
0.34
0.91
7.91
LPST
66.14
29.44
–
0.72
3.70
HBBL
78.26
17.08
–
0.61
4.05
DDCM
64.89
26.99
0.19
1.65
6.28
3.3 Food composition of males and females, fish sizes, two seasons, and four sites
The diet composition of male and female G. giuris over fish size, season and sites consisted of small fish, Acetes spp., Uca spp., molluscs and others (Table 2). The fish fed primarily on Acetes spp (52.83 %), followed by other (26.42 %) and small fish (17.79 %). This species could randomly ingest Uca spp. (1.89 %) and molluscs (1.08 %) based on the prey occurrence. Meanwhile, prey weight analysis showed that this species fed predominantly Acetes spp. (71.47 %), followed by small fish (23.23 %) and other prey (∼5.30 %). The biovolume prey analysis indicated that this goby fed primarily on Acetes spp., followed by small fish (Table 2). Likewise, males and females of different sizes in the dry-wet season pattern ingested mainly Acetes spp, followed by small fish and others based on the occurrence frequency analysis. However, the fish predominantly ingested Uca spp., followed by others and small fish according to prey weight and biovolume analyses (Table 2). Diet composition was seen not vary with sex (PERMANOVA, Pseudo-F = 0.26, p = 0.80), fish sizes (Pseudo-F = 2.58, p = 0.07), but varied over season (Pseudo-F = 4.96, p < 0.001), and site (Pseudo-F = 5.58, p < 0.001). The variations in the diet between males and females was not dependant on fish size (Pseudo-F = 1.74, p = 0.19), season (Pseudo-F = 1.09, p = 0.34) and site (Pseudo-F = 1.18, p = 0.32). Other preys regulated variation in food composition between two seasons as their biovolume changed with season (Mann-Whitney, U = -3.40, p < 0.001). Similarly, the difference in food composition among the four sites was regulated by Acetes spp. and other prey, reaching the highest values in CRCT (Kruskal-Wallis, χ2Acetes spp. = 23.55, χ2other = 18.55, pAcetes spp < 0.001, pother < 0.001).
4 Discussion
The goby G. giuris displayed high feeding intensity due to its high proportion of fish with an empty stomach. Similar results have been found in other gobies living in VMD, such as Boleophthalmus boddarti (Dinh, 2015), Eleotris melanosoma (Dinh et al., 2017a), P. serperaster (Dinh et al., 2017c), and Periophthalmodon schlosseri (Tran et al., 2019) and G. sparsipapillus (Tran et al., 2021).
Glossogobius giuris in various fish sizes, seasons, and sites was a generalist feeder as different food types were found in its stomach, enabling the species to sufficiently adapt to various environmental conditions. A generalist feeding habits were also found for Pomatoschistus minutus and P. microps (Salgado et al., 2004; Leitão et al., 2006), Periophthalmus barbarus (Udo, 2002), P. argentilineatus (Kruitwagen et al., 2007), Pseudapocrytpes elongatus (Tran, 2008), B. boddarti (Dinh, 2015), P. serperaster (Dinh et al., 2017c), P. schlosseri (Zulkifli et al., 2012; Tran et al., 2019) and G. sparsipapillus (Tran et al., 2021). By contrast, Economidichthys pygmaeus was found to be a specialist feeder, ingesting copepods and chironomids (Gkenas et al., 2012). The difference in feeding habits of these gobies indicates that the feeding strategy was species-specific.
Glossogobius giuris was a carnivore, feeding primarily Acetes spp., secondarily small fish, and rarely molluscs, Uca spp. and other prey, as demonstrated by the greatest and lowest percentage of these prey items in the fish diet. Yet, in Mithamain Haor, Bangladesh, this species was a carnivore consuming mainly small fish and crustacean (Hossain et al., 2016). Similarly, G. sparsipapillus living in VMD fed mostly on Acetes spp. and small fish (Tran et al., 2021). Meanwhile, P. elongatus (Tran, 2008) and P. serperaster (Dinh et al., 2017c) in VMD ingested primarily detritus and diatoms, whereas Boleophthalmus pectinirostris (Yang et al., 2003) and Boleophthalmus boddarti (Ravi, 2013; Dinh, 2015) were seen to feed mainly diatoms. It is therefore suggestive that the fish diet is species-specific and regulated by food availability. For example, P. serperaster ingested mostly diatoms in Malaysia (Khaironizam & Norma-Rashid, 2000), but detritus in the VMD (Dinh et al., 2017c). The stomach of P. microps contented Mysidacea in the upper Tagus estuary (Salgado et al., 2004), but not in fish found in the Mondego estuary (Leitão et al., 2006).
Some gobies like P. serperaster (Dinh et al., 2017c) show the intraspecific variation of dietary composition. But, in this study, the diet composition of immature G. giuris was not significantly different from the mature group, which was found in G. sparsipapillus (Tran et al., 2021). The intraspecific change in diet composition was also found in P. waltoni in Iraq (Barak, 1994), P. barbarus in the Imo River estuary (Udo, 2002) and Rumuolumeni Creek of Nigeria (Bob-Manuel, 2011), P. argentilineatus in Japan (Nanjo et al., 2008) in East Africa (Kruitwagen et al., 2010), P. schlosseri in Malaysia (Zulkifli et al., 2012) and B. boddarti in India (Ravi, 2013).
The detritus input regulated from high rainfall in the wet season affected the diet composition of G. giuris in this study, but corresponding results were not found for G. sparsipapillus (Tran et al., 2021). The seasonal variations in diet composition were found in B. boddarti in India (Ravi, 2013) and P. septemradiatus in VMD (Dinh et al., 2020).
5 Conclusion
Glossogobius giuris was carnivorous fish, consuming mainly small Acetes spp, small fish, and others. The diet of this goby did not change with sex, size but varied with season and site. The present study contributes important biological data of this goby species, which can be used to advance sustainable fish management and aquaculture.
Funding
This study is funded by the Ministry of Education and Training of Vietnam under Grant No B2020-TCT-13. Phan Hoang Gieo was funded by Vingroup JSC and supported by the Master, PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), Institute of Big Data, code VINIF.2021.TS.146.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. United Kingdom, PRIMER-E, Plymouth; 2008.
- Ontogenetic and diel changes in diets of two sympatric mudskippers Periophthalmus modestus and Periophthalmus magnuspinnatus on the tidal flats of Suncheon Bay, Korea. Fisheries Science. 2013;79(4):629-637.
- [Google Scholar]
- The piscivorous feeding of mudskipper Periophthalmus waltoni Koumaus from Khor Al-Zubair, Northwest Arabian Gulf. Pak. J. Zool.. 1994;26:280-283.
- [Google Scholar]
- Food and feeding ecology of the mudskipper Periopthalmus koelreuteri (Pallas) Gobiidae at Rumuolumeni Creek, Niger Delta, Nigeria. Agric. Biol. J. North Am.. 2011;2(6):897-901.
- [Google Scholar]
- Spatial and seasonal variability in the diet of round goby (Neogobius melanostomus): stable isotopes indicate that stomach contents overestimate the importance of dreissenids. Can. J. Fish. Aquat. Sci.. 2012;69(3):573-586.
- [Google Scholar]
- PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth: United Kingdom; 2006.
- Species composition of gobiidae distributed in the coastal areas, Soc Trang Province. VNU J. Sci. Nat. Sci. Technol.. 2014;30(3):68-76.
- [Google Scholar]
- Data of survey on the species composition of fishes in Hau Basin at An Phu district, An Giang province. Can Tho Univ. J. .ence. 2008;10:213-220.
- [Google Scholar]
- Preliminary study result of length – weight of tank goby, Glossogobius giuris, distributing in Soc Trang. Can Tho Univ. J. Sci.. 2014;2014(2):220-225.
- [Google Scholar]
- Preliminary study on dietary composition, feeding activity and fullness index of Boleophthalmus boddarti in Mekong Delta, Vietnam. Tap chi Sinh hoc. 2015;37(2):252-257.
- [Google Scholar]
- Food and feeding habits of the broadhead sleeper Eleotris melanosoma from coastline in Soc Trang. In: Noi H.a., Le C.X., eds. Proceedings of the 7th National Scientific Conference on Ecology and Biological Resources. Publishing House for Science and Technology; 2017. p. :1873-1879.
- [Google Scholar]
- Population biology of the goby Glossogobius giuris (Hamilton 1822) caught in the Mekong Delta. Asian Fish. Sci.. 2017;30(1):26-37.
- [Google Scholar]
- Seasonal variation of food and feeding in burrowing goby Parapocryptes serperaster (Gobiidae) at different body sizes. Ichthyol. Res.. 2017;64(2):179-189.
- [Google Scholar]
- Aspects of reproductive biology of the red goby Trypauchen vagina (Gobiidae) from the Mekong Delta. J. Appl. Ichthyol.. 2018;34(1):103-110.
- [Google Scholar]
- Variation in diet composition of the mudskipper Periophthalmodon septemradiatus from Hau River, Vietnam. Bull. Marine Sci.. 2020;96(3):487-500.
- [Google Scholar]
- Diet and feeding habits of Economidichthys pygmaeus (Perciformes: Gobiidae) in Lake Pamvotis, NW Greece. J. Appl. Ichthyol.. 2012;28(1):75-81.
- [Google Scholar]
- Review of the dwarf Glossogobius lacking head pores from the Malili lakes, Sulawesi, with a discussion of the definition of the genus. Raffles Bull. Zool.. 2015;63:14-26.
- [Google Scholar]
- Food and feeding habit of Bele Glossogobius giuris (Hamilton and Buchannan, 1822) collected from Mithamain Haor of Kishoreganj districts, northeastern Bangladesh. Internat. J. Fish. Aquat. Studies. 2016;4(5):84-88.
- [Google Scholar]
- The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. J. Anim. Ecol.. 1950;19(1):36-58.
- [Google Scholar]
- Stomach contents analysis - a review of methods and their application. J. Fish Biol.. 1980;17(4):411-429.
- [Google Scholar]
- A new record of the mudskipper Parapocryptes serperaster (Oxudercinae: Gobiidae) from peninsular Malaysia. Malay. J. Sci.. 2000;19:101-104.
- [Google Scholar]
- Influence of morphology and amphibious life-style on the feeding ecology of the mudskipper Periophthalmus argentilineatus. J. Fish Biol.. 2007;71(1):39-52.
- [Google Scholar]
- Importance of different carbon sources for macroinvertebrates and fishes of an interlinked mangrove–mudflat ecosystem (Tanzania) Estuar. Coast Shelf Sci.. 2010;88(4):464-472.
- [Google Scholar]
- Provinces and city in the Mekong Delta. In: Le T., ed. Geography of Provinces and Cities in Vietnam. Ha Noi: Education Publishing House; 2006. p. :49-94.
- [Google Scholar]
- Feeding ecology, population structure and distribution of Pomatoschistus microps (Krøyer, 1838) and Pomatoschistus minutus (Pallas, 1770) in a temperate estuary, Portugal, Estuarine. Coastal Shelf Sci.. 2006;66(1–2):231-239.
- [Google Scholar]
- Food habits of fishes in the mangrove estuary of Urauchi River, Iriomote Island, southern Japan. Fish. Sci.. 2008;74(5):1024-1033.
- [Google Scholar]
- Index of preponderance—a method of grading the food elements in the stomach analysis of fishes. Indian J. Fish.. 1961;8:54-59.
- [Google Scholar]
- Morphometric and meristic variations in Glossogobius giuris distributed in different locations in the Mekong Delta. TNU J. Sci. Technol.. 2021;226(10):31-38.
- [Google Scholar]
- The zoobenthos of the Can Gio mangrove ecosystem. Ha Noi: Natural Science and Technology Publishing House; 2013.
- Length-weight relationship, growth pattern and condition factor of Glossogobius giuris caught from coastal areas in the Mekong Delta. AACL Bioflux. 2021;14(3):1478-1485.
- [Google Scholar]
- Food and feeding habits of the mudskipper, Boleophthalmus boddarti (Pallas, 1770) from Pichavaram mangroves, southeast coast of India. Internat. J. Marine Sci.. 2013;3(12):98-104.
- [Google Scholar]
- Global register of migratory species - from global to regional scales (final report of the R&D-Projekt 808 05 081). Bonn, Germany: Federal Agency for Nature Conservation; 2004.
- Feeding ecology of the gobies Pomatoschistus minutes (Pallas, 1770) and Pomatoschistus microps (Krøyer, 1838) in the upper Tagus estuary, Portugal. Sci. Marina. 2004;68(3):425-434.
- [Google Scholar]
- Diet composition and feeding habit of Glossogobius sparsipapillus caught from estuarine regions in the Mekong Delta. Egypt. J. Aquat. Res.. 2021;47(3):313-319.
- [Google Scholar]
- Some aspects of biology and population dynamics of the goby Pseudapocryptes elongatus (Cuvier, 1816) in the Mekong Delta. Malaysia: Universiti Malaysia Terengganu; 2008. PhD thesis
- Fishes of Mekong Delta. Vietnam, Can Tho: Can Tho University Publisher; 2013.
- Species composition and biodiversity index of gobiid assemblage in estuarine areas of the Mekong Delta, Vietnam. Egypt. J. Aquat. Biol. Fish.. 2020;24(7):931-941.
- [Google Scholar]
- Digestive tract morphology, food composition and feeding habits of the giant mudskipper Periophthalmodon schlosseri (Pallas, 1770) from the coastline in Tran De, Soc Trang. VNU J. Sci. Nat. Sci. Technol.. 2019;35(3):30-38.
- [Google Scholar]
- Tropic attributes of the mudskipper, Periophthalmus barbarus (Gobiidae: Oxudercinae) in the mangrove swamps of Imo River estuary, Nigeria. J. Environ. Sci.. 2002;14(4):508-517.
- [Google Scholar]
- Feeding and growth. In: Wootton R.J., ed. Fish Ecology. New York, United States: Chapman & Hall; 1996. p. :98-131.
- [Google Scholar]
- Selective feeding by the mudskipper (Boleophthalmus pectinirostris) on the microalgal assemblage of a tropical mudflat. Marine Biol.. 2003;143(2):245-256.
- [Google Scholar]
- Food preference of the giant mudskipper Periophthalmodon schlosseri (Teleostei : Gobiidae) Knowledge Manage. Aquat. Ecosyst.. 2012;405
- [Google Scholar]







