Translate this page into:
Facile technique towards clean fuel production by upgrading waste cooking oil in the presence of a heterogeneous catalyst
⁎Corresponding authors at: Department of Petroleum and Chemical Engineering, College of Engineering, Sultan Qaboos University, Muscat, Oman (A.H. Al-Muhtaseb) and Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung City, Taiwan (V.K. Ponnusamy). muhtaseb@squ.edu.om (Ala'a H. Al-Muhtaseb), kumar@kmu.edu.tw (Vinoth Kumar Ponnusamy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Herein, we utilized waste triglycerides “cooking oil” as an abundant source for biodiesel (methyl esters) production via transesterification over a novel synthesized heterogeneous catalyst (Zn-MgO-ZrO2). The catalyst was prepared by the co-precipitation technique and analyzed by using X-ray diffraction, Scanning Electron Microscope, temperature desorption analysis and surface analysis to better understand the surface chemistry of the catalyst. Moreover, the biodiesel production process through transesterification by using Zn-MgO-ZrO2 was optimized. The optimal biodiesel yield was 92.3 wt% when the optimal set of process variables were at a temperature of 80 °C, molar ratio 12 (methanol and oil), time 4 h and catalyst amount was 4 wt%. The synthesized heterogeneous catalyst is reusable with almost equal efficiency as for the fresh catalyst. Different techniques also examined the quality of produced biodiesel according to the standard procedures defined by the American Society for Testing and Materials (ASTM) and European Union (EU) Standards.
Keywords
Biodiesel
Waste triglycerides
Heterogeneous catalyst
Transesterification
Optimization
Reusability
1 Introduction
Moving towards industrialization, demand for energy and fossil-based fuels have been increased devastatingly (Chua et al., 2020). Currently, the major source for energy and transportation are fossil-based fuels and due to their declining reserves, it leads to a search for alternative sources (Osman, 2020; Osman et al., 2020). Besides, the major concern of global warming which is linked to the combustion of fossil-based fuels. There are mainly three approaches for climate change mitigation, which are conventional mitigation, negative emissions and radiative forcing geoengineering technologies. The utilization of renewable energy is one of the conventional mitigation approaches for climate change abatement (Fawzy et al., 2020). Thus, to address both issues regarding fossil fuels, the potential alternative is required and has been under consideration in the last few decades. Biodiesel is the most commonly studied and commercially produced bio-fuel (Ben-Iwo et al., 2016). Besides, the reported fuel properties of biodiesel produced from biomass are comparable to fossil diesel and can be used directly in a conventional diesel engine without any prior modifications (Abu-Jrai et al., 2017). Transesterification being a reversible equilibrium reaction, is affected by several process parameters such as temperature, time, catalysts loading and methanol to oil molar ratio. To obtain a maximum conversion, a detailed parametric study for their effect on the yield has to be considered (Bokhari et al., 2016). The oil with high free fatty acid content is to be pretreated before transesterification for biodiesel production. However, several bifunctional heterogeneous catalysts have been reported earlier as it promotes simultaneous esterification and transesterification and tending to reduce the overall product cost (Alhassan et al., 2015). Jamil et al. reported transition metal oxides (ZrO2) in a modified form and efficiently used for methyl esters production (Jamil et al., 2018).
Herein we synthesized a dual nature Zn-MgO-ZrO2 catalyst with active sites (acidic and basic nature) to reaction media and used for the production of methyl esters from waste triglyceride. Furthermore, the produced biodiesel was characterized to ensure the quality to be used as a drop-in fuel. It was observed that all measured properties complied with the international standards ASTM and EN.
2 Materials and methodology
2.1 Materials
Waste cooking oil with free fatty acid content 3.9 and molecular weight 882 was collected from the faculty café at Sultan Qaboos University, Oman. Chemicals that have been used herein were of analytical grade and purchased from Sigma-Aldrich which includes ZrO2 (zirconium oxide), KOH (potassium hydroxide), K2CO3 (potassium bicarbonate), CH3OH (methanol), n-C6H6 (hexane), ZnNO3 (zinc nitrate) and MgNO3·6H2O (magnesium nitrate hexahydrate) from Merck-UK.
2.2 Catalyst synthesis and characterization
In catalyst synthesis first co-precipitation method was adopted to prepare the based material, which is MgO-ZrO2. In the co-precipitation method, firstly, all desired amounts of both materials (ZrO2 & MgNO3·6H2O) were taken following the ratio Mg: Zr of 0.4 and poured into a beaker containing 100 ml of deionized water. The solid mixture materials in the beaker changed into a basic medium by adding 1 M KOH and 0.25 M K2CO3 drop-wise into the beaker until the pH of the overall mixture rise to 10, and pH is monitored continuously with stirring of the solution as well. The mixture was allowed to settle overnight and the precipitated material was filtered and washed thoroughly with deionized water until the filtrate reached pH = 7. The solid material was dried overnight at 120 °C and finally calcined at 650 °C for 4 h at 4 °C/min to form the precursor material (MgO-ZrO2). Followed by wet-impregnation of zinc nitrate for the mixed-oxide catalyst (Zn-MgO-ZrO2), it was also calcined at 650 °C for 4 h at 4 °C/min. Finally, the synthesized heterogeneous catalyst was obtained Zn-MgO-ZrO2.
The synthesized catalyst was characterized through several techniques such as X-ray powder diffraction (XRD) technique using PANalytical, Xpert PRO instrument, Scanning Electron Microscopy (SEM) analysis was carried out while utilizing JEOL JSM-7800F-Japan, temperature-programmed desorption TPD technique with two different gases were used as CO2-TPD used for determination of basic sites and NH3-TPD to determine acidic sites by using Thermo Finnigan equipment model TPDRO 1100 Series and BET by ASAP 2020, made by Micromeritics Instruments Inc., (the USA).
2.3 Biodiesel production through transesterification
Waste cooking oil is transformed into biodiesel through transesterification. All experiments are conducted based on the process parameters defined in Table 1.
Parameter
Range
Low
High
Temperature (°C)
60
100
Time (h)
1
6
Methanol to oil (molar ratio)
6
15
Catalyst (wt. %)
1
6
Firstly, the waste cooking oil was poured into a reaction vessel placed on the heating plate and heated up to 60 °C with continuous stirring, as shown in Fig. 1. Meanwhile, the desired amount of synthesized heterogeneous catalyst is stirred with methanol and added into the reaction vessel through one of the necks. The reaction mixture is stirred continuously with continuous heating for the required duration followed by cooling of the reaction mixture to room temperature. Once the reaction mixture is cooled, it is filtered and finally poured into separating funnel and allowed to stay overnight. The reaction mixture showed two distinct layers, a bottom layer known as glycerol and the upper layer, which is the desired biodiesel. The glycerol is separated, and biodiesel is washed thoroughly with warm deionized water in a separating funnel. Moreover, the biodiesel was passed through several standard procedures such as ASTM 5002 to determine the density, ASTM D613 for cetane number, ASTM D2500 for cloud point, ASTM D97 for pour point, ASTM D6371 for cold filter plugging point, ASTM D93 for flashpoint, and finally acid value through ASTM D664 to examine its quality. The yield of biodiesel is calculated using relation as mentioned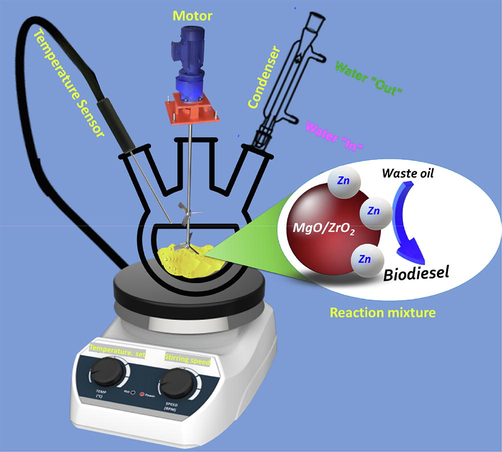
Schematic representation of the experimental apparatus of biodiesel production.
3 Results and discussions
3.1 Catalyst characterization
3.1.1 XRD analysis
The XRD analysis of pristine ZrO2 and its modified form Zn-MgO-ZrO2 was performed to examine the crystallinity and the results were compared with standard JCPDS library. Fig. 2a shows the XRD patterns of catalysts (ZrO2 and Zn-MgO-ZrO2), which depicts that pristine ZrO2 is highly crystalline based on the sharp diffraction lines in its pattern. Further on, the pattern representing the modified catalyst Zn-MgO-ZrO2 shows the sharps diffraction lines, which depicts that the crystallinity of the material has been sustained. The pattern representing the pristine ZrO2 shows the characteristic diffraction lines at 24, 29, 32 and 34° (JCPDS card no. 00–027-099), which represent its monoclinic phase (Jamil et al., 2018). Moreover, the pattern representing Zn-MgO-ZrO2 contains the characteristic diffraction lines of parent material along with some additional diffraction lines, such as at 36° (1 0 1) and 42.3°, which shows the addition of active metals zinc oxide and magnesium oxide, respectively.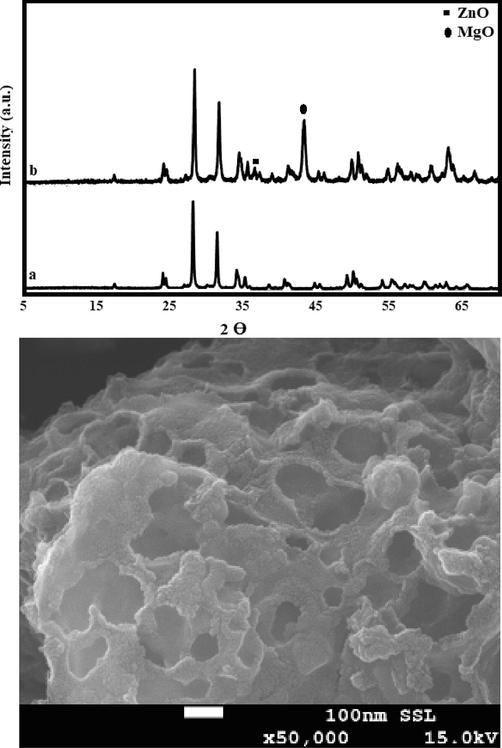
a) XRD analysis for synthesized catalysts for pure ZrO2 and mixed metal oxide (Zn-MgO-ZrO2) catalyst and b) SEM image of synthesized catalyst (Zn-MgO-ZrO2).
3.1.2 Microscopic analysis
The surface morphology of the synthesized catalyst is shown in Fig. 2b, which showed that it is porous with a rough surface. The porous catalyst with large pore channels can offer better diffusivity of reactants, which extends the availability of active sites for reactants and thus tending to give a better product yield (Jamil et al., 2016; Tatarchuk et al., 2019). Based on Fig. 3, it can be reported that synthesized catalyst can offer a better pore channel with high porosity for the process involving triglycerides.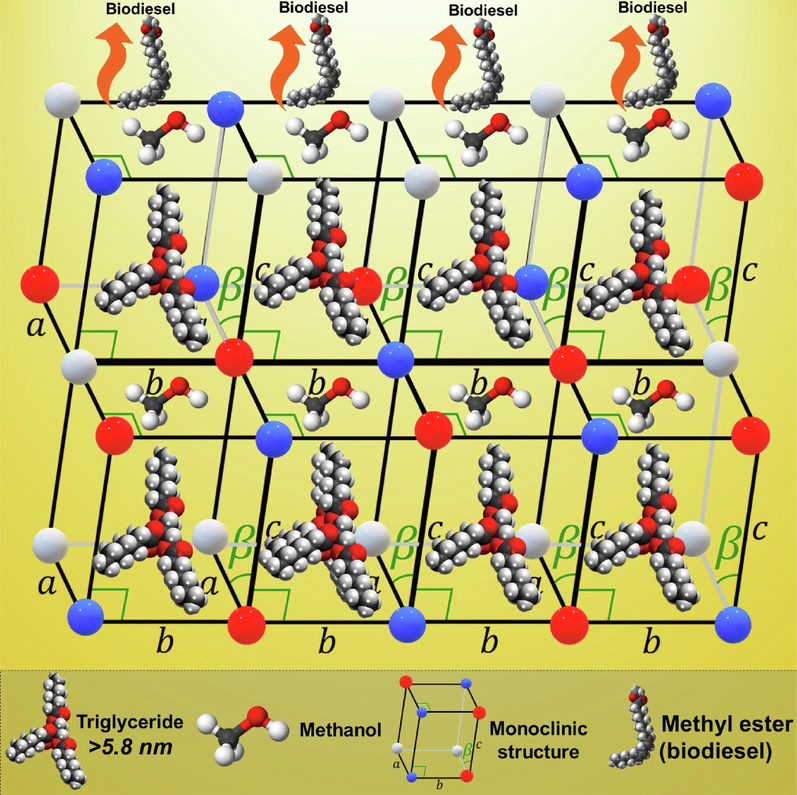
Schematic molecular representation of the transformation of triglycerides along with methanol over the novel mixed metal oxide catalyst system for the production of the biodiesel.
3.1.3 Brunauer-Emmett-Teller (BET) analysis
The catalysts were further analyzed to determine the surface area, pore volume and pore diameter by the BET analysis given in Table 2. It can be noticed that initially, the surface area of pristine ZrO2 support was 11.92 m2/g. However, when modified with alkaline metal oxide MgO, it increased to a large extent to a value of 46.18 m2/g. Along with this the pore diameter, the volume also increased. Moreover, by increasing the surface area and opening up new active new pore channels and paths, this will ultimately help in getting better contact between the active sites and the reactant leading to a better product yield (Nandan et al., 2013; Naushad et al., 2016;). The diameter of the triglyceride molecule is almost 5.8 nm thus the catalyst which offers high pore diameter than this will be most suitable to conduct the conversion of triglycerides without blocking the pore channels of catalyst as shown in Fig. 3. It shows the molecular level of transformation of triglycerides along with methanol over the monoclinic porous structure of the mixed metal oxide Zn-MgO-ZrO2 and biodiesel yield observed was 92.3%.
Sample
Surface area (m2/g)
Pore diameter (nm)
Pore volume (cm3/g)
ZrO2
11.92
10.50
0.041
MgO-ZrO2
46.18
17.13
0.121
Zn-MgO-ZrO2
37.25
15.21
0.107
3.1.4 Temperature programmed desorption
Fig. 4a shows the temperature-programmed desorption CO2-TPD analysis results for the pristine ZrO2 and its modified form Zn-MgO-ZrO2 which is done to determine the existence of basic sites in the solid mixed metal oxide catalyst material. The curve representing the pristine ZrO2 shows the broad peak appearing at the temperature range of 100–200 °C and this is attributed to weaker strength sites of basic nature. Moreover, the curve of Zn-MgO-ZrO2 shows three different strengths peaks representing the presence of active sites on the surface of the mixed metal oxide catalyst. The weak basic sites that exist in the pristine ZrO2 were also observed in Zn-MgO-ZrO2 catalyst. A high strength peak was observed at a temperature range of 250–350 °C which can be related to moderate basic sites. A small peak was observed at a higher temperature of 700 °C, which is related to highly basic sites due to the presence of monodentate.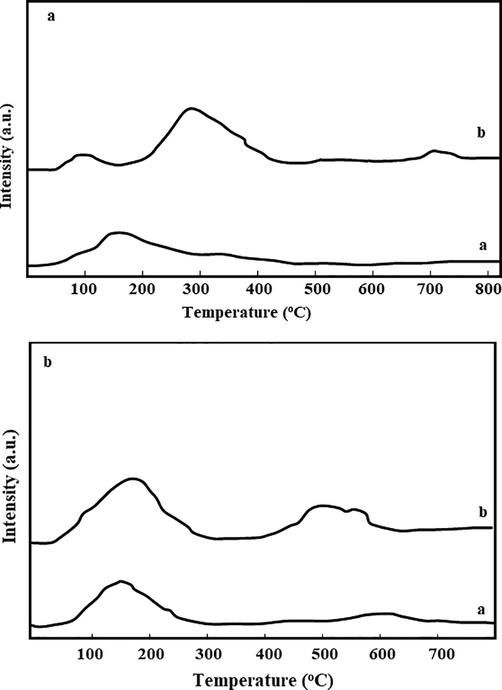
a) CO2-TPD analysis for ZrO2 and Zn-MgO-ZrO2 catalyst along with NH3-TPD analysis for pure ZrO2 and Zn-MgO-ZrO2 catalyst.
The NH3-TPD analysis was performed to determine the presence of acidic sites within the synthesized catalysts and the curves represent the desorption behaviour of ammonia with increasing temperature as shown in Fig. 4b. It can be observed from the TPD-NH3 curves representing the behaviour of pristine ZrO2 that only one peak appears in the curve and it lies within the temperature range of 100–300 °C. The acidic sites present between 100 and 300 °C can be related to weak acidic sites. Moreover, Zn-MgO-ZrO2 curve with additional metallic oxides gives three peaks in the TPD-NH3 curve, which can be attributed to the redistribution of active charges of material after modification (Konaka et al., 2013). The peak appearing at 100 °C can be attributed to the presence of weak acidic sites; however, while moving further, a large intense peak appears which can be related to the presence of moderate acidic sites. Moreover, the small intensity peak appearing at 700 °C can be related to the presence of strong acidic sites. Thus, based on TPD analysis, it can be concluded that the synthesized catalyst (Zn-MgO-ZrO2) has both acidic and basic active sites that can actively participate in the reaction for better conversion and product yield.
3.2 Parametric studies
The modified catalyst is applied for biodiesel production over the novel mixed metal oxide catalyst as shown from the process flow in Fig. 5. As shown in Fig. 6, all four parameters are plotted against the product biodiesel yield obtained from the transesterification of waste cooking oil. Fig. 6a represents that initially when the process temperature is low, the biodiesel yield is low as well, as temperature increased similar trend for biodiesel yield was observed until 80 °C. This increment in the yield of biodiesel initially was low at low-temperature, methanol and triglyceride were not at the exited state to replace the functional groups. However, when the temperature increased, the alkyl group from methanol is replaced with the formation of alkyl esters known to be biodiesel. However, if the temperature is increased beyond 80 °C, the biodiesel yield tends to decrease. Thus, due to the common fact that above boiling point methanol is thought to be in vapour phase above the reaction media in a reaction vessel, which ultimately tends to make methanol less available thus tending to decrease the product yield.
Process flow sheet to produce biodiesel over the novel mixed metal oxide catalytic system.
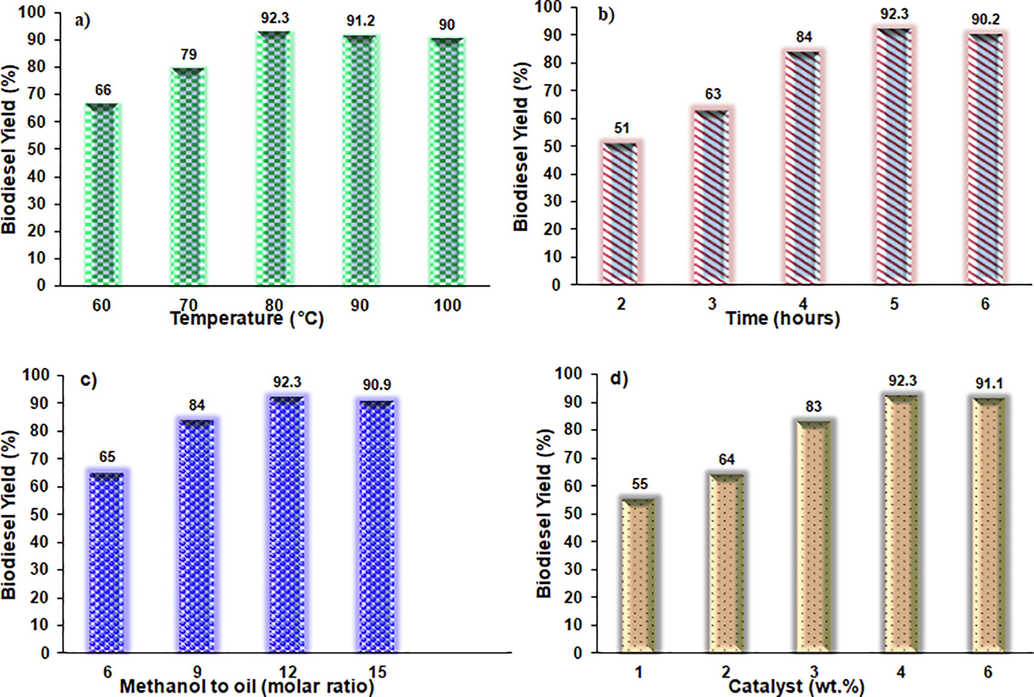
Study of parameters effect on biodiesel yield.
Similarly, the effect of time on the biodiesel product yield is obvious in Fig. 6b. Initially, when the reaction time was low at 2 h, the biodiesel yield was significantly low; however, an ascending trend in the biodiesel product was observed when the reaction time was increased and observed to be maximum when the provided time for the reaction reached up to 5 h. However, it can be observed that when the reaction time is increased beyond 5 h, biodiesel tends to decrease. It has been reported that if time is increased beyond the certain limit for transesterification reaction, the yield is decreased. Thus, enough time should be provided depending upon the nature of catalyst for maximum production of methyl esters and avoiding the reversible reaction to proceed.
Fig. 6c presents the effect of the molar ratio of methanol to oil on biodiesel yield. Based on the stoichiometric calculation, transesterification reaction requires a molar ratio of reactants to be three; however, since the reaction is reversible, methanol is provided in excess. Herein, the initial molar ratio of reactants was kept at 6 and it was observed that the formation of methyl esters is less but enhanced by increasing the molar ratio. Thus, by increasing ratio, it tends to provide additional methyl groups to form methyl esters. However, when the ratio was increased beyond 12, the biodiesel was observed to be less as compared to the yield when the ratio was kept at 12. This can be attributed to the fact that increasing the molar ratio of reactants tends to increase the amount of methanol in the reaction media, which tends to cause the hindrance in the product biodiesel separation from the by-product.
Fig. 6d that initially, when the amount of catalyst was low, the biodiesel was significantly low. However, upon increasing the amount of catalyst, the methyl esters production showed an increasing trend. This result confirms adding up more catalyst in the reactor; it enhances the availability of charged sites for reaction to occur. Despite this, the biodiesel yield was increased by adding up catalyst up to a definite limit. For instance, 4 wt% catalyst weight in the reaction vessel was shown to result in the maximum biodiesel yield, beyond that yield starts decreasing. Thus, the descending order of product biodiesel yield upon increasing the amount of catalyst in reaction vessel after a certain limit can be related to the observation that high catalyst amount in vessel tends to give mass transfer limitation and also causes difficulty in mixing and causes decreased product yield. Thus, based on this parametric study optimized set of process parameters can be reported such as temperature 80 °C, when the processing time is 4 h while using a molar ratio of reactants 12 and loading catalyst up to 4 wt% to get an optimum yield of product biodiesel of 92.3 wt%.
3.3 Reusability of the synthesized catalyst
As shown in Fig. 7, the used catalyst was evaluated in two ways, one without calcination only by washing with methanol (to remove the remaining reactants) and applied to reaction media. However, another experiment was conducted in which the used catalyst (after washing with methanol) was calcined at 650 °C and applied to the reaction media. However, when the catalyst was applied without calcination, biodiesel production was less. This can be attributed to the fact that the decrement in the yield might be due to the deposition of molecules on the surface of the catalyst and blocking the active sites, which cause less availability of active sites in the reaction media. However, when the used catalyst was calcined and loaded to reaction vessel methyl esters production was almost the same compared to the fresh catalyst. Thus, it can be concluded that the synthesized novel mixed metal oxide catalyst herein is highly active and can be reused after calcining for multiple times without losing its catalytic activity.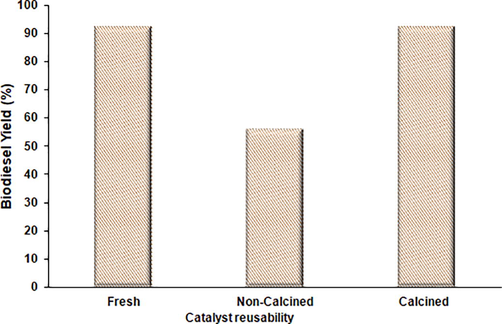
Reusability study for the synthesized mixed metal oxide catalyst.
3.4 Properties determined for biodiesel
The measured fuel properties of biodiesel are compared with the standard values and ranges defined by ASTM and EN standards as shown in Table 3. The measured acid value for the biodiesel is 0.39 mg KOH/g, which is less than the maximum limit defined by ASTM and EN standards. Density was 874 kg/m3, which is within the range defined by ASTM and EN standards. Viscosity is 3.69 mm2/s; this refers to the fact that produced biodiesel will not affect the engine performance. Biodiesel’s flashpoint was 175 °C this satisfies the standards limit. aNot defined.
Property
WCO
Date seed biodiesel [Jamil et. al. 2018b]
EN 14,214
ASTM 6751
Acid Value (mg KOH/g)
0.39
0.11
0.50 max
0.80 max
Density (kg.m−3) at 25 °C
874
881
860–900
-a
Viscosity (mm2.s−1) at 40 °C
3.69
3.85
3.5–5.0
1.9–6.0
Flashpoint (°C)
175
142
120 min
93 min
Calorific Value (MJ/kg)
44.13
44.21
-a
-a
Cetane number
70.24
61.12
51 min
47 min
Cloud point (°C)
1.6
3.69
-a
–
Pour point (°C)
−3.4
−1.23
-a
-a
Cold Filter Plugging Point (°C)
−2.54
−0.61
-a
-a
Free Glycerin (%)
0.011
0.011
0.020 max
0.020 max
Total Glycerin (%)
0.160
0.162
0.250 max
0.240 max
The calorific value for biodiesel produced is 44.13 MJ/kg, which is similar to fossil diesel fuels. The cetane number of biodiesels refers to the anti-knocking of the engine, so it directly affects the efficiency of the engine. For the produced biodiesel herein, it has a value of 70.24, which is higher than the minimum and within the limit defined by ASTM and EN standards. In the current research work, the cloud point and pour point of produced biodiesel are 1.6 °C and −3.4 °C, respectively. Thus, based on all characteristic values of biodiesel produced herein, it can be stated that it can be efficiently used as a fuel in a diesel engine without any prior modifications.
3.5 Conclusions
The present study revealed that the synthesized novel mixed metal oxide heterogeneous catalyst (Zn-MgO-ZrO2) is highly active in biodiesel production from waste triglyceride “cooking oil”. The dual nature catalyst was with high surface area, pore diameter and volume also supported the specifications for biodiesel production. The optimal yield of biodiesel obtained was 92.3 wt% when the synthesized catalyst was applied, which depicts its effectiveness. The reusability of the synthesized catalyst was evaluated and found to be reusable after modification. Moreover, the fuel properties of biodiesel were according to standard values given by ASTM and EN, ensuring its potential to be used as an effective fuel.
Acknowledgement
One of the authors (Asma A. Alothman) is grateful to the Researchers Supporting Project number (RSP-2020/243), King Saud University, Riyadh, Saudi Arabia for financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abu-Jrai, A.M., Jamil, F., Al-Muhtaseb, A.a.H., Baawain, M., Al-Haj, L., Al-Hinai, M., Al-Abri, M., Rafiq, S., 2017. Valorization of waste Date pits biomass for biodiesel production in presence of green carbon catalyst. Energy Conversion and Management 135, 236-243.
- Synthesis of waste cooking oil-based biodiesel via effectual recyclable bi-functional Fe2O3MnOSO42−/ZrO2 nanoparticle solid catalyst. Fuel. 2015;142:38-45.
- [Google Scholar]
- Biomass resources and biofuels potential for the production of transportation fuels in Nigeria. Renew. Sustain. Energy Rev.. 2016;63:172-192.
- [Google Scholar]
- Optimisation on pretreatment of rubber seed (Hevea brasiliensis) oil via esterification reaction in a hydrodynamic cavitation reactor. Bioresour. Technol.. 2016;199:414-422.
- [Google Scholar]
- Biodiesel synthesis using natural solid catalyst derived from biomass waste — A review. J. Ind. Eng. Chem.. 2020;81:41-60.
- [Google Scholar]
- Upgrading of bio-oil from palm kernel shell by catalytic cracking in the presence of HZSM-5. Int. J. Green Energy. 2016;13(4):424-429.
- [Google Scholar]
- Jamil, F., Al-Muhtaseb, A.a., Myint, M.T.Z., Al-Hinai, M., Al-Haj, L., Baawain, M., Al-Abri, M., Kumar, G., Atabani, A.E., 2018. Biodiesel production by valorizing waste Phoenix dactylifera L. Kernel oil in the presence of synthesized heterogeneous metallic oxide catalyst (Mn@MgO-ZrO2). Energy Conversion and Management 155, 128-137.
- Conversion of biodiesel-derived crude glycerol into useful chemicals over a zirconia-iron oxide catalyst. Ind. Eng. Chem. Res.. 2013;52(44):15509-15515.
- [Google Scholar]
- Acid functionalized carbon–silica composite and its application for solketal production. Microporous Mesoporous Mater.. 2013;179:182-190.
- [Google Scholar]
- Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem. Eng. J.. 2016;300:306-316.
- [Google Scholar]
- Mass spectrometry study of lignocellulosic biomass combustion and pyrolysis with NOx removal. Renew. Energy. 2020;146:484-496.
- [Google Scholar]
- Upcycling brewer's spent grain waste into activated carbon and carbon nanotubes for energy and other applications via two-stage activation. J. Chem. Technol. Biotechnol.. 2020;95(1):183-195.
- [Google Scholar]
- A review on removal of uranium(VI) ions using titanium dioxide-based sorbents. J. Mol. Liq.. 2019;293:111563
- [Google Scholar]







