Translate this page into:
Facile fabrication of chitosan-cl-poly(AA)/ZrPO4 nanocomposite for remediation of rhodamine B and antimicrobial activity
⁎Corresponding author at: College of Materials Science and Engineering, Shenzhen Key Laboratory of Polymer Science and Technology, Guangdong Research Center for Interfacial Engineering of Functional Materials, Nanshan District Key Lab for Biopolymers and Safety Evaluation, Shenzhen University, Shenzhen 518055, PR China. gaurav8777@gmail.com (Gaurav Sharma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
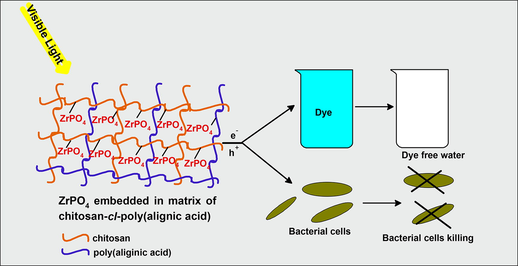
Abstract
The chitosan based nanohydrogel reinforced nanocomposite was fabricated by facile sol–gel polymerization technique. A chitosan-cl-poly(AA)/ZrPO4 nanocomposite was discovered as a capable photocatalyst for the elimination of rhodamine dye. The designed chitosan-cl-poly(AA)/ZrPO4 nanocomposite was examined by various characterization approaches such as Scanning electron microscopy (SEM), X-Ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and Transmission electron microscopy (TEM). The photocatalytic experiment outcomes revealed that the nanocomposite had good remediation potential as 82% of rhodamine B dye was remediated within 3 h of process. The antimicrobial behavior of nanocomposite was studied against Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus bacteria. Cell toxicity of the synthesized chitosan-cl-poly(AA)/ZrPO4 nanocomposite was performed against Vibrio fischeri as model organism. The chitosan-cl-poly(AA)/ZrPO4 nanocomposite demonstrated the potential antibacterial behavior.
Keywords
Nanocomposite
Zirconium Phosphate
Chitosan
Photocatalysis
Antimicrobial
1 Introduction
Owing to extraordinary progress in urbanization and industrialization, the environmental contamination has been a great fear in present context of times. Pollution is the entrance of pollutes into the natural atmosphere that causes hostile change. Huge amount of carbon-based and inorganic contaminants has been introduced within water bodies from domestic and industrial sources (Ghorai et al., 2014; Hosseini Asl et al., 2019). Constituents of contaminates, can be either foreign substances or naturally happening pollutants. The carbon-based pollutants such as insecticides, phenols and colorants etc. causes various stern threats to the atmosphere and human well-being (Motshekga et al., 2015; Sharma et al., 2017; Zhang and Wang, 2010). The above contaminants have been released from the different industries as fabric, paper & pulp, medical, paint, bleaching and tannery etc. To unravel this problem, manufacturing industries waters must be treated to confiscate the noxious pollutants previously they are released into the water sources. Several chemical and physical procedures have been established and are active for environmental remediation such as coagulation, adsorption, reverse osmosis, extraction, chemical precipitation, ion exchange, membrane filtration, and photocatalysis etc. (Lei et al., 2019; Naushad, 2014; Sharma et al., 2015). The tenacious carbon-based pollutants have stern health threats and essential be detached before receiving into water, air and soil. Development of these carbon-based pollutants released from manufacturing effluents over time period in the bodies of living beings can root serious impairment as these cannot be remediated biologically. The existence of contaminants above acceptable quantities in environment can be reasons for health issues as abdominal pain, liver impairment, breathing problems, gastrointestinal bleeding, kidney disfunction and even cancer in organisms, etc. (Al-Ghouti et al., 2003). Photocatalysis is one of the existing procedures active for the exclusion of pollutants from environment, and still has its enormous significance in the coming eras (Cheng et al., 2016). With the stable evolutions of varied photocatalyst materials, the arena of photocatalysis has become wider and explicit in nature for precise pollutants encompassing antibiotics, heavy metals, dyes, phenols and insecticides etc. The number of photocatalyst of higher photocatalytic competence and selectivity have been produced, e.g. metal oxides, bimetallic and trimetallic nanoparticles, ferrites, metal sulphides, nanocomposites and metal organic frameworks (MOFs) (Fresno et al., 2014; Sharma et al., 2018a; Sharma et al., 2019). Out of these the nanocomposites have shown extraordinary role in photocatalysis, and are widely explored for exclusion of carbon-based pollutants from wastewater due to their numerous advantages. Such as their organic counterpart provides the large surface for attachment of inorganic part hence increase its mechanical strength and also increase its adsorption capacity (Zhang et al., 2014). The various organic materials used in preparation of nanocomposites includes, graphene, carbon nitrides and biopolymers etc. The combination of nanoparticles with polymers and hydrogels is a new and quickly emerging class of materials, gaining extensive application in several areas, chiefly in pharmacy, agriculture and environmental remediation (Sharma et al., 2018c; Sui et al., 2019; Vanaamudan et al., 2018). Biopolymers are specially focused materials for fabrication of hydrogels, nanofibers, biopolymeric microspheres and nanospheres as these are easily available and biocompatible in nature. Crosslinked hydrophilic biopolymeric hydrogels matrix are among the most promising supports for synthesis of their nanocomposites with inorganic materials as their three-dimensional network provides suitable sites for attachment, and even their properties can be tailored by simple modifying the functionalities in their structure (Prusty and Swain, 2018).
Thus, nanohydrogels are used to improve the properties of pre synthesized inorganic particles (Naushad et al., 2019). Nature has unceasingly joined organic and inorganic constituents, on the nanoscale, to create smart materials with amazing functions and properties (mechanics, density, colour, hydrophobicity, permeability etc.). Nanocomposites hydrogels are composed of nanosized inorganic particles mixed with some organic matrix these have greater surface area and the particles at interface adopts key roles, as these explains their final properties. Currently, nanocomposites have virtuous future perceptions, where using these, exigent and novel applications are executed to accomplish enormous harmony among human actions and environment (Packirisamy et al., 2019; Rabel et al., 2019; Sharma et al., 2014; Singh et al., 2019).
The present work provides an insight into fabrication of zirconium phosphate embedded chitosan-based nanocomposite fabricated by simple sol–gel method. Thus nanocomposite was explored for its photocatalytic and antimicrobial action.
2 Materials and techniques
2.1 Chemicals and instruments
Zirconium oxychloride, orthophosphoric acid, acetic acid, ammonium per sulfate purchased from CDH Pvt. Ltd. India. Aliginic acid was attained from Loba Chemie Pvt. Ltd. India. N,N’-methylene bisacryl amide was purchased from S-D fine Chem. Limited, India.
Shaker, Centrifuge machine (Instruments, Chemical Private Limited), Fourier transform infrared (FTIR) spectrophotometer (Nicolet 5700 FTIR Spectrophotometer), X-Ray Diffractrometer (Philips 1830 diffractometer), UV–visible spectrometer (INCO Instrument and Chemical Private Limited), Transmission electron microscopy (Techni G2 20 S-Twin), Scanning electron microscopy (JSM 6100) were used here.
2.2 Synthesis of chitosan-cl-poly(AA)/ZrPO4 nanocomposite
The nanocomposite was fabricated by means of sol–gel/polymerization method. Inorganic counterpart was synthesized by mixing 0.05 M orthophosphoric acid dropwise into 0.1 M zirconium oxchloride solution with continuous stirring. The obtained sol of zirconium phosphate was reserved in mother liquor for about 24 h for complete digestion. Chitosan-cl-poly(AAc) was synthesized as discussed in our previous works (Sharma et al., 2017). The sol of zirconium phosphate was added into the prepared gel of chitosan-cl-poly(AAc). To the above blend, ammonium persulfate (initiator) and N,N’–methylene bisacyrlamide (crosslinker) were added. The subsequent blend was stirred for 6 h at 70 °C by means of magnetic stirrer. The obtained chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was collected with ethanol. Subsequently, the resulting material was washed numerous times using water–ethanol. Lastly, the nanocomposite was dried under inert conditions in nitrogen atmosphere at 50 °C.
2.3 Characterization
The Fourier Transform Infrared (FTIR) spectrum of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was studied using FTIR (Nicolet 5700 FTIR Spectrophotometer). For FTIR, chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was powdered and mixed with KBr to make pellets and spectrum was noted. X-ray diffraction pattern was logged using a Philips 1830 diffractometer. The surface topographies of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was considered using JSM 6100 scanning electron microscope. Sample was mounted rigidly on a specimen holder. The dimensional analysis was done using transmission electron microscopy (Techni G2 20 S-Twin). The nanocomposite was ultrasonicated for 2 h in ethanol and was loaded on carbon grid and examined under the TEM instrument below different resolutions. For determination of the optical band gap of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite a suspension of it was made in ethanol. The suspension was then ultrasonicated for 90 min. The UV–vis spectrum of suspension was attained by employing double-beam spectrophotometer and Tauc relation is used for calculating the direct band gap.
2.4 Rhodamine B photocatalytic degradation
The photocatalytic nature of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was tested for the degradation of rhodamine B dye. The photocatalytic tests were achieved using photoreactor surrounded with thermostatic water current to maintain a 25 °C ± 1 continual temperature. Chitosan-cl-poly(AAc)/ZrPO4 nanocomposite(100 mg) was inserted into 2 × 10−6 M solution of rhodamine B dye to make a slurry. Firstly, the slurry was set aside in dark to reach the adsorption–desorption steadiness and later uncovered to sunlight for further photocatalysis. Approximately 4 mL of the rhodamine B solution was taken out at numerous breaks of period and centrifuged to impound particles of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite. The absorbance was noted in the range of 300–750 nm and kinetics for the photocatalytic degradation of rhodamine B was inspected at 554 nm. Following above procedure, the reusability study was performed for five consecutive cycles. The percent degradation of rhodamine B was found as (Gupta et al., 2015; Rupa et al., 2007):
2.5 Antimicrobial activity and cell toxicity of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite
The antibacterial activity of the synthesized chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was analyzed against Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus as described previously(Travan et al., 2009). The nanocomposite preparations were sterilized at 121 °C for 20 min. The selected cultures were grown overnight at 37 °C under continuous shaking at 150 rpm. The colony forming unites were counted on the tested plates with added chitosan-cl-poly(AAc)/ZrPO4 nanocomposite and without chitosan-cl-poly(AAc)/ZrPO4 nanocomposite as control.
The cell toxicity of the synthesized chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was performed by using Microtox machine and the study was performed against Vibrio fischeri as model organism. The toxicity analysis was done by toxicity test protocol. chitosan-cl-poly(AAc)/ZrPO4 nano-composite (5.0 mg/mL) was suspended in double distilled water, to form a homogenous mixture and osmotic adjusting solution were added. The endpoint obtained by the Microtox assay, which notices the decrease in the light intensity emanated by the luminescent bacterium V. fischeri afterward the exposure for 15 and 30 min (Hira et al., 2018).
3 Result and discussion
3.1 Synthesis and characterization of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite
The chitosan-cl-poly(AAc)/ZrPO4 nanocomposite has been synthesized using sol–gel polymerization process in existence of crosslinker N,N’-methylene bis-acyrlamide. The reaction yield for synthesis of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was found to and 88%. The FTIR spectrum Fig. 1(a) shows the characteristics peaks in chitosan-cl-poly(AAc)/ZrPO4 nanocomposite. The peaks signals at 1114 cm−1, 1589 cm−1and 3390 cm−1are assigned to the C–O stretching, C = O stretching and O–H stretching vibrations for the chitosan molecules (Sharma et al., 2018b; Sharma et al., 2017). The representative peak at 1051 cm−1 is for ionic phosphate stretching Fig. 1(a). The peak that detected at about 2347 cm−1 is recognized to the manifestation of C–H bond. A broad band from 650 to 500 cm−1 at is due to the presence of metal oxide connection (Bhatt et al., 2019).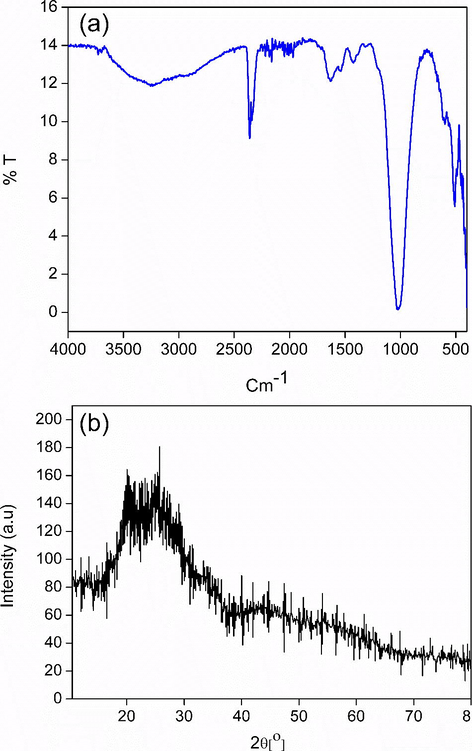
(a) FTIR spectrum (b) XRD pattern of chitosan-cl-poly(AA)/ZrPO4 nanocomposite.
X-ray diffraction pattern of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was revealed in Fig. 1(b). The peaks at 2θ = 11.5°, 19.8° and 24.7° and 28.7° represents the presence of zirconium phosphate with diffraction planes (0 0 2) (1 1 0) and (1 1 2) (Fu et al., 2018), whereas the peaks at 2θ = 31.8°, 33.5°, 38.2, 48.5, 54.1° and 59.2° reveal the presence of Zr-O linkage of zirconium phosphate with chitosan-cl-poly(AAc) counterpart conforming to diffraction planes of (1 1 1), (2 0 0), (2 2 0) and (3 1 1) correspondingly (JCPDS file no. 27–0997) (Kumar et al., 2016). The presence of low intensity small peaks overlapping around 2θ = 10° and 20° confirmed presence of chitosan-cl-poly(AAc)counterpart (Kumar and Koh, 2012). Therefore, the XRD results confirm the formation of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite. Surface micrographs of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was considered by means of scanning electron microscope. Fig. 2. (a, b, c and d) displays the SEM images of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite at different magnification. Pictures reveals that dur to presence of ZrPO4 the surface morphology of nanocomposite appears to be irregular with rough texture. Thus SEM reveals the presence of both components in chitosan-cl-poly(AAc)/ZrPO4 nanocomposite i.e., ZrPO4 and chitosan-cl-poly(AAc). TEM images of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite are shown in Fig. 3.(a and b) at diverse magnification. TEM pictures indicated appearance of ZrPO4 embedded in matrix of chitosan-cl-poly(AAc) with average size of particles between 30 and 70 nm. Fig. 4 depicts that the optical band gap of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite is 3.1 eV which lies in ultraviolet regions for semiconductors hence nanocomposite can be explored for its photocatalytic nature.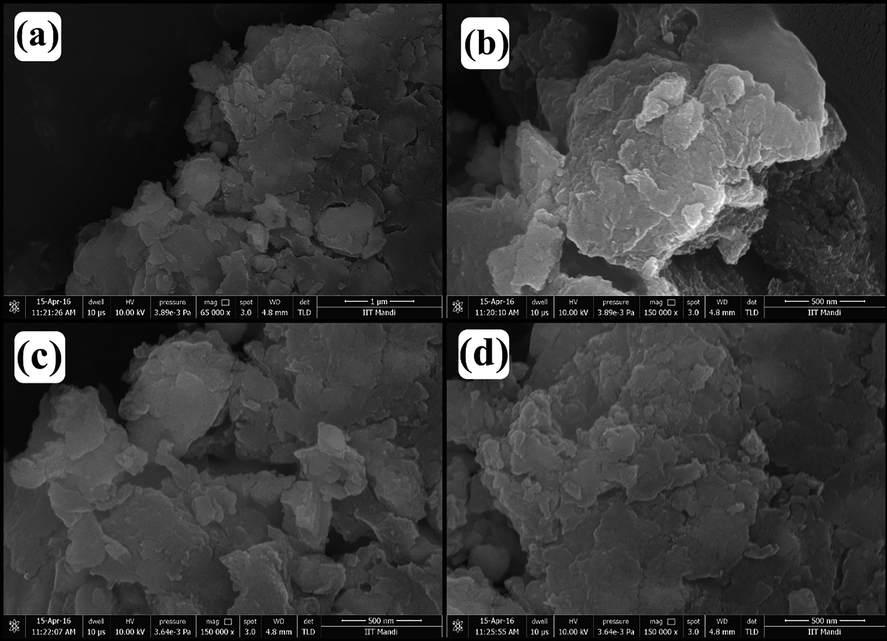
SEM images of chitosan-cl-poly(AA)/ZrPO4 nanocomposite.
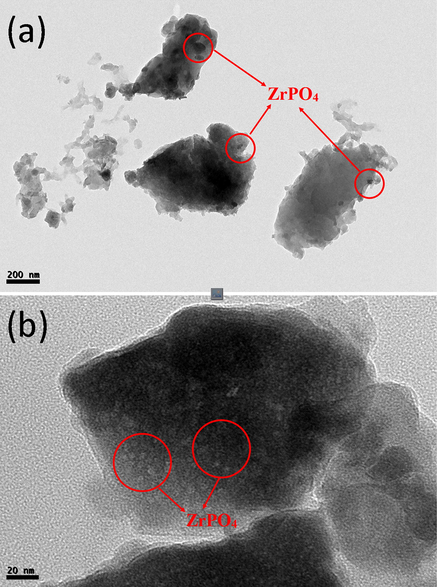
TEM images of chitosan-cl-poly(AA)/ZrPO4 nanocomposite.
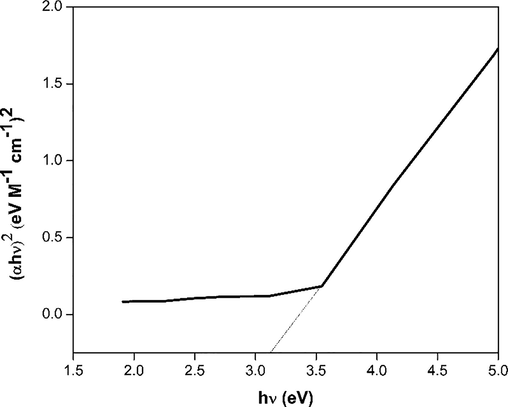
Band Gap of chitosan-cl-poly(AA)/ZrPO4 nanocomposite.
3.2 Photocatalytic degradation of rhodamine B
Photocatalytic degradation of the rhodamine B dye using chitosan-cl-poly(AAc) nanocomposite is shown in Fig. 5(a, b and c). Fig. 5(a) decapitates the change in absorbance with exposer period. The progression of photocatalytic activity befalls in two steps; adsorption–desorption equilibrium and photocatalysis. It was detected that maximum 20% of rhodamine B dye get adsorbed after one hour of contact period to achieve equilibrium and 62% of dye undergoes photocatalysis in 2 h of photoperiod and it was observed that about 82% of the rhodamine B dye was removed within 3 h of complete process Fig. 5 (b). The rate of photocatalytic degradation of rhodamine B dye was reported to follow pseudo first order kinetic model:
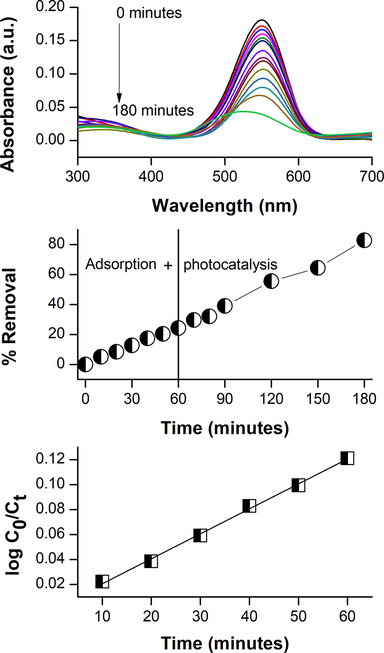
(a) Absorbance spectra for degradation of rhodamine B dye by chitosan-cl-poly(AAc)/ZrPO4 nanocpmposite (b) percent degradation of rhodamine B (c) kinetics for degradation process.
The removal efficiency of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite for dye was also examined by chemical oxygen demand (COD) investigation. The COD is an active procedure to quantify the concentration of organic matter existing in reaction. The COD examination allows to measure total amount of oxygen essential for the complete oxidation of organic content to carbon dioxide and water (Meng et al., 2019; Priya and Jeyanthi, 2019). Fig. 6(a) displays the decrease in COD of dye solution at several reaction intervals. The COD of reaction solution lowered to 39% in 3 h indicating noteworthy mineralization of dye. The reusability of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was investigated for consecutive 5 cycles and it was detected that after 5th cycle 61% dye was remediated (Fig. 6b). Thus, proving effectiveness of nanocomposite for multiple use.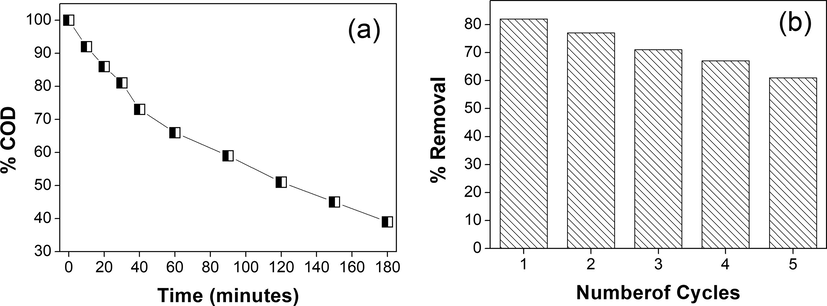
(a) COD for degradation of rhodamine B dye by chitosan-cl-poly(AAc)/ZrPO4 nanocpmposite (b) reusability.
3.3 Antimicrobial activity and cell toxicity and chitosan-cl-poly(AAc)/ZrPO4 nanocomposite
The antibacterial activities of the synthesized chitosan-cl-poly(AAc)/ZrPO4 nanocomposite was analyzed against Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus as described previously. The results exhibited that the antimicrobial activity of chitosan-cl-poly(AAc)/ZrPO4 is higher towards all tested microorganisms (Fig. 7). Whereas E.coli strain was found to be much more resistant than that of S. aureus and P. aeruginosa. In a previous report, the nanocomposite of cellulose and copper were tested for their antimicrobial action against Klebsiella pneumoniae and Staphylococcus aureus , which showed morphology and concentration of metal content affect the antibacterial activity (Pinto et al., 2013). In another study, a chitosan nanocomposite with Ag nanoparticles showed high antimicrobial action against S. aureus, P. aeruginosa, Candida albicans and E. coli. Thus, presence of chitosan and ZrPO4 nanoparticles make our nanocomposite as promising antibacterial agent.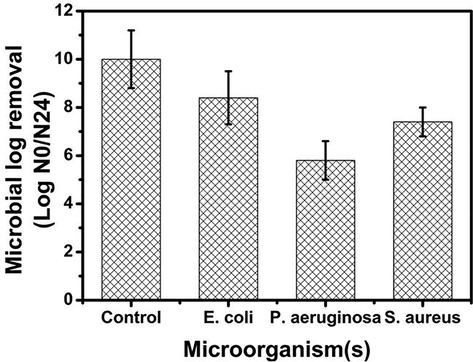
Antimicrobial activities of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite.
The cell toxicity of the synthesized chitosan-cl-poly(AAc)/ZrPO4 was performed by using 81.9% toxicity test. The acute toxicity(EC50) values of chitosan-cl-poly(AAc)/ZrPO4 nanocomposite were 257 and 121 µg/mL towards the V. fischeri after 15 and 30 min of incubation (Table 1). The significantly higher EC50 values for chitosan-cl-poly(AAc)/ZrPO4 detected herein demonstrates the more biocompatible nature of synthesized nanocomposite. Literature studies suggested that reactive oxygen species generated by nanoparticles are responsible for toxicity. Generally the toxicity of nanoparticles depends on amount of dose, size, and type of cell (Regiel-Futyra et al., 2017). Hence, in our study we found that our chitosan-cl-poly(AAc)/ZrPO4 nanocomposite is nontoxic and biocompatible in nature.
Type of particles
parameter
EC50 value (µg/mL)
chitosan-cl-poly(AA)/ZrPO4
EC50-15min
257 ± 0.32
EC50-30min
121 ± 0.41
4 Conclusion
Novel hydrogel-based nanocomposite was fabricated by simplistic sol–gel/polymerization method. The chitosan-cl-poly(AAc)/ZrPO4 was successfully used for remediation of rhodamine B dye from aqueous medium. The particle diameter lies between 30 and 70 nm for ZrPO4. Diverse characterization procedures verified the fabrication of the nanocomposites. The percent remediation for rhodamine B was found to be 82%. The chitosan-cl-poly(AAc)/ZrPO4 was tested for its antimicrobial activity against different bacteria’s. It was observed that chitosan-cl-poly(AAc)/ZrPO4 nanocomposite showed promising antibacterial nature. On the other hand, cytotoxicity studies reveled that nanocomposite was non-toxic and biocompatible. Thus, chitosan-cl-poly(AAc)/ZrPO4 nanocomposite with desirable properties is useful material for wastewater treatment.
Acknowledgment
One of the authors (Mu. Naushad) is grateful to the Researchers Supporting Project number (RSP-2019/8), King Saud University, Riyadh, Saudi Arabia for the support. The financial support from China Postdoctoral Science Foundation Grant (2018M643168) is greatly acknowledged.
References
- The removal of dyes from textile wastewater: a study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J. Environ. Manage.. 2003;69(3):229-238.
- [Google Scholar]
- Bhatt, R., V, A., Rathod, S.B., P, P., 2019. Self-assembled chitosan-zirconium phosphate nanostructures for adsorption of chromium and degradation of dyes. Carbohydrate Polymers 208, 441-450
- Rhodamine (B) photocatalysis under solar light on high crystalline ZnO films grown by home-made DC sputtering. Optik. 2018;174:77-85.
- [Google Scholar]
- Enhanced adsorption and photocatalysis capability of generally synthesized TiO2-carbon materials hybrids. Adv. Powder Technol.. 2016;27(5):1949-1962.
- [Google Scholar]
- Photocatalytic materials: recent achievements and near future trends. J. Mater. Chem. A. 2014;2(9):2863-2884.
- [Google Scholar]
- Two-dimensional cardanol-derived zirconium phosphate hybrid as flame retardant and smoke suppressant for epoxy resin. Polym. Degrad. Stab.. 2018;151:172-180.
- [Google Scholar]
- Synthesis and characterization of magnetic nanocomposite Fe3O4@TiO2/Ag, Cu and investigation of photocatalytic activity by degradation of rhodamine B (RhB) under visible light irradiation. Optik. 2019;179:646-653.
- [Google Scholar]
- Enhanced Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solution Using a Nanocomposite of Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica. ACS Appl. Mater. Interfaces. 2014;6(7):4766-4777.
- [Google Scholar]
- A cellulose acetate based nanocomposite for photocatalytic degradation of methylene blue dye under solar light. Ionics. 2015;21(6):1787-1793.
- [Google Scholar]
- Pectin-guar gum-zinc oxide nanocomposite enhances human lymphocytes cytotoxicity towards lung and breast carcinomas. Mater. Sci. Eng., C. 2018;90:494-503.
- [Google Scholar]
- Porous adsorbents derived from coal fly ash as cost-effective and environmentally-friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: A review. J. Cleaner Prod.. 2019;208:1131-1147.
- [Google Scholar]
- Magnetically recoverable ZrO2/Fe3O4/chitosan nanomaterials for enhanced sunlight driven photoreduction of carcinogenic Cr(vi) and dechlorination & mineralization of 4-chlorophenol from simulated waste water. RSC Adv.. 2016;6(16):13251-13263.
- [Google Scholar]
- Physiochemical, Optical and Biological Activity of Chitosan-Chromone Derivative for Biomedical Applications. Int. J. Mol. Sci.. 2012;13(5)
- [Google Scholar]
- Synergetic photocatalysis/piezocatalysis of bismuth oxybromide for degradation of organic pollutants. J. Alloy. Compd.. 2019;809:151840
- [Google Scholar]
- Degradation of methylene blue by photocatalysis of copper assisted ZnS nanoparticle thin films. Optik. 2017;130:1168-1172.
- [Google Scholar]
- Efficient COD reduction from sulfide minerals processing wastewater using Fenton process. Miner. Eng.. 2019;132:110-112.
- [Google Scholar]
- Preparation and antibacterial activity of chitosan-based nanocomposites containing bentonite-supported silver and zinc oxide nanoparticles for water disinfection. Appl. Clay Sci.. 2015;114:330-339.
- [Google Scholar]
- Surfactant assisted nano-composite cation exchanger: development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem. Eng. J.. 2014;235:100-108.
- [Google Scholar]
- Photodegradation of toxic dye using Gum Arabic-crosslinked-poly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Cleaner Prod.. 2019;241:118263
- [Google Scholar]
- Synthesis of novel Sn1-xZnxO-chitosan nanocomposites: structural, morphological and luminescence properties and investigation of antibacterial properties. Int. J. Biol. Macromol.. 2019;138:546-555.
- [Google Scholar]
- Antibacterial Activity of Nanocomposites of Copper and Cellulose. Biomed Res. Int.. 2013;2013:6.
- [Google Scholar]
- Removal of COD, oil and grease from automobile wash water effluent using electrocoagulation technique. Microchem. J.. 2019;150:104070
- [Google Scholar]
- Nano silver decorated polyacrylamide/dextran nanohydrogels hybrid composites for drug delivery applications. Mater. Sci. Eng., C. 2018;85:130-141.
- [Google Scholar]
- A green chemistry to produce iron oxide – Chitosan nanocomposite (CS-IONC) for the upgraded bio-restorative and pharmacotherapeutic activities - Supra molecular nanoformulation against drug-resistant pathogens and malignant growth. Int. J. Biol. Macromol.. 2019;138:1109-1129.
- [Google Scholar]
- Development of noncytotoxic silver–chitosan nanocomposites for efficient control of biofilm forming microbes. RSC Adv.. 2017;7(83):52398-52413.
- [Google Scholar]
- Effect of deposition of Ag on TiO2 nanoparticles on the photodegradation of Reactive Yellow-17. J. Hazard. Mater.. 2007;147(3):906-913.
- [Google Scholar]
- Fabrication and characterization of trimetallic nano-photocatalyst for remediation of ampicillin antibiotic. J. Mol. Liq.. 2018;260:342-350.
- [Google Scholar]
- Guar gum-crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Int. J. Biol. Macromol.. 2018;114:295-305.
- [Google Scholar]
- Novel development of nanoparticles to bimetallic nanoparticles and their composites: a review. J. King Saud Univ. – Sci.. 2019;31(2):257-269.
- [Google Scholar]
- Sharma, G., Naushad, M., Al-Muhtaseb, A.a.H., Kumar, A., Khan, M.R., Kalia, S., Shweta, Bala, M., Sharma, A., 2017. Fabrication and characterization of chitosan-crosslinked-poly(alginic acid) nanohydrogel for adsorptive removal of Cr(VI) metal ion from aqueous medium. Int. J. Biol. Macromolecules 95, 484-493
- Modification of Hibiscus cannabinus fiber by graft copolymerization: application for dye removal. Desalin. Water Treat.. 2015;54(11):3114-3121.
- [Google Scholar]
- Fabrication, characterization and antimicrobial activity of polyaniline Th(IV) tungstomolybdophosphate nanocomposite material: Efficient removal of toxic metal ions from water. Chem. Eng. J.. 2014;251:413-421.
- [Google Scholar]
- Sharma, G., Sharma, S., Kumar, A., Al-Muhtaseb, A.a.H., Naushad, M., Ghfar, A.A., Mola, G.T., Stadler, F.J., 2018c. Guar gum and its composites as potential materials for diverse applications: a review. Carbohydrate Polymers 199, 534-545
- Graphene and carbon nanotube reinforced epoxy nanocomposites: a review. Polymer. 2019;180:121724
- [Google Scholar]
- Non-cytotoxic Silver Nanoparticle-Polysaccharide Nanocomposites with Antimicrobial Activity. Biomacromolecules. 2009;10(6):1429-1435.
- [Google Scholar]
- Chitosan-Guar gum blend silver nanoparticle bionanocomposite with potential for catalytic degradation of dyes and catalytic reduction of nitrophenol. J. Mol. Liq.. 2018;271:202-208.
- [Google Scholar]
- Adsorption of Pb(II) from Aqueous Solution by Chitosan-g-poly(acrylic acid)/Attapulgite/Sodium Humate Composite Hydrogels. J. Chem. Eng. Data. 2010;55(7):2379-2384.
- [Google Scholar]
- Magnetically separable attapulgite-TiO2-FexOy composites with superior activity towards photodegradation of methyl orange under visible light radiation. J. Ind. Eng. Chem.. 2014;20(5):3884-3889.
- [Google Scholar]







