Translate this page into:
Fabrication of fluorescent carbon nanodots from laboratory paper waste for Fe3+ ions detection
⁎Corresponding author. alshatwi@ksu.edu.sa (Ali A. Alshatwi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, we fabricated non-toxic, highly stable, eco-friendly carbon nanodots (CD) from laboratory paper waste through a facile synthetic approach. The paper waste was converted to carbon by thermal decomposition. The carbon powder obtained was dispersed in acid using a sonication process. Subsequently, the CD was separated by centrifugation and filtration. The morphological properties of the prepared CD were analyzed using atomic force microscopy (AFM). The optical behavior of the CD was assessed using a UV–vis and fluorescence spectrophotometer. The AFM results clearly indicated that the CD had a spherical shape and sizes of ∼3–10 nm. The CD exhibited excitation-dependent photoluminescence. Finally, we assessed the metal ion selectivity of the paper waste derived CD, and utilized it as a fluorescent probe to detect Fe3+ ions. This CD fabrication approach is sustainable, cheap, and uses a renewable precursor. It also may enable the valorization of laboratory paper waste to high-value products.
Keywords
Carbon nanodots
Atomic force microscopy
Fluorescent material
Fe3+ ions
1 Introduction
Globally, waste generation has increased exponentially in every year. The generated waste is discarded into water resources or landfilled, which can create environmental problems. Additionally, waste management has become a serious problem recently (Bi et al., 2014). Several countries have attempted to recycle the waste, but the recycling process has several disadvantages such as being expensive and generating hazardous by-products (Zhu et al., 2016). Every year the solid waste generation has been increased due to rapid industrialization, urbanization and increased population rate. The solid wastes are collected and discarded in dumpsites or landfills (Nizami et al., 2015). Reuse, recycling and energy harvesting is still at an initial stage. Also, the old practice for land filling system or dumping of solid waste that may cause several environmental issues (Anjum et al., 2016). According to Ouda et al., number of dumping sites are reach their capacities with in few years (Ouda et al., 2013). Hence, there is necessary to exploiting the solid waste in useful way. In recent years, a few studies have reported that waste materials can be valorized by producing high-value products using waste materials as the precursor. For instance, Bi et al. (2014) reported the production of carbon microbelt aerogel from waste paper. Athinarayanan et al. (2018a) demonstrated that cellulose nanostructures could be fabricated using Bassia eriophora waste. Interestingly, carbon nanodots, cellulose nanostructures, and lignin nanoparticles have been synthesized from lignocellulosic biomass of Phoenix dactylifera using a facile method (Athinarayanan et al., 2019). Additionally, Periasamy et al. (2018) studied biocompatible silica phytoliths extracted from sorghum waste for three-dimensional culture of human mesenchymal stem cells. Alshatwi et al. (2015) suggested that high-value biogenic silica nanostructures could be derived from rice husk waste. Fascinatingly, antioxidative and biocompatible lignin has been isolated from Borassus flabellifer biomass (Athinarayanan et al., 2018b). Our earlier studies, we have demonstrated that cellulose nanofibrils fabrication from palmyra palm biomass (Athinarayanan et al., 2020a). Also, we have fabricated carbon nanodots and hydroxyapatite from fish scale using integrated process (Athinarayanan et al., 2020b). Moreover, some studies have produced carbon nanostructures and activated carbon from various agricultural residues for valorization.
Carbon nanodots (CDs) is an emergent nanostructure due to their unique properties including high photo stability, biocompatibility, strong fluorescence and eco-friendly (Athinarayanan et al., 2020b; Athinarayanan et al., 2020c; Yang et al., 2017; Liu et al., 2018). These activities have been utilized for different potential applications such as optical sensing, bio-imaging, photovoltaic and photo-catalysis (Athinarayanan et al., 2020b; Yang et al., 2017; Liu et al., 2018; Rani et al., 2020). CDs have, when compared with metal and semi metal-based quantum dots (Liu et al., 2018). Recently, several studies demonstrated that naturally available solid waste is exploited as a precursor for CDs fabrication. For instance, the natural solid wastes including fish scale (Athinarayanan et al., 2020b), palmyra palm leaf (Athinarayanan et al., 2020c), Phoenix dactylifera biomass (Athinarayanan et al., 2019), hair (Guo et al., 2016), walnut shells (Cheng et al., 2017), cranberry bean (Zulfajri et al., 2019) and sugarcane bagasse pulp (Thambiraj and Shankaran, 2016) were utilized as carbon source for CDs fabrication. Interestingly, naturally occurring solid waste derived CDs have been utilized as fluorescent probe for metal ions detection. In this regards, some studies developed CDs based Fe3+ ions detection. Volvariella volvacea mushroom derived CDs was exploited for Fe3+ ions detection, whereas limits of detection of Fe3+ ions is16 nM (Zulfajri et al., 2020). Also, Senol and Bozkurt demonstrated that seville orange derived CDs have potential to detect Fe3+ ions and its limit of detection is 0.53 µM (Senol and Bozkurt, 2020).
Currently, laboratories are generating large quantities of wastes, including solvents, tissue paper, and solvent containers. However, these wastes are not being utilized properly but are often being dumped into open sites and discarded directly into the trash (Braghiroli et al., 2018). Furthermore, packing industries, stationary-consuming sectors, homes, hotels, and cosmetics industries are generating large quantities of tissue paper waste (Masternak-Janus and Rybaczewska-Błażejowska, 2015). However, only a few studies have utilized different kinds of paper wastes to produce high-value products, including activated carbon, graphene oxide quantum dots, carbon aerogel, and carbon nanodots (Bi et al., 2014; Wei et al., 2014; Adolfsson et al., 2015). The laboratory paper waste is mainly composed by cellulose and small quantity of lab reagents that cellulose can act as carbon source for different carbon based nanostructures. The paper waste not using appropriately. Thus, it is necessary to find an alternative method of proper utilization of laboratory paper waste. With this impetus, in the present investigation, we utilized laboratory paper waste as a precursor to fabricate a high-value fluorescent probe via a facile method.
2 Materials and methods
2.1 Materials
Waste tissue paper was collected from our laboratory. Silver nitrate, calcium chloride, cadmium chloride, cobalt chloride, copper sulfate, iron (III) chloride, iron (II) chloride, magnesium chloride, molybdenum (III) chloride, trisodium phosphate, sodium dihydrogen phosphate, and zinc chloride were obtained from Nice Chemicals (Mumbai, India). The obtained chemicals are analytical grade and used without further purification.
2.2 Fabrication of carbon nanodots
Carbon nanodots (CD) were fabricated using laboratory paper waste as the precursor. Briefly, the paper waste was thermal decomposed at 600 °C for 1 h under absence of air in muffle furnace (ThermoScientific, USA). The obtained carbon powder was mixed with sulfuric acid and ultrasonicated for 60 min at 750 W and 20 KHz using a probe sonicator (VCX 750, Sonics, USA). Then, the mixture was centrifuged (Avanti J-26 XPI, Beckman Coulter, USA) at 15000 rpm for 15 min to remove large carbon particles. Subsequently, the supernatant was collected and filtered using a sterile and polyethersulfone with 0.22 μm pore size syringe filter (TPP, Switzerland). The obtained liquid fraction was purified using dialysis process. Finally, the obtained CD were dried and used for subsequent studies.
2.3 Characterization
The optical properties of the prepared CD were studied using UV–Vis-NIR spectroscopy (Cary 5000, Agilent, Saudi Arabia) and fluorescence spectroscopy. The morphology of the CD was examined using atomic force microscopy. X-ray powder diffraction (XRD) data were obtained using an X-ray diffractometer (Bruker, AXS) with Cu-Kα radiation (0.15406 nm). The thermal behavior of the CD was assessed by thermogravimetric analysis (TGA Q500, TA instruments).
2.4 Metal ion detection
The synthesized CD (25 µg/mL) solution was used for metal ion selectivity analysis. Ag+, Ca2+, Mg2+, Zn2+, Fe2+, Cu2+, Co2+, Mo2+, Cd2+, and Fe3+ ion solutions (250 µM in 100 µL) were mixed with 100 µL of CD in 96 well plates. The fluorescence intensity of each plate was read using a Promega GloMax multimode microplate reader using different filters (UV: Ex 365 nm, Em 410–460 nm; Blue: Ex 490 nm, Em 510–570 nm; Green: Ex 525 nm, Em 580–640 nm; Red: Ex 625 nm, Em 660–720 nm). Additionally, different concentrations of Fe3+ ion solution (5–250 µM in 100 µL) were added to 96 well plates. Then, 100 µL of CD (25 µg/mL) per well was added to the different concentrations of Fe3+. Subsequently, the fluorescent intensity of the 96 well plates was measured by Promega GloMax multimode microplate reader using a blue filter (Ex 490 nm, Em 510–570 nm). The fluorescence intensity was measured three times.
3 Result and discussion
Large quantities of laboratory paper waste are generated each year and thrown away without any proper usage. The laboratory paper waste is mainly composed by cellulose and small quantity of reagents. Thus, the paper waste can be act as carbon precursor for carbon based nanostructures fabrication. In this study, we attempted to valorize the laboratory paper waste by fabricating carbon nanomaterials. Fig. 1 shows the schematic process of the CD fabrication from laboratory paper waste. The tissue paper waste is a cellulosic material that carbonizes at 600 °C and absence of air in muffle furnace. The carbonized material obtained in this study contained carbon nanostructures and large size carbon particles. The black powder was dispersed in sulfuric acid using an ultrasonication process for 60 min. During the sonication process, agglomerated carbon nanostructures were dispersed well in aqueous medium. Following the centrifugation process, the large-size carbon structures were removed. After that, the supernatant was separated carefully. The supernatant was filtered using a syringe filter (0.22 μm), and dialyzed. The obtained CD was kept under visible and 365-nm UV light (Fig. 2). The CD exhibited brown color in visible light and emitted blue color in UV light because of its fluorescence. Fig. 3 depicts the UV–vis absorption spectra of the prepared CD. The CD exhibits an optical absorption peak at 285 nm, and the results revealed the π–π* transition of aromatic units or non-bonding electrons (Al-Hadi et al., 2016). The characteristic behavior of the CD was size-based photoluminescent emission. Thus, we assessed the detailed photoluminescent behavior of the synthesized CD. Fig. 4 shows the photoluminescent behavior of the CD at various excitations. The emission peak is red-shifted as the wavelength of excitation increases through the range of 370 nm to 520 nm due to the different sizes of CD and the presence of different emissive trap sites on the CD. The CDs photoluminescent (PL) property mechanisms continuously under discussion. Gan et al. demonstrated that CDs probable photoluminescent mechanism involving excitation-independent and excitation-dependent PL (Gan et al., 2016). The surface traps, quantum confinement effect, synergistic models and electronegativity of heteroatoms were proposed for the carbon nanomaterials including the CDs, showing excitation-dependent PL (Gan et al., 2016; Dager et al., 2019). Recently, some studies demonstrated that photoluminescent behavior was coined from CDs fluorophores and intrinsic structure, with slight or no graphitic formation (Gan et al., 2016; Dager et al., 2019; Liu et al., 2016). Though, the probabilities of fabricated CDs have fluorophores are insignificant. There are two possibilities related to fluorophores presence; fluorophores are attached on the CDs surface or present individually in the CDs (Dager et al., 2019). Fig. 5 represents the XRD spectra of paper waste derived CDs. The XRD pattern exhibited a broad peak at 22° of 2 theta value, which corresponded to (0 0 2) plane of graphitic carbon. Additionally, the XRD spectra of CDs shows the broadened peak owing to small size of the CDs formation. The results are exactly matched with earlier studies (Athinarayanan et al., 2020c). The topographical pictures were used for determining the roughness and size of the fabricated CDs (Athinarayanan et al., 2020b; Al-Hadi et al., 2017). The three-dimensional morphology of the synthesized CD was analyzed using atomic force microscopy (AFM), and images of the CD are shown in Fig. 6. The AFM images clearly indicate that the CDs are very tiny and spherical in shape. It was found to be in the range of 3–10 nm.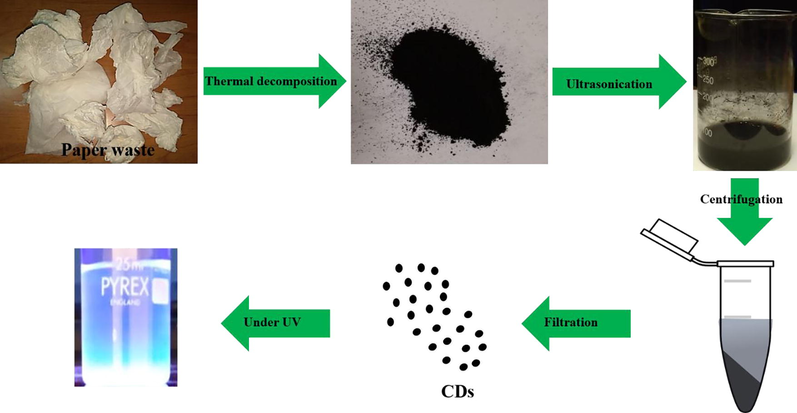
Schematic diagram of carbon nanodots fabrication using thermal decomposition approach.
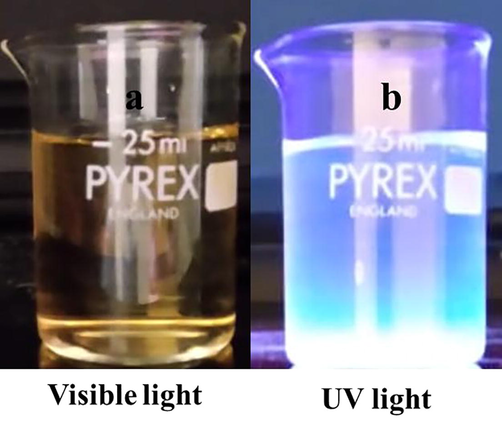
Laboratory paper waste carbon nanodots kept under (a) visible and (b) UV light at 365 nm.
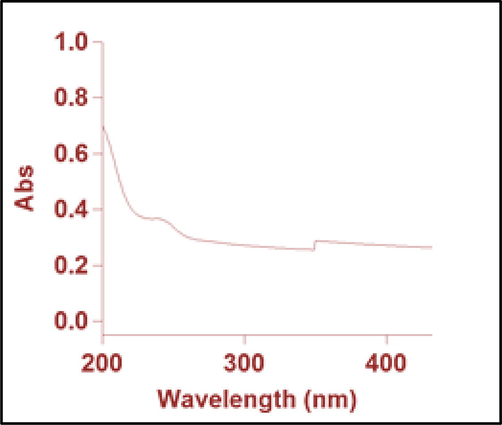
UV–Vis absorption spectra of carbon nanodots derived from laboratory paper waste.
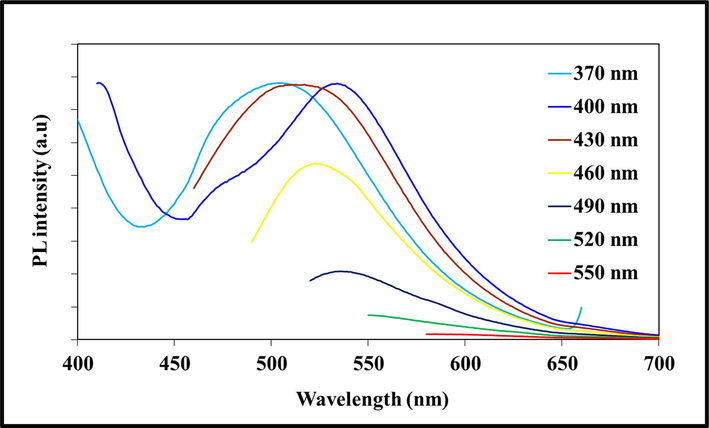
Photoluminescence emission spectra of carbon nanodots at various excitation wavelengths.
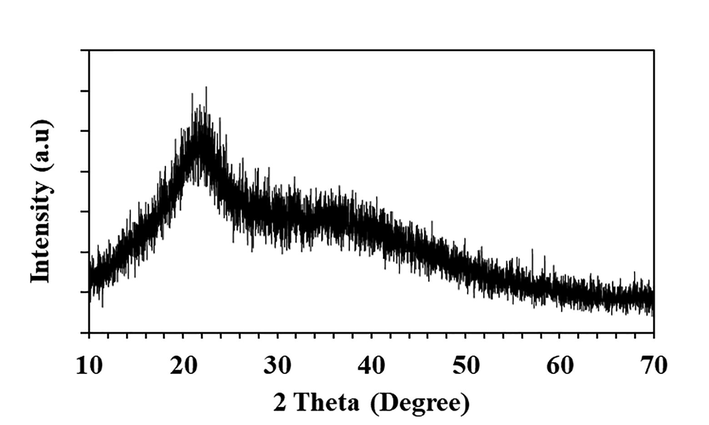
XRD pattern of paper waste derived carbon nanodots.
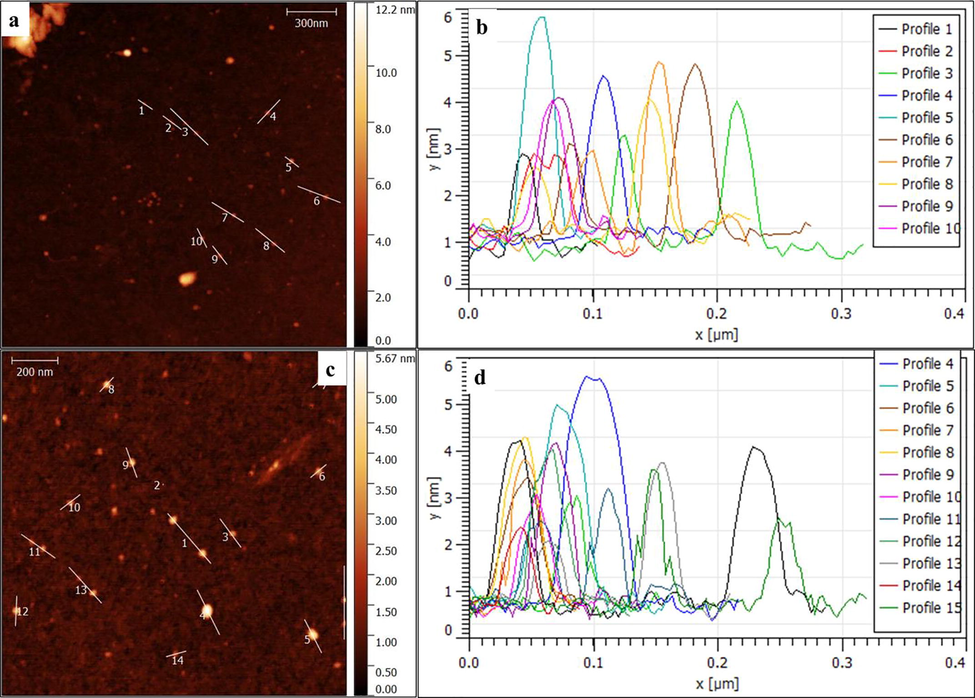
(a & c) Atomic force microscopic images of carbon nanodots and (b & d) profile height distribution.
Previously several studies reported that CDs have been utilized for Fe3+ ions detection. For instance, Zulfajri et al. demonstrated that CDs derived from mushroom was used as fluorescent probe for Fe3+ ions detection, while detection limit is 16 nM (Zulfajri et al.2020). Similarly, Senol and Bozkurt reported that orange derived CDs used to detect the Fe3+ ions with detection limit is 0.53 µM (Senol and Bozkurt, 2020). Selectivity plays an important role in metal ions detection. The paper waste derived CDs fluorescence quenching behavior have been evaluated using different metal ions. The influence of different metal ions on the florescence of the CD is shown in Fig. 7. The CD fluorescence intensity was not affected significantly by Ag2+, Cd2+, Zn2+, Mo2+, Mg2+, or Co3+ ions, but it changed in the presence of Fe3+, Fe2+, Cu2+, and Ca2+ that may be possible electron transfer or intramolecular charge transfer mechanism (Huang et al., 2017). In particular, a remarkable effect was observed in the presence of Fe3+ ions, which could arise from the interaction between the functional groups on the surface of the CD and Fe3+ (Huang et al., 2017). As similar, Qi et al. reported that N-doped CDs fluorescence is quenched by Fe3+ ions due to an extraordinary interaction between N-CDs surface phenolic hydroxyl groups and Fe3+ ions (Qi et al., 2019). In addition, N-CDs fluorescence quenching in Fe3+ ion solution may give to nonradiative electron transfer, wherein limited electrons were shifted to Fe3+ ions d-like states (Qi et al., 2019). To evaluate the quantitative Fe3+ ions detection, paper waste derived CDs solution mixed with different concentrations of Fe3+ ions (5–250 μM). After that, we measured the fluorescence intensity of the CD in the presence of different concentrations of Fe3+ ions. The fluorescence intensity was gradually decreasing with increasing the Fe3+ ions concentration (Fig. 8). These result suggested that fluorescence sensor is exactly sensitive with different concentration of Fe3+ ions. Also, the Fe3+ ions can efficiently and simply interact with paper waste derived CDs. Earlier studies demonstrated that CDs derived from various resources was exploited as fluorescent probe for Fe3+ ions detection (Table 1). In Fig. 8, the fluorescence intensity of different concentration of Fe3+ ions (5–250 µM) shows excellent linearity with the correlation coefficient of R2 = 0.9904. Thus, the CD derived from the paper waste could be applied as a sensing platform for detection of Fe3+ ions. Our preliminary studies result clearly indicate that paper waste derived CDs could be suitable to detect the Fe3+ ions in real samples.
Fluorescence quenching response of laboratory paper waste derived carbon nanodots in different metal ions.
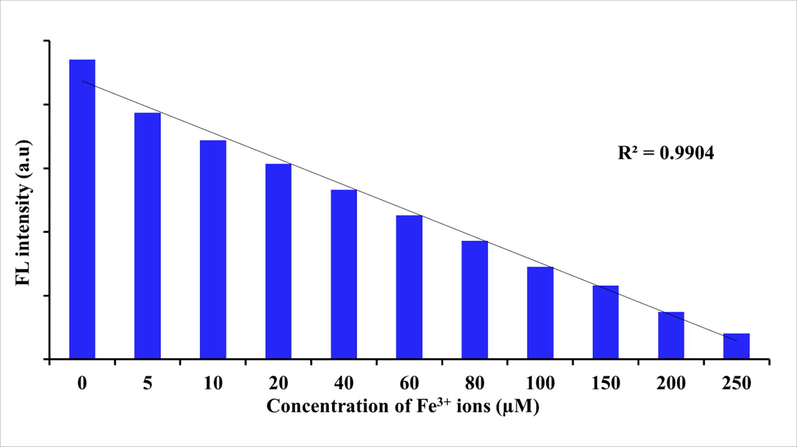
Influence of different concentration of Fe3+ ions on the fluorescence intensity of CDs at the excited wavelength 490 nm.
S.No
Precursor
linear range (μM)
Reference
1
Seville orange
2–8
Senol and Bozkurt, 2020
2
Mushroom
1–100
Zulfajri et al., 2020
3
blueberry
12.5–100
Aslandaş et al., 2015
4
cranberry beans
30–600
Zulfajri et al., 2019
5
Laboratory paper waste
5–250
This study
4 Conclusion
We are currently fabricating carbon nanodots by a facile and low-cost method using laboratory paper waste as the precursor. The paper waste is naturally bio-degradable and is an important source for eco-friendly fabrication of carbon nanostructures. The laboratory paper waste is used to prepare novel fluorescent probe carbon nanodots applicable for organic electronics. The non-toxic and biocompatible nature of the CD makes it highly appropriate for biological applications including bio-imaging, drug delivery, and bio-sensing. Additionally, the CD metal ion selectivity indicated that it could be useful for the detection of Fe3+ ions. This innovative material of the carbon nanostructures group has an optimistic future due to its distinctive nature.
Acknowledgments
We gratefully acknowledge the financial support of the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia (Grant No: RSP-2020/178). We acknowledge the Researchers Support & Services Unit, Deanship of Scientific Research, King Saud University, Saudi Arabia for their English editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and characterization of mono-disperse carbon quantum dots from fennel seeds: photoluminescence analysis using machine learning. Sci. Rep.. 2019;9(1):1-12.
- [Google Scholar]
- Life cycle analysis of tissue paper manufacturing from virgin pulp or recycled waste paper. Manage. Prod. Eng. Rev.. 2015;6:47-54.
- [Google Scholar]
- An argument for developing waste-to-energy technologies in Saudi Arabia. Chem. Eng. Trans.. 2015;45:337-342.
- [Google Scholar]
- Biocompatibility assessment of rice husk-derived biogenic silica nanoparticles for biomedical applications. Mater. Sci. Eng., C. 2015;47:8-16.
- [Google Scholar]
- The presence of carbon nanostructures in bakery products induces metabolic stress in human mesenchymal stem cells through CYP1A and p53 gene expression. Environ. Toxicol. Pharmacol.. 2016;41:103-112.
- [Google Scholar]
- Extraction of ultrafine carbon nanoparticles from samooli Bread and evaluation of their in vitro cytotoxicity in human mesenchymal stem cells. Process Biochem.. 2017;52:250-258.
- [Google Scholar]
- Facile green and one-pot synthesis of seville orange derived carbon dots as a fluorescent sensor for Fe3+ ions. Microchem. J.. 2020;159:105357.
- [CrossRef] [Google Scholar]
- Liquid nitrogen-assisted synthesis of fluorescent carbon dots from Blueberry and their performance in Fe3+ detection. Appl. Surf. Sci.. 2015;356:747-752.
- [Google Scholar]
- Carbon quantum dots from carbonized walnut shells: structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater. Sci. Eng., C. 2017;79:473-480.
- [Google Scholar]
- Production, characterization, and potential of activated biochar as adsorbent for phenolic compounds from leachates in a lumber industry site. Environ. Sci. Pollut. Res.. 2018;25:26562-26575.
- [Google Scholar]
- Photoluminescent carbon dots derived from sugarcane molasses: synthesis, properties, and applications. RSC Adv.. 2017;7:47840-47847.
- [Google Scholar]
- Carbon microbelt aerogel prepared by waste paper: an efficient and recyclable sorbent for oils and organic solvents. Small. 2014;10:3544-3550.
- [Google Scholar]
- Biomass-derived nitrogen-doped carbon quantum dots: highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci.. 2019;539:332-341.
- [Google Scholar]
- Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev.. 2016;116:9305-9374.
- [Google Scholar]
- Biocompatibility analysis of Borassus flabellifer biomass-derived nanofibrillated cellulose. Carbohydr. Polym.. 2020;235:115961.
- [Google Scholar]
- Phoenix dactylifera lignocellulosic biomass as precursor for nanostructure fabrication using integrated process. Int. J. Biol. Macromol.. 2019;134:1179-1186.
- [Google Scholar]
- Simultaneous fabrication of carbon nanodots and hydroxyapatite nanoparticles from fish scale for biomedical applications. Mater. Sci. Eng., C. 2020;117:111313.
- [CrossRef] [Google Scholar]
- Synthesis and cytocompatibility analysis of carbon nanodots derived from palmyra palm leaf for multicolor imaging applications. Sustainable Chem. Pharm.. 2020;18:100334.
- [CrossRef] [Google Scholar]
- Fabrication and cytotoxicity assessment of cellulose nanofibrils using Bassia eriophora biomass. Int. J. Biol. Macromol.. 2018;117:911-918.
- [Google Scholar]
- Borassus flabellifer biomass lignin: Isolation and characterization of its antioxidant and cytotoxic properties. Sustainable Chem. Pharm.. 2018;10:89-96.
- [Google Scholar]
- One-step hydrothermal synthesis of nitrogen-doped conjugated carbonized polymer dots with 31% efficient red emission for in vivo imaging. Small. 2018;14(15):1703919.
- [CrossRef] [Google Scholar]
- Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. New J. Chem.. 2014;38(3):906.
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis and photoluminescent mechanistic investigation of highly fluorescent nitrogen doped carbon dots from amino acids. Mater. Res. Bull.. 2017;89:26-32.
- [Google Scholar]
- Valorization of cellulose and waste paper to graphene oxide quantum dots. RSC Adv.. 2015;5(34):26550-26558.
- [Google Scholar]
- Cranberry beans derived carbon dots as a potential fluorescence sensor for selective detection of Fe3+ ions in aqueous solution. ACS Omega. 2019;4(13):15382-15392.
- [Google Scholar]
- Utilization of carbon dots derived from Volvariella volvacea mushroom for a highly sensitive detection of Fe3+ and Pb2+ ions in aqueous solutions. Chemosensors. 2020;8(3):47.
- [Google Scholar]
- An assessment of the potential contribution from waste-to-energy facilities to electricity demand in Saudi Arabia. Energy Conserv. Manage.. 2013;75:402-406.
- [Google Scholar]
- Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl. Surf. Sci.. 2016;390:435-443.
- [Google Scholar]
- A review of carbon quantum dots and their applications in wastewater treatment. Adv. Colloid Interface Sci.. 2020;278:102124.
- [CrossRef] [Google Scholar]
- Extraction and biocompatibility analysis of silica phytoliths from sorghum husk for three-dimensional cell culture. Process Biochem.. 2018;70:153-159.
- [Google Scholar]
- Simple approach to synthesize amino-functionalized carbon dots by carbonization of chitosan. Sci. Rep.. 2016;6:31100.
- [Google Scholar]
- Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci. Rep.. 2016;6:1-7.
- [Google Scholar]
- Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: consensus, debates and challenges. Nanoscale. 2016;8(15):7794-7807.
- [Google Scholar]







