Translate this page into:
Fabrication and functionalization of magnesium nanoparticle for lipase immobilization in n-propyl gallate synthesis
⁎Corresponding author. kanwarss2000@yahoo.com (Shamsher Singh Kanwar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
An extracellular lipase partially purified from Bacillus thermoamylovorans BHK67 was effectively immobilized onto modified magnetic MgFe2O4 nanoparticles (NPs). NPs were prepared by the sol-gel auto-combustion method and characterized by Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), Ultra-Violet–Visible Spectroscopy (UV–vis) and atomic force microscopy (AFM). Protein loading reached a saturated amount of about 0.20 mg lipase per milligram of MgFe2O4 NPs with 78.9% binding efficiency. The NPs-bound lipase also showed stability following exposure to n-propanol and iso-propanol or FeCl2 and MgCl2 metal ions at (1 mM) at 55 °C. NPs-bound lipase also retained 50% of its original hydrolytic activity even after 8th cycle, as well as after 12 h of incubation at 55 °C. NPs-bound lipase in an esterification reaction of n-propanol and gallic acid (25 mM) performed for 12 h at 55 °C produced n-propyl gallate with a conversion rate of 82%. Synthesized n-propyl gallate possessed strong antioxidant activity, which was confirmed by DPPH assay, and in addition has anticancerous activity which was tested on a human L132 cell line.
Keywords
B. thermoamylovorans BHK67
NPs-bound lipase
Thermostability
n-Propyl gallate
Esterification
Antioxidant
1 Introduction
Enzymes are the biocatalysts which are present in living organisms and they boost the rate of a biochemical reaction. Enzymes are known to human society since prehistoric period and a number of bioprocess are achievable only because of the enzymes. Currently enzymes can be purified with greater effortlessness using various methods of enzyme purification and these purified enzymes either in free or immobilized form are used in a number of biotechnological processes (Sharma et al., 2016). Enzymes are now used to enhance the production of previously known products or development of new products or processes. At present almost 4000 enzymes are well-known, and of these about 200 are in commercial use (Sharma et al., 2010, 2016; Li et al., 2011; Gurung et al., 2013). Bacteria are considered superior sources of these enzymes than the higher organisms because of the ease with which bacterial cells may be mass cultured and genetically manipulated (Sood et al., 2016). Lipases are enzymes of extraordinary importance to industry, hydrolysing carboxylic ester bonds that appear in a myriad of food, detergent, pharmaceutical, and materials science applications. The thermotolerant enzymes are always in ample demand to perform catalysis in reactions that need higher temperature to keep reactants in a liquefied state. The lipase production by B. thermoamylovorans has been previously reported following optimization of a few physico-chemical conditions that included temperature, time and pH (Deive et al., 2012). However, no attempt was made to purify and/or characterize the lipase from Bacillus thermoamylovorans in previous studies. In the present study in order to reduce the cost of purifying lipase, we have used partially purified lipase to achieve the production of medicinally important i.e. n-propyl gallate ester by employing iron NPs-bound lipase.
Immobilization of enzymes onto some solid matrices is gaining much attention nowadays because immobilized biocatalysts reduce the operational cost of a process and enhance the reusability of the biocatalysts. Immobilized enzymes often show outstanding thermal and functioning stability at wider pH values, ionic strengths and are often more thermally stable than the native soluble form of enzymes (Sharma et al., 2017). Magnetic NPs offers much advantages as supporting material for immobilization of enzymes over other materials because of lower mass transfer resistance, selective, high surface area for enzyme binding, less fouling effect, nonchemical separation from biocatalyst system by an applied a magnetic field and low leaching problem (Laurent et al., 2008; Johnson et al., 2008; Kanwar et al., 2015). Ester synthesis is done often in a water-limited medium by using lipase. n-Propyl gallate can be prepared either from gallic acid and n-propanol by esterification reaction or by transesterification reaction between tannic acid and n-propanol. n-Propyl gallate is an antioxidant commonly used in various products like foods, cosmetics, hair products, adhesives and lubricants (Lorente et al., 2011). It protects living cells against damaging effect of hydrogen peroxide and oxygen free radicals. The biological effects of n-propyl gallate include antimicrobial activity, ultraviolet (UV) radiation protection, chemoprotection, antimutagenesis, antitumorigenesis, antiteratogenesis and anticarcinogenesis (Safety assessment sheet of propyl gallate, 2007). In the present study, Fe3O4/γFe2O3 nano-particles were prepared, employed for lipase immobilization and subsequently exploited to achieve esterification of n-propanol and gallic acid to synthesize n-propyl gallate.
2 Methodology
2.1 Chemicals
p-Nitrophenylformate (p-NPF), p-nitrophenylpalmitate (p-NPP), p-nitrophenylbutyrate (p-NPB), p-nitrophenylbenzoate (p-NPBz), p-nitrophenylmyristate (p-NPM), p-nitrophenyl stearate (p-NPS) and p-nitrophenol (p-NP) were purchased from Alfa Aesar, Manchaster, England; FeCl2, MgCl2, KCl, NH4Cl, CaCl2, FeCl3, NaCl and HgCl2 were purchased from S.D. Fine-Chem. Ltd., Hyderabad, India. Tetraethoxy silane (TEOS) was purchased from Sigma–Aldrich Chemical Co. St Louis, USA. K2HPO4, NaNO3, KCl, MgSO4.7H2O, FeSO4.7H2O, FeNO3, MgNO3, Ammonium sulfate and yeast extract Ammonium sulphate, gallic acid, n-propanol, molecular sieves, methanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Dulbecco’s Modified Eagle’s medium (DMEM) were procured from HIMEDIA Laboratory Ltd., Mumbai, India. DMSO, propane 1-ol, iso-propanol, ethanol, n-octanol, acetonitrile, Citric acid, Ethylene glycol and Tris buffer were purchased from Merck Darmstadt, Germany. A human transformed L132 cell line was purchased from National Centre of Cell Science, Pune, Maharashtra, India.
2.2 Production of bacterial extracellular lipase
The thermophilic lipase producing isolate was grown in the (broth) medium containing (g/L) yeast extract (5.0), potassium chloride (0.6), sodium nitrate (3.0), magnesium sulphate heptahydrate (0.6), dipotassium hydrogen phosphate (0.1), ferrous sulphate heptahydrate (0.01), and cotton seed oil (10 mL/L). The seed culture was transferred (8%, v/v) to 50 mL production broth (250 mL Erlenmeyer flask) kept for 24 h under shaking (110 rpm) at 55 °C.
2.3 Partial purification of lipase
The partial purification of the extracellular B. thermoamylovorans lipase was executed using approach of ammonium sulphate salting out (Borkar et al., 2009). Ammonium sulphate (141 g) was added to 500 mL of cell-free broth; 40% saturation followed by dialysis. The precipitates were dissolved in 0.05 M Tris buffer pH 8 and extensively dialyzed against 2 L of the same buffer at a regular interval of 2 h so as to completely remove ammonium sulphate.
2.4 Synthesis, surface modification and characterization of MgFe2O4 magnetic NPs
MgFe2O4magnetic NPs were synthesized by sol–gel auto combustion method (Sharma et al., 2015a,b). The structural analysis and morphology of magnetic NPs were determined by Fourier Transform Infra Red spectroscopy (FTIR), X-ray diffraction (XRD), Ultra-Violet–Visible Spectroscopy (UV–vis) and Atomic force microscopy (AFM) done at University Sophisticated Instrumentation Centre (USIC), Himachal Pradesh University, Shimla, India.
2.5 Immobilization of lipase onto modified MgFe2O4 magnetic NPs by covalent binding
The partially purified lipase from B. thermoamylovorans was successfully immobilized onto modified magnetic MgFe2O4 NPs. For immobilization of lipase onto modified nano-particles, the nanoparticles (30 mg) suspended in minimum volume of Tris HCl buffer, (pH 8.0) were ultrasonicated for 5 min at room temperature. Lipase dissolved in 0.05 M Tris HCl buffer (20 mL, pH 8.0) was added to NPs suspension with simultaneous N2bubbling followed by shaking at 140 rpm for 24 h at 37 °C to achieve optimal binding of lipase onto modified magnetic NPs. NPs-bound biocatalyst was separated by a magnet and supernatant was separately collected. Collected NPs were given several washing with Tris buffer (0.05 M, pH 8.0 and were dispersed into same buffer for further use. The NPs-bound lipase suspension was kept at 55 °C, overnight under gentle stirring. Thereafter, the NPs-bound lipase suspension was centrifuged and NPs were separated. Protein binding on magnetite NPs was calculated by subtracting the total protein used for immobilization from that of total protein recovered in the supernatant.
2.5.1 Lipase activity
Lipase activities of supernatant and immobilized biocatalyst were measured by a previously reported (Winkler and Stuckman, 1979; Sharma et al., 2016) method by measuring the micromoles of p-nitrophenol released from p-nitrophenylpalmitate (p-NPP). One unit (U) of lipase activity was defined as μmol(s) of p-nitrophenol released per minute by hydrolysis of p-NPP by 1 mL of soluble enzyme or 1 mg of immobilized biocatalyst (weight of matrix included) at 55 °C under standard assay conditions. The concentrations of proteins in test samples were estimated by dye binding method (Bradford, 1976).
2.6 Characterization of magnetic MgFe2O4NPs-bound lipase
2.6.1 Effect of different substrate on NPs-bound lipase
To study the substrate specificity of NPs-lipase, different chromogenic substrates namely p-NPF, p-NPP, p-NPB, p-NPBz, p-NPM and p-NPS were used. Each of the above substrates was prepared as a stock (5 mM) in iso-propanol. The reaction was performed using 40 μL of NPs-bound lipase and Tris buffer (0.05 M) of pH 8.0 at 55 °C for 10 min.
2.6.2 Effect of reaction temperature on NPs-bound lipase
To study the effect of reaction temperature, enzyme activity was assayed at selected reaction temperature (45, 55, 65 and 75 °C) with 5 mM substrate p-NPP. The reaction was performed using 40 μL of NPs-bound lipase and Tris buffer (0.05 M) of pH 8.0 for 10 min at the selected temperature.
2.6.3 Effect of metal ions on the activity of NPs-bound lipase
To examine the effect of various salt-ions (FeCl2, MgCl2, KCl, NH4Cl, CaCl2, FeCl3, NaCl and HgCl2) on the activity of NPs-bound lipase, the biocatalyst was pre-incubated at 55 °C for 10 min separately with each of the selected salt-ions (1 mM) in 1:2 ratios (v/v). NPs-bound lipase was checked for residual lipase activity using 40 μL of NPs-bound lipase and Tris buffer (0.05 M) of pH 8.0 at 55 °C for 10 min.
2.6.4 Effect of organic solvents on NPs-bound lipase
Ideally biocatalyst must be able to work in different organic solvents in order to carry out various synthetic reactions effectively. NPs-bound lipase was pre-exposed to different solvents (2%, v/v) i.e. methanol, ethanol, acetonitrile, iso-propanol, DMSO, n-propanol and n-octanol at 55 °C for 10 min (Saun et al., 2014). NPs-bound lipase was checked for residual lipase activity using 40 μL NPs-bound lipase and Tris buffer (0.05 M) of pH 8.0 at 55 °C for 10 min.
2.6.5 Reusability of NPs-bound lipase
One of the important parameter of immobilized enzyme is its recovery and reusability. Magnetic NPs bind covalently to lipase enzyme and found to be excellent lipase carriers resulting in stability of enzyme molecules. Reusability of the biocatalyst was determined by recycling NPs-bound lipase up to 11 cycles of hydrolysis of p-NPP. After each cycle, NPs-bound lipase was separated using magnet and was given three Tris HCl (0.05 M, pH 8.0) washes to prepare it for fresh cycle of enzymatic assay with p-NPP using 40 μL of NPs-bound lipase and Tris buffer (0.05 M) of pH 8.0 at 55 °C for 10 min.
2.6.6 Kinetic study of NPs-bound lipase
The Km and Vmax of free lipase and NPs-bound lipase were determined by measuring the reaction velocities at the different concentration of the p-NPP i.e. (3–5.5 mM). The reciprocal of the reaction velocity was plotted against the reciprocal of the substrate concentration to determine the Km and Vmax value by Lineweaver-Burk plot (Lineweaver and Burk, 1934). The reaction was performed using 40 μL of enzyme (free and NPs-bound) and Tris buffer (0.05 M) of pH 8.0 at 55 °C for 10 min.
2.6.7 Thermostability of free lipase and NPs-bound lipase
Thermostability of NPs-bound lipase was compared with that of free lipase by incubating free and NPs-bound lipase at 55 and 65 °C. Lipase activity of both free and immobilized lipase were recorded after 0, 1, 3, 4, 5, 7, 9, 11, 13 and 15 h at 55 °C and after 0, 1, 3, 5 and 7 h at 65 °C. The activity measured immediately before incubation was defined as 100% of hydrolytic activity. The reaction was performed using 40 μL of free enzyme and Tris buffer (0.05 M, pH 8.0) at 55 and 65 °C.
2.7 Synthesis of n-propyl gallate using magneticNPs-bound lipase
The immobilized lipase enzyme (100 mg) was added to 25 mM gallic acid in 5 mL n-propanol in the presence of molecular sieves (1 mg/mL) under shaking at 55 °C and 100 rpm for 12 h. After incubation the content was obtained by drying to recover n-propyl gallate. After completion of the reaction, the immobilized NPs bound-lipase was isolated from reaction mixture using magnet. The supernatant was dried at 40 °C in a vacuum evaporator and dissolved in methanol. Synthesized n-propyl gallate was analysed by HPLC for the detection of the ester group.
2.8 Antioxidant activity and cytotoxicity assay of synthesized n-propyl gallate
DPPH is a stable free radical and it has unpaired electron, which is translocated. DPPH stock solution was prepared by dissolving 0.5 mM DPPH in 50 mL methanol. Control was prepared by adding 500 µL distilled water and 500 µL DPPH solution. Synthesized n-propyl gallate was added in the test tubes in different amounts to make 500 µL by adding distilled water, followed by the addition of 500 µL of DPPH solution. Reaction mixture was incubated at 37 °C for 30 min. Ascorbic acid (1.0 mg/mL) was used as a positive control. After incubation A517values were recorded. Lower A517 values represented higher DPPH scavenging activity of the test sample/compound. The percentage DPPH-scavenging activity was calculated as follows;
To check the cytotoxicity of synthesized n-propyl gallate, if any, MTT assay was conducted on L132 murine cell line. Approximately 10,000 cells per well in a 96-wells microtiter plate were incubated with increasing concentration of synthesized n-propyl gallate (25–495 µg/mL) for 24 h at 37 °C. MTT (20 μl; 5 mg/mL prepared in distilled water) was added to each of the wells followed by 1 h incubation in dark. Thereafter, the DMEM was completely removed/discarded and DMSO (100 μL/well) was added. The purple coloured formazan end product extracted in the DMSO in each case was checked at A570for reduced MTT using a microplate reader (Thermo Electron Corporation, China), values were recorded and % viability in each of the regimens was determined. The control well didn’t contain any synthesized n-propyl gallate added to the L132 cells. Culture medium containing methanol was used as a solvent control and untreated cells preparation was used as negative control. The cell growth inhibition (%) was calculated according to the following formula;
3 Results
3.1 Concentration of B. thermoamylovorans lipase
The extracellular bacterial lipase (25.8 U/mL; 1.397 mg protein/mL) secreted by B. thermoamylovorans in the Nutrient broth (1000 mL) was subjected to (0–40%) ammonium sulphate precipitation. The precipitates were dialysed for 24 h against 0.05 M Tris HCl (pH 8.0). After dialysis, an activity of 58.8 U/mL with 8.4-fold purification and 11.4% yield were recorded (Table 1). Dialysed lipase preparation showed strong lipase activity and hence was directly used for immobilization study. CFE: Cell free extract. ASP: Ammonium sulphate precipitation (0–40%).
Purification Stage
Volume (mL)
Total activity (U)
Total protein (mg)
Specific activity (U/mg)
Fold purification
Yield (%)
CFE
900
23232.6 ± 10.12
1257.3 ± 7.89
18.5
1.0
100.0
Dialysed after ASP
45
2650.3 ± 5.67
16.9 ± 1.12
156.2
8.4
11.4
3.2 Synthesis and surface modification of MgFe2O4 magnetic NPs
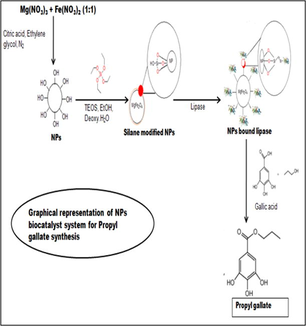
Magnetic NPs were modified to attach partially purified lipase onto the surface of NPs (Fig. 1). Surface modification of magnetic NPs was based on well-known Stober’s process (Stober et al., 1968) in which silica is formed through the hydrolysis and condensation of sol-gel precursors, such as tetraethoxysilane (TEOS). The incredible property of these silanes is that they quickly react with water in the presence of shorter chain alcohol such as ethanol or ammonia to form monodispersed silica particles. Possibly the chemical group ‘silane’ helps in chemical binding with lipase as these nanoparticles are so small for physical binding of the protein/lipase. This property was employed in coating of NPs with silanes, which not only prevents the oxidation of magnetic core but also helps in functionalizing NPs for effective immobilization of biomolecule onto their surface. N2 gas was used for the storage of modified magnetic NPs.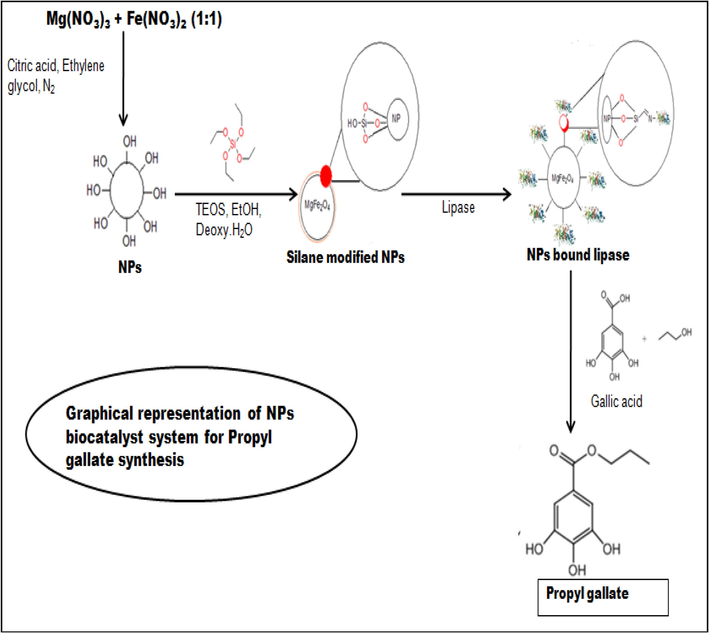
Reaction chemistry involved in the synthesis of silane-coated MgFe2O4 NPs and their use in biocatalysis.
3.3 Characterization of synthesized magnetic NPs
3.3.1 FTIR, XRD, AFM and UV–vis spectroscopy analysis of synthesized MgFe2O4 magnetic NPs
The prepared NPs were characterized by FTIR (Shimadzu FTIR-8400S, Japan) and the results were recorded (Fig. 2). The main absorption bands at 1635 cm−1 resulted from the anti-symmetrical and symmetrical stretching vibration bands of COO− (Kaur and Srivastava, 2013). The peak at 1635 cm−1 could not be attributable to the water bound bending as the sample was initially dried in oven at 55 °C. Also absorption band at 1715 cm−1 might be associated with C⚌O of carboxylate group. The other bands recorded at 1457, 1385 and 868 cm−1 corresponded to the stretching and bending vibrations of H—C—H, C—H and C—C, respectively. The characteristic absorption bands between 580 cm−1 were assigned to the vibration of the bond between the oxygen atom and the metal ions (F—O), confirming the formation of hexaferrite, which corresponded to the vibrations of the tetrahedral and octahedral sites. The Fe—O stretching vibration band of the bulk magnetite is usually at 580 cm−1 and the band shifted to high wave numbers because of the finite size of the nanoparticles. The peak appearing at 1092 cm−1 was due to Si—OCH3 group. Amide I and Amide II bands were localized in the 1700–1600 cm−1 and 1600–1500 cm−1 region, respectively. Alpha helix is localized in the 1660–1650 cm−1 region (Marsh et al., 2000; Hu and Laskin, 2016). Furthermore, 1631 and 1595 cm−1 signals confirmed the binding of lipase to nanoparticles by covalent immobilization (Rani et al., 2015).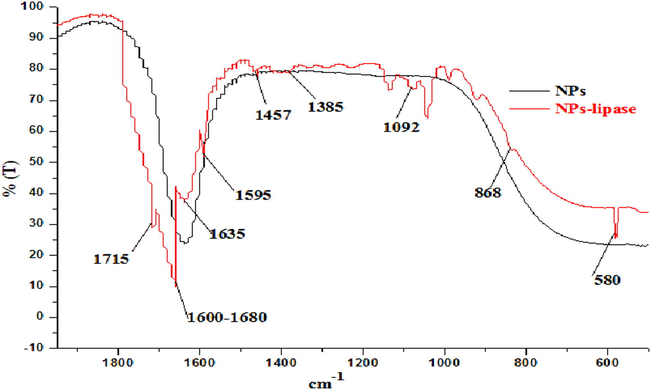
FTIR analysis of synthesized MgFe2O4 NPs (in black) as well as NPs bound lipase (in red).
The synthesized NPs were studies with CUKα radiation at voltage of 30 kV and current of 20 mA with a scan rate of 0.030/s by X-ray diffraction spectroscope (Philips PAN analytical, The Netherland). XRD analysis is used to determine the phase distribution, crystallinity and purity of the synthesised NPs. The major XRD peak was obtained at 2θ = 36.1° which strongly suggested that MgFe2O4 is the major phase with inverse spinel structure having crystallite size of the order of 42.3 nm (Fig. 3a). UV–vis spectra of MgFe2O4NPs, was recorded in the range of 200–800 nm using a UV Spectrophotometer (Lambda Bio 20, Perkin Elmer, USA). Magnetic MgFe2O4 NPs also possessed a broad absorption in the <350 nm (Fig. 3b). Broad absorption of MgFe2O4 NPs was possibly due to the presence of iron oxides. Iron oxides have three kinds of optical transitions: (i) the Fe3+ ligand field transition or the d-d transition, (ii) the ligand to metal charge-transfer transitions, and (iii) the pair excitations resulting from the simultaneous excitations of two neighbouring Fe3+ cations that are magnetically coupled (Dhiman et al., 2012). AFM (NTEGRA NT-MDT Scanning probe microscope, Russia) images showed that the synthesized magnetic MgFe2O4NPs have grain size of 57.2 nm with roughness of 0.641 nm (Fig. 3c). The thresh-holding can be effective only for higher roughness or substrate curvature, which is most effective feature of NPs immobilization.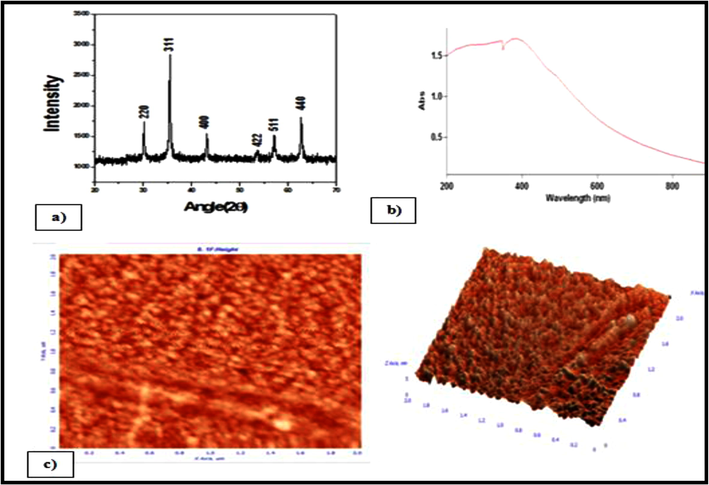
The XRD pattern (a), UV–vis Spectrum (b) and AFM 2D & 3D analysis (c) of synthesized MgFe2O4 NPs.
3.4 Immobilization of lipase on silane-coated MgFe2O4 magnetic NPs
Different amounts of partial purified lipase (0.376 mg/mL) were loaded to determine the protein loading efficiency of lipase onto magnetite NPs (Table 2). It was found that with an increase in protein loading the amount of immobilized lipase initially increased and reached a saturated amount of about 0.20 mg lipase per milligram of magnetic NPs with 78.9% binding efficiency. However, it was found that with an increase in the loaded protein, % binding of lipase onto nano-particles decreased. This could be explained by the fact that steric hindrance becomes stronger with an increasing adsorbed amount of enzyme on the surface of NPs.
Dilution
Lipase (mL)
Total protein loaded (mg)
Total protein in supernatant (mg)
Total bound protein/30 mg of NPs
Total bound protein/mgNPs
Binding of lipase on NPs (%)
D1
5
1.88
0.794
1.086
0.0362
57.7
D2
10
3.76
1.191
2.569
0.0856
68.3
D3
15
5.64
1.551
4.089
0.1363
72.5
D4
20
7.52
1.580
5.940
0.1980
78.9
D5
25
9.40
3.621
5.879
0.1956
62.6
3.5 Characterization of immobilized NPs-bound lipase of B. thermoamylovorans
3.5.1 Effect of different substrate on NPs-bound lipase
To study the substrate specificity of NPs-bound lipase, different chromogenic substrates namely p-NPF, p-NPP, p-NPM, p-NPS and p-NP were used (Fig. 4a). Both free lipase as well as NPs-bound lipase showed maximum activity with p-NPP i.e. 58.24 ± 1.18 U/mL and 53.03 ± 1.29 U/mL, respectively.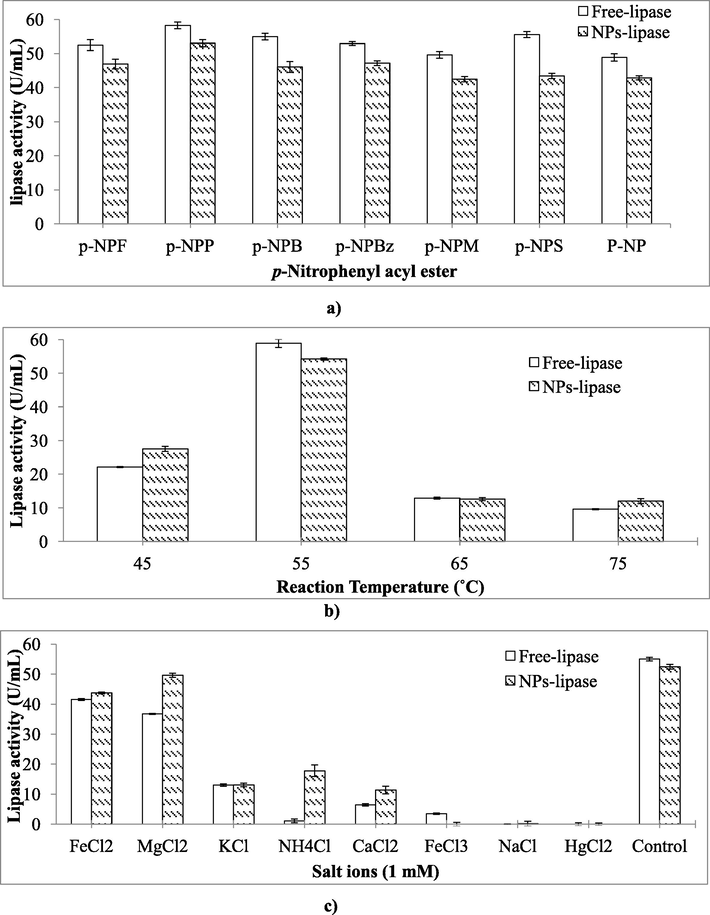
(a–c): Effect of different p-nitrophenyl acyl esters (a), reaction temperature (b); and (c) different salt/metal ions on NPs-bound lipase.
3.5.2 Effect of reaction temperature on NPs-bound lipase
The effect of temperature on the NPs-bound lipase was evaluated (Fig. 4b). The results showed that both free lipase as well as NPs-bound lipase show maximum activity at temperature 55 °C i.e. 58.9 ± 1.34 U/mL and 55.2 ± 1.13 U/mL respectively. However, it was found that nano-particles bound lipase was active even at temperature of 75 °C (12.0 U/mL).
3.5.3 Effect of metal ions on the activity of NPs-bound lipase
To examine the effect of various salt-ions (NaCl, FeCl2, FeCl3, NH4Cl, HgCl2, CaCl2, KCl and MgCl2) on the activity of NPs-bound lipase, the biocatalyst was pre-incubated at 55 °C for 10 min separately with each of the selected salt-ions (1 mM) in 1:1 ratio (v/v). Most of the tested ions/salts led to a decrease in the activity of the free as well as NPs-bound lipase while FeCl2 and MgCl2 (1 mM) showed tolerance towards lipase (Fig. 4c).
3.5.4 Effect of different solvents on the activity of NPs-bound lipase
NPs-bound lipase showed greater activity in the presence of alcohols when used as solvents. n-Propanol and iso-propanol were fairly tolerated by the lipase (Table 3). Other selected organic solvents drastically inhibited the lipase activity.
Solvents (2%)
Log p value
Activity free-lipase (U/mL)
Activity NPs-bound lipase (U/mL)
Relative activity NPs-bound lipase (%)
Relative activity free-lipase (%)
Control
–
53.88 ± 1.23
50.67 ± 1.45
100.00
100.00
Methanol
−0.74
26.36 ± 0.69
28.15 ± 0.98
55.15
48.93
Ethanol
−0.58
14.98 ± 0.45
21.75 ± 0.23
42.92
27.81
Acetonitrile
−0.33
16.69 ± 0.14
16.74 ± 0.17
33.03
30.97
iso-Propanol
–
36.39 ± 0.83
36.24 ± 0.78
71.58
67.53
n-Propanol
–
35.23 ± 1.15
33.38 ± 1.89
37.97
65.39
DMSO
1.22
14.93 ± 0.12
30.62 ± 1.11
60.43
27.72
n-Octanol
2.9
22.45 ± 0.34
28.27 ± 0.12
55.79
22.45
3.5.5 Reusability of NPs-bound lipase
Examination of hydrolytic activity of NP-bound lipase for reusability up to 11 cycles gave amazing results (Fig. 5a). The nano-biocatalyst was simply and rapidly separated under an external magnetic field after each cycle was ready to use for next round of hydrolytic cycle (Fig. 5a). Results showed that NPs-bound lipase retained ∼50% of its initial hydrolytic activity after 8 cycles. This indicated highly effective and efficient immobilization of lipase onto nano-particles exhibiting enormous strength and stability.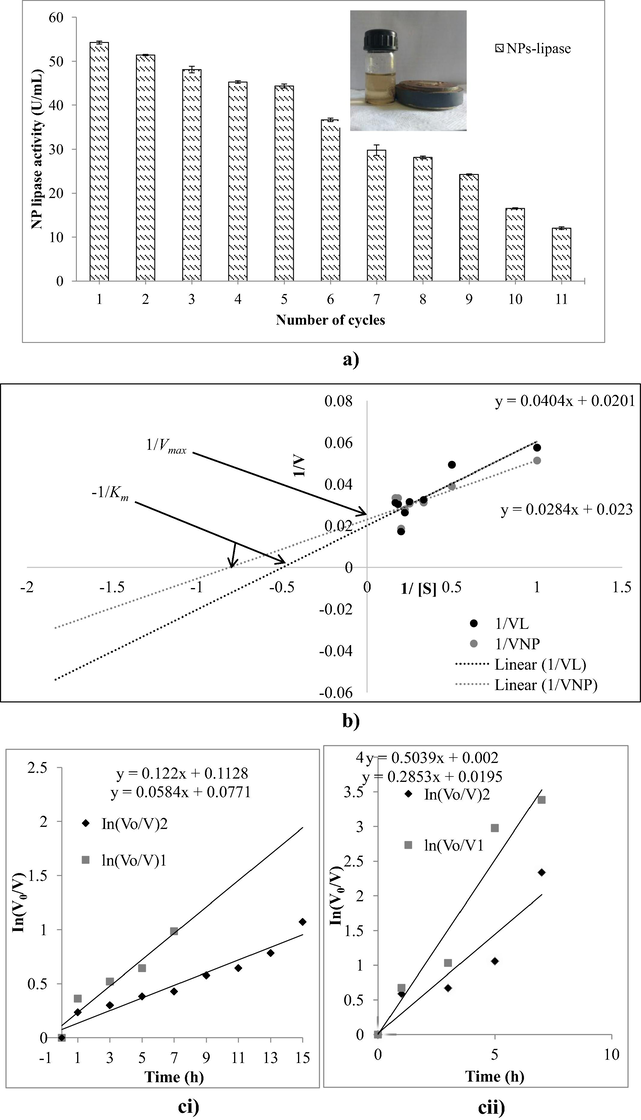
(a) Separation of NPs after each cycle using magnet and reusability of NPs-bound lipase in continuous cycles of hydrolysis. (b) Line weaver-Burk plot and, (c) thermostability profile of free and NPs bound lipase of Bacillus thermoamylovorans for p-NPP at 55 °C (i) and 65 °C (ii).
3.5.6 Determination of Km and Vmax for free lipase and NPs-bound lipase
The rate of reaction Vmax and Km using the best colorimetric substrate p-NPP were studied by employing (1–5.5 mM) of p-NPP concentration in (0.05 M) Tris-buffer (pH 8.0) under shaking at (55 ± 1 °C) (Fig. 5b). The values (Km and Vmax) for free lipase (2.52 ± 0.012 mM, 58.82 ± 1.09 μmol/mL/min) and NPs-bound lipase (1.42 ± 0.009 mM, 47.61 ± 1.16 μmol/mL/min) were observed. The lower Km value i.e. 1.42 mM for NPs-bound lipase indicates higher affinity of immobilized lipase for its substrate, which could be the fact that larger surface area provided by nano-particles have been completely occupied by enzyme molecule for its attachment thereby maximizing the exposure of available active sites to its substrate. The decrease in Vmax of NPs-bound lipase as compared to that of free lipase could be reasoned as the confinement of enzyme molecule on non-porous solid support thereby affecting its movability in an aqueous solution. However, increased affinity and enhanced stability of lipase have proved successful for effective immobilization of lipase on magnetic nano-particles. The values of Km, Vmax, Kcat and specificity constant for free and nano-particle immobilized were presented (Table 4).
Km (mM)
Vmax (U/mL/min)
Kcat (s−1)
Specificity constant
Free-lipase
2.52 ± 0.012
58.82 ± 1.09
147.05 ± 3.67
58.13 ± 1.12
NPs-bound lipase
1.42 ± 0.009
47.61 ± 1.16
119.04 ± 2.19
88.33 ± 2.12
3.5.7 Thermostability of free lipase and NPs-bound lipase at 55 °C and 65 °C
Thermostability experiments of free lipase and NPs-bound lipase at 55 °C and 65 °C were performed (Fig. 5c). The half-life (t1/2) of purified lipase was approximately 5.68 h at 55 °C. NPs-bound lipase retained almost 50% of its initial activity even after ∼12 h of incubation at 55 °C. Such observations and recorded results showed that nano-particles bound lipase was highly stable and exhibited thermostability at higher temperatures even after 12 h. t1/2 of both free and immobilized lipase at 55 °C and 65 °C (Table 5).
Temp (°C)
t1/2Free-lipase (h)
t1/2NPs-bound lipase (h)
55
5.68 ± 0.34
11.94 ± 0.39
65
1.37 ± 0.03
2.43 ± 0.04
3.5.8 Synthesis and HPLC analysis of n-propyl gallate using NPs bound lipase
The NPs-immobilized partially purified lipase from B. thermoamylovorans was tested for its ability to synthesize n-propyl gallate (Fig. 6). Analysis of n-propyl gallate was performed using 515-HPLC pump (Waters) equipped with Reverse phase Lichrosorb C18-5 µm (4 × 125 mm) column (Waters) and 2998 photodiode assay detector (Waters). Samples were dissolved in methanol and a volume of 10 µL was injected into HPLC. The solvent system/mobile phase comprised of methanol: water in the ratio of 70:30 at a flow rate of 1 mL/min for 8 min. The absorbance analysis was carried out at 275 nm. HPLC analysis confirmed the presence of n-propyl gallate in the reaction mixture. At the end of the reaction 17.47 of n-propyl gallate (i.e. ∼82% conversion rate of gallic acid into product) was produced (Fig. 7a–c).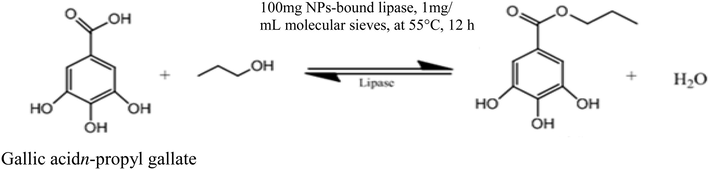
Schematic representation of synthesis of propyl gallate by magnetic NPs-bound lipase.
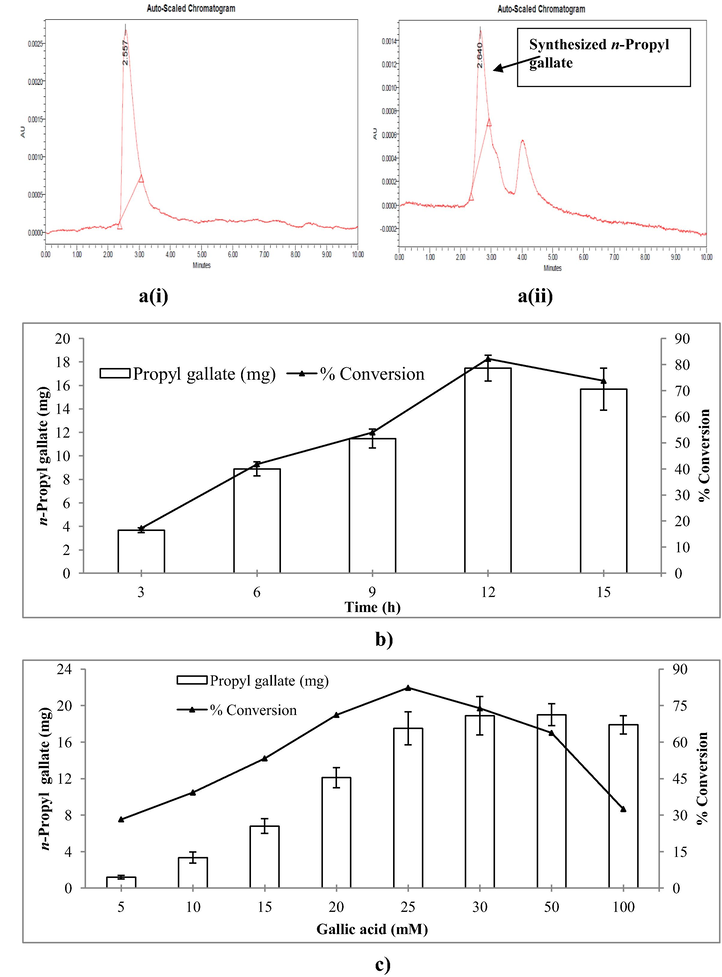
(a) HPLC chromatogram of standard and sample n-propyl gallate showing RT of 2.557 min (i) and 2.640 min (ii), (b and c) represents% conversion of gallic acid into n-propyl gallate with changing reaction time and gallic acid concentration, respectively.
3.5.9 Antioxidant activity and cytotoxicity assay of synthesised n-propyl gallate
A maximum scavenging of 54.0% of DPPH free radicals was observed in 45 µg/mL sample (Fig. 8a). The maximum % inhibition (cytotoxicity) i.e. 27.9% towards L132 cell was found in the wells (Fig. 8b) in which 225 μg/mL synthesized n-propyl gallate was added as compared to the control (methanol without n-propyl gallate). MTT assay proved that synthesized n-propyl gallate had anticancerous activity and indicated by the toxicity towards the selected mammalian cancerous cell line.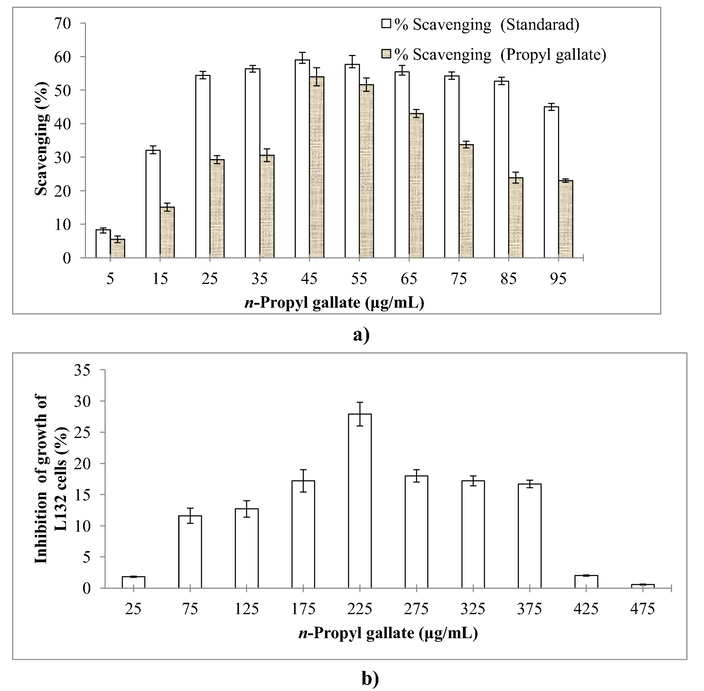
(a) Scavenging activity and (b) cytotoxicity assay of synthesized n-propyl gallate.
4 Discussion
In the light of inherent beneficial properties of nano-particles, a successful attempt was made to immobilize the B. thermoamylovorans BHK67 lipase onto iron-based magnetic nano-particles. The average crystallite size of the synthesized magnetic NPs was found to be 42.3 nm. XRD analysis also showed that magnetic NPs possessed a crystalline structure of inverse spinel making the produced NPs suitable for technological applications. Additionally, AFM measurements of synthesized magnetic NPs indicate the effective thresh-holding with higher roughness which is most effective feature of NPs immobilization. The adsorption capacity of lipase onto Fe3O4 NPs could reach 115 mg/g (i.e. 0.115 mg/mg NP) (Chen et al., 2014), which was concluded to be higher than previous research (Lee et al., 2008; Huang et al., 2008). In an another experiment with immobilization of Candida rugosa lipase on alkyl silane coated Fe3O4 NPs, amount of immobilized lipase was found to be about 0.20 mg lipase per mg of NPs (Wang et al., 2010). However, in the present research immobilized lipase reached a saturated amount of 0.20 mg lipase per milligram of magnetic NPs with 78.9% binding efficiency which was noticeably higher than previous reports.
The activity profiles of the free and immobilized lipases at temperatures ranging from 45 to 75 °C were measured. A greater loss in the activity of free lipase was observed as compared to immobilized enzyme. The celite-bound lipase was quite thermostable as it showed half life of 238, 195 and 176.5 min compared to free lipase that showed half life of 180, 139.5 and 131.5 min when incubated at 50, 55 and 60 °C, respectively (Saun et al., 2014). However, in our study free and immobilized lipase showed highest activity at 55 °C and remained active up to 75 °C. NPs bound lipase showed half life of 11.94 and 2.43 h compared to free lipase that showed half life of 2.43 h and 1.37 h at 55 °C and 65 °C respectively. Reusability and recovery of immobilized enzyme are amongst greatly desired and important parameters that have to be considered in order to ensure efficient and effective immobilization. The immobilized lipase on collagen retained 64% of its initial activity after 5 cycles (Dewei et al., 2016). The decrease of activity was attributed to possible denaturation or leaching (detachment) of lipase molecules form collagen. In case of porcine pancreas lipase (PPL) covalently immobilized on the surface of silica-coated modified magnetite nano-particles, immobilized lipase retained 63.5% of its initial activity after 6 cycles (Sun et al., 2015). However, in our study NPs-bound lipase showed enormous stability and retentivity. It retained 67.6% activity after 6th cycle and 51% after 8th cycle. This confirmed that the lipase bound to silane coated NPs was highly stable and overcomes enzyme leaching problem reported by earlier workers (Hartmann and Kostrov, 2013).
Measurement of Michaelis–Menten kinetic parameters (Vmax and Km) unveiled the considerable enhancement in the immobilization of enzyme onto a particular support system. Reports have been presented determining Vmax and Km of immobilized enzyme with nano-particles as support system. Lipase immobilized on alkyl functionalized Fe3O4-SiO2 nano-particles showed immobilized enzyme with Km = 3.6 mM and Vmax = 131.4 U mg−1 in comparison to free enzyme that showed Km = 0.09 mM and Vmax = 133.3 U mg−1 (Sharma et al., 2015a,b). In an another study, the lipase bound to silica-coated magnetite NPs showed Km and Vmax of 0.123 mM and 0.56 μmol/min/mg, respectively as compared to free lipase (0.312 mM and 0.0132 μmol/min/mg, respectively), which therefore, represented a higher affinity of immobilized lipase towards the substrate (Sun et al., 2015). In the present study, kinetic parameters for free lipase and NPs-bound lipase were calculated from Lineweaver-Burk plot using p-NPP as substrate. The values Km and Vmax for free lipase 2.52 mM, 58.8 μmol/mL/min and NPs-bound lipase 1.42 mM, 47.6 μmol/mL/min, respectively were recorded. A decrease in the Km for immobilized enzyme indicated an increase affinity of the enzyme for its substrate (p-NPP). It was found that NPs-bound lipase showed enhanced activity in case of n-propanol and iso-propanol. Previously Saun et al. (2014) found that celite bound lipase from B. aerius showed inhibitory effect towards selected organic solvents (1%). Therefore the tolerance of NPs-bound lipase in different solvents suggested its likely use in organic synthesis, enantio-selectivity and associated applications. Further, NPs-bound lipase was used to synthesize n-propyl gallate by esterification reaction performed between n-gallic acid and n-propanol.
At the end of the reaction 17.47 mg of n-propyl gallate (i.e. ∼82% conversion rate of gallic acid into product) was successfully produced which was far better than previous report (Tran et al., 2012). The electron-donating ability of gallates, which are food and pharmaceutical antioxidants, is quantitatively assessed on the basis of their electrochemical characteristics (Gunckel et al., 1998). Gallic acid and its esters, in turn, are hydroxybenzoic derivatives used as antioxidant additives in both food and pharmaceutical industries (n-propyl gallate and n-octyl gallate), which are known to protect against oxidative damage induced by reactive oxygen species (Fiuza et al., 2004). n-Propyl gallate has antioxidant activity which was determined by DPPH assay. DPPH assay confirmed that n-propyl gallate have substantial antioxidant activity. Gallic acid derivatives such as n-propyl gallate are known to cause apoptosis in tumour cell lines and to inhibit lymphocyte proliferation (Fiuza et al., 2004). Anticancerous activity of synthesized n-propyl gallate was evaluated on L132 cell line. The recorded results showed 27.9% growth inhibition of cells. In the future studies, the n-propyl gallate synthesis may be scaled up on volumetric basis and its effect on triggering apoptotic cascade in the cancerous cell line (s) may be explored.
5 Concluding remarks
In present study, a lipase from B. thermoamylovorans BHK67 was effectively immobilized onto modified MgFe2O4 NPs. The NPs-bound lipase also showed strong stability following exposure to methanol, propane-2-ol and propane-1-ol. All of the tested salt ions reduced the NPs-bound lipase activity by 50–70% except FeCl2 and MgCl2. It was found that NPs-bound lipase sustained active over 11 cycles of repetitive use and retained 50% of its initial hydrolytic activity even after 8th cycle. Immobilized lipase retained almost 50% of its initial activity after 12 h of incubation at 55 °C and 50% of its initial activity after 3 h at 65 °C. NPs-bound lipase was used to synthesize n-propyl gallate by esterification reaction. At the end of the reaction 17.47 mg of n-propyl gallate was produced. Synthesized n-propyl gallate have antioxidant activity which was confirmed by DPPH assay, and also have anticancerous activity which was tested on L132 cell line. So the overall study showed that remarkable properties of high stability and reusability exhibited by the lipase bound on magnetic NPs may offer undoubtedly promising results at industrial scale and multi-purpose NPs as whole can serve as superlative host systems for various biomolecules.
Conflict of interest
The authors declare no conflict of interests publishing this article in this journal.
Acknowledgements
The financial support in the form of DST-JRF to one of the authors (AS) in the form of a Junior Research Fellowship by Department of Science and Technology, New Delhi (India) is thankfully acknowledged.
References
- Purification and characterization of extracellular lipase from a new starin Pseudomonas aeruginosa SRT9. Braz. J. Microbiol.. 2009;40:358-366.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Synthesis of amine-functionalized Fe3O4@C nano-particles for lipase immobilization. J. Mater. Chem.. 2014;2:18334-18339.
- [Google Scholar]
- Process for extracellular thermostable lipase production by a novel Bacillus thermoamylovorans strain. Bioprocess. Biosyst. Eng.. 2012;35:931-941.
- [Google Scholar]
- Collagen-immobilized lipases show good activity and reusability for butyl butyrate synthesis. Appl. Biochem. Biotechnol.. 2016;16:1-15.
- [Google Scholar]
- Solution combustion preparation of Fe2O3-nanoflakes: Synthesis and characterization. Adv. Mater. Lett.. 2012;3:300-333.
- [Google Scholar]
- Phenolic acid derivatives with potential anticancer properties-a structure-activity relationship study. Part 1: methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem.. 2004;12:3581-3589.
- [Google Scholar]
- Antioxidant activity of gallates: an electrochemical study in aqueous media. Chem. Biol. Interact.. 1998;114:45-59.
- [Google Scholar]
- A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res. Int.. 2013;2013:1-18.
- [Google Scholar]
- Immobilization of enzymes on porous silica-benefits and challenges. Chem. Soc. Rev.. 2013;42:6277-6289.
- [Google Scholar]
- Secondary structures of ubiquitin ions soft-Landed onto self assembled monolayer surfaces. J. Phys. Chem.. 2016;20:4927-4936.
- [Google Scholar]
- Covalent immobilization of lipase from Candida rugosaonto poly (acrylonitrile-co-2-hydroxyethyl methacrylate) electrospun fibrous membranes for potential bioreactor application. Bioresour. Technol.. 2008;99:5459-5465.
- [Google Scholar]
- Novel method for immobilization of enzymes to magnetic nano-particles. J. Nanopart. Res.. 2008;10:1009-1025.
- [Google Scholar]
- Nanoparticles in biochemical engineering: synthesis and chemistry. In: Thakur V.K., Thakur M.K., eds. Handbook of Sustainable Polymers: Struct. Chem.. USA: Stanford; 2015. p. :685-710.
- [Google Scholar]
- Effect of pH on magnetic properties of doped barium hexaferrite. Int. J. Res. Mech. Eng. Technol.. 2013;3:171-173.
- [Google Scholar]
- Magnetic iron oxide nano-particles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev.. 2008;108:2064-2110.
- [Google Scholar]
- Immobilization of lipase on hydrophobic nano-sized magnetite particles. J. Mol. Catal. B: Enzym.. 2008;57:62-66.
- [Google Scholar]
- Biocompatibility of Fe3O4@Au composite magnetic nano-particles in vitro and in vivo. Int. J. Nanomed.. 2011;6:2805-2819.
- [Google Scholar]
- The determination of enzyme dissociation constants. J. Am. Chem. Soc.. 1934;56:658-666.
- [Google Scholar]
- Synthesis of propyl gallate by transesterification of tannic acid in aqueous media catalysed by immobilised derivatives of tannase from Lactobacillus plantarum. Food Chem.. 2011;128:214-217.
- [Google Scholar]
- Orientation of the infrared transition moments for an alpha-helix. Biophys. J. 2000:2499-2510.
- [Google Scholar]
- Novel approach of alkaline protease mediated biodegradation analysis of mustard oil driven emulsified bovine serum albumin nanospheres for controlled release of entrapped Pennisetum glaucum (Pearl Millet) amylase. Am. J. Adv. Drug Delivery. 2015;3:135-148.
- [Google Scholar]
- Comparative study of free and immobilized lipase from Bacillus aerius and its application in synthesis of ethyl ferulate. J. Oleo Sci.. 2014;63:911-919.
- [Google Scholar]
- Physical adsorption of lipase onto mesoporous silica. Int. J. Curr. Adv. Res.. 2017;6:3837-3841.
- [Google Scholar]
- Production, purification, characterization, and applications of lipases. Biotechnol. Adv.. 2010;19:627-662.
- [Google Scholar]
- Sol–gel auto combustion processed soft Z-type hexananoferrites for microwave antenna miniaturization. Ceram. Int.. 2015;41:7109-7114.
- [Google Scholar]
- Gallic acid-based alkyl esters synthesis in a water-free system by celite-bound lipase of Bacillus licheniformis SCD11501. Biotechnol. Prog.. 2015;31:715-723.
- [Google Scholar]
- Purification and characterization of an extracellular high molecular mass esterase from Bacillus pumilus. J. Adv. Biotechnol. Bioeng.. 2016;4:9-16.
- [Google Scholar]
- Carboxylesterases: Sources, characterization and broader applications. Insight Enzyme Res.. 2016;1:1-8.
- [Google Scholar]
- Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci.. 1968;26:62-69.
- [Google Scholar]
- Immobilization of Yarrowia lipolytica lipase on macroporous resin using different methods: characterization of the biocatalysts in hydrolysis reaction. Biomed. Res. Int.. 2015;15:1-12.
- [Google Scholar]
- Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. J. Biotechnol.. 2012;158:112-119.
- [Google Scholar]
- Magnetic mesoporous silica nanoparticles: fabrication and their laccase immobilization performance. Bioresour. Technol.. 2010;101:8931-8935.
- [Google Scholar]
- Glucogenhyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Biotechnol.. 1979;138:663-670.
- [Google Scholar]







