Fabrication and characterization of surgical sutures with propolis silver nano particles and analysis of its antimicrobial properties
⁎Corresponding authors. gayu.bt14@gmail.com (Gayathri Devi Selvaraju), sumathijones@gmail.com (C. SumathiJones), sudesh.yadav@student.uts.edu.au (Sudesh Yadav)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The objective of this work was to synthesize propolis – silver nanoparticles, coat the prepared nanoparticles on surgical sutures, characterize the coated sutures and to study the antimicrobial activity of coated sutures against human pathogenic microorganisms.
Methods
Silver nanoparticles were synthesized with ethanolic propolis extract and analyzed by UV spectrophotometry analysis. Absorption peak was detected at 430 nm to confirm silver nanoparticle synthesis. Propolis – silver nanoparticles were coated over plain Cal Gut surgical sutures with the help of sodium alginate by slurry dipping technique. The characterization of coated sutures was done by Fourier-Transform Infrared analysis (FTIR) analysis and Scanning Electron Microscopy (SEM). The antimicrobial activity of propolis silver nanoparticles was determined by disc diffusion method against pathogenic bacteria like Staphylococcus aureus, E.coli and Aspergillus flavus obtained from clinical samples.
Results
FTIR confirmed the presence of silver through its various peaks and the various functional groups were detected. The SEM characterization revealed the particles size and shape of the nanoparticle at a magnification of 20KV. The size is about 20 nm and its mostly spherical in shape. The surface of the coated sutures was rough indicating the coating of propolis – silver nanoparticles (AgNPs) over the sutures. The in-vitro antimicrobial assay showed that the propolis – silver nanoparticles and the also coated sutures possessed a significant anti-microbial activity against the clinical pathogenic microorganisms.
Conclusion
Propolis is a natural product which is an asset to the mankind. The combined effect of the propolis and silver nanoparticles and also the antimicrobial activity of propolis nanoparticle coated sutures were extensively studied. So, this synergistic drug can be used as antimicrobial materials for clinical practice and bio medical use. It is proved that propolis silver nanoparticle loaded sutures are a beneficial in preventing the surgical site infections.
Keywords
Propolis
Silver nanoparticles
Sutures
Antimicrobial activity
Surgical site infections
Clinical pathogens
1 Introduction
Propolis is a biomaterial which is naturally found in the honey comb along with the bee’s wax. It is collected and deposited by honey bees from various tree buds. It is a mixture of substances like enzymes, and used mainly for sealing holes in the comb and cleaning the comb cells (Greenaway et al., 1990). Beside these, propolis is called as the chemical weapon against human pathogenic microorganisms (Urushisaki et al., 2011), as well as bee pathogens (Simone-Finstrom et al., 2012). Propolis has a very complex composition of chemicals in the form of bioactive components (Marcucci, 1995).
Propolis is used in various fields such as food and meat preservatives, drugs for cancer, cold sores, diabetes, wound healing, oral diseases etc., It is proved to have anti-inflammatory, antibacterial, antifungal and antiviral properties. It has been used as a traditional medicine and has been reported to prevent against many acquired diseases in humans (Burdock, 1998; Banskota et al., 2001). It has high amount of flavonoid compound called pinocembrin acting as an antifungal agent. These anti-inflammatory and antimicrobial properties contribute in wound healing nature of propolis. Propolis alcoholic extract which can be applied topically was more effective in reducing mast cells during wounds caused by oral surgery thereby reduced inflammation.
Antimicrobial activity of propolis is one of the most expansively investigated studies. Several studies have been conducted in various laboratories on its antimicrobial activity (Fernandes et al., 1997). Its antibacterial activity can be considered on two levels. Firstly, it has direct action on the microorganism, and secondly it stimulates the immune system and thereby activates the natural defense mechanism of the organism. Generally, it is observed that Gram positive bacteria are more sensitive to propolis extract than Gram-negative bacteria. This is because of the structural differences in their plasma membrane. Gram-negative bacteria produces more hydrolytic enzymes that have the ability to break down the bioactive ingredients of propolis (Stepanović et al., 2003).
Nanotechnology refers to the amalgamation of ideas and techniques related to biology and nano technology. The use of nanoparticles has many applications including drug delivery, health monitoring and diagnosis. One of its important goals is therapeutic drug delivery, where drug carrying nanoparticles are introduced into the body and they aid in targeted drug delivery. They have gained a wide range of applications in biomedicine, food, textile industries and personal home care products (Liao et al., 2019).
Though there are various conventional methods like physical and chemical methods, the use of biological methods leads to the production of lipid coated silver nanoparticles that gives a greater solubility, a critical factor for biomedical applications. Various resources such as plants, insects, bacteria, algae and fungi were reported for the silver nanoparticle synthesis. The combination of natural products and silver nanoparticles may synergistically improve their antimicrobial effects and allows its easy incorporation into the matrix thus attaining uniform distribution. Hence, in this study we have investigated the combined properties of two components such as silver nanoparticles and ethanolic extract of propolis (Kischkel et al., 2020).
Surgical site infections are the most common infections that occur during long hospital stay. According to national nosocomial infections surveillances system Staphylococcus aureus, Enterococcus spp. and Escherichia coli are among the most frequently isolated pathogens at surgical sites. Sutures are biomaterials widely used for wound closure because it ligates injured blood vessels and draw divided tissues together in clinical settings. Coating sutures with natural antibiotics which release drug in a controlled way can be useful against these infections. Synergistic effect of propolis and silver nanoparticles would enhance wound healing and this might be a novel approach in developing suture materials. For the above purpose, sutures were treated with propolis extract using a slurry dipping method in previous studies (Augustine and Rajarathinam, 2012).
Hence, the present study made an attempt to design and develop propolis loaded silver nanoparticles and their incorporation on the surface of the selected plain calgut sutures. Evaluation of the coated sutures for their efficiency, safety, and stability were carried out by standard procedures. Antimicrobial activities of the coated suture were analyzed for the bacterial human associated pathogens. The sutures were also characterized by UV spectrometry, SEM and FTIR. The results proved that there was an effective antimicrobial activity against the pathogenic microbes.
2 Materials and methods
2.1 Collection of samples
The propolis sample (stingless bee propolis) was collected from Vibis natural bee farm, Kadachenenthal, Madurai, Tamil Nadu, India.
2.2 Ethanolic extract of propolis
50 g of raw propolis was dissolved in 1 L of solvent mixture containing ethanol and distilled water in the ratio (7:3) and incubated at room temperature (25 °C) in an open shaker for 7–10 days in dark. The dissolved brown color solution was filtered using Whatman number 4 filter paper and then the residues were removed. The filtrate was then evaporated using rotary vacuum evaporator. The resulting brown colored viscous substance was used as ethanolic extract of propolis (EEP) (Wojtyczka et al., 2013).
2.3 Preparation of silver nitrate and synthesis of silver nanoparticles
0.005 g of silver nitrate is weighed approximately and mixed with 50 ml of milli-Q water (1000 ppm). 5 g of ethanolic propolis extract was dissolved in 500 ml of distilled water and NaOH was added to adjust the pH. To this mixture, 500 ml of 50 mM AgNO3 solution was added in an Erlenmeyer flask and was stirred continuously in a magnetic stirrer for 4 h at 120 rpm in room temperature. When stirred continuously at RT for about an hour, the light brown color solution turns into dark brown color which indicates the onset of formation silver nanoparticles. Once the reaction commences the mixture is incubated overnight in a shaker in dark. The next day, it is kept under sunlight for the photo catalytic action to take place that enhances the color and formation of silver nanoparticles. During this process, the silver nitrate is oxidized to silver ions. The reaction was monitored by measuring the absorbance at regular interval of time. The synthesized solution was then centrifuged at 7000 rpm for 20 min at 4 °C. The pellet was collected then frozen followed by lyophilization. This process yields pure synthesized propolis silver nanoparticles which can be further used for characterization and biological assays (Kumar et al., 2016).
2.4 Revival of microorganisms and inoculum preparation
Infectious pathogenic microorganisms which were isolated from wounds were revived from the previously stored glycerol stocks. The cultures used for the study was Gram -positive Staphylococcus aureus, Gram-negative Escherichia coli and fungi Aspergillus niger. For the antimicrobial assay, organisms were sub-cultured once onto fresh nutrient broth following incubation 37 °C overnight, the broth cultures were used for further studies and assays.
2.5 Tests for antimicrobial activity
The agar well diffusion method is widely used to evaluate the antimicrobial activity of the extracts. Muller Hinton agar plates were prepared and sterilized. The bacterial cultures were inoculated by spreading the microbial inoculum over the agar surface. Sterile well punctures were used to punch wells of 5 mm diameter and were impregnated with 100 µl of different concentrations of nanoparticles (100 mg, 75 mg, 50 mg, 25 mg and 12.5 mg). Equal volume of sterile distilled water was used as control. The agar plates were incubated at 37 °C for 24 h and the zone of clearance was measured for the inhibition of the bacterial growth (Shubharani et al., 2019).
2.6 Antifungal activity
Antifungal activity of propolis – silver nanoparticles (AgNPs) against Aspergillus flavus was performed by disc diffusion assay. About 100 μl of the fungal culture was uniformly spread over the potato dextrose agar plates. Then, 10 μl of varied concentrations of propolis – silver nanoparticles (AgNPs) was added on the wells. Equal volume of sterile distilled water was used as control. The plates were then incubated at 28 °C for 2–3 days. The zone sizes were then measured and noted to find the maximum zone of inhibition (Huang et al., 2020).
2.7 Coating of propolis – silver nanoparticles (AgNPs) on surgical sutures by immobilization
The propolis silver nanoparticles were coated on surgical sutures by the slurry dipping technique by using sodium alginate as an immobilizing agent. 10 ml sodium alginate (2% w/v) were prepared and 10 ml sodium chloride (0.1% w/v) was mixed under constant stirring in a magnetic stirrer at room temperature for 8 h. The propolis – silver nanoparticles (AgNPs) (100 mg/ml) were added and kept for 30 min under stirring for the formation of alginate-propolis silver nanoparticles colloid.
Sutures (Cal Gut, plain) were purchased from local surgical then cut to 2 cm in length and immersed in the prepared mixture solution for 10 min, followed by air drying for 5 min. This process was repeated 10 times for proper coating of sutures. After coating, the sutures were immersed in 4% calcium chloride solution for about 30 min to ensure the complete cross linkage and was washed with double distilled water and kept for further use (Augustine and Rajarathinam, 2012).
2.8 Characterization of the coated sutures
The propolis silver nanoparticles were coated on surgical sutures and were characterized by UV–Visible spectroscopy analysis using a UV – Vis spectrophotometer (Thermoscientific Genesys 50), Fourier transform infrared spectroscopy (FTIR) analysis and scanning electron microscopy (SEM) analysis.
2.8.1 UV–Visible spectral analysis
Absorbance was taken for the propolis silver nanoparticle solution in the wavelength of 230–730 nm. Ethanolic extract of propolis was placed as blank solution. The absorbance values were noted and tabulated and represented graphically.
2.8.2 Fourier-Transform Infrared (FTIR) spectrometry analysis
Fourier-Transform Infrared (FTIR) Spectrometry is a standard assay to identify various types of chemical bonds and functional group present in the components of propolis silver nanoparticle and to characterize the nanoparticles. FTIR spectrometry in the range of 4000–800 cm−1 was performed at a resolution of 4 cm−1 using an FTIR spectrophotometer (Shimadzu, Japan).
2.8.3 Scanning electron microscopy (SEM)
The morphological characteristics of the nanoparticles were identified using scanning electron microscopy (SEM). A pinch of the prepared nanoparticle powder was casted on to a carbon-coated copper grid and then analyzed by the microscope.
2.9 Antimicrobial study of the coated suture
The alginate-propolis – silver nanoparticles (AgNPs) coated sutures were checked for their antimicrobial assays for the bacterial and fungal cultures. Fresh overnight cultures inoculums were spread on the nutrient agar plates and used for the assay. The coated sutures of 2 cm in length were then placed on the cultures and incubated at 37 °C overnight. Uncoated sutures were maintained as control and the respective zones of inhibitions were measured (Baygar, 2020).
3 Results and discussion
3.1 Ethanolic extraction of propolis
Propolis was extracted using 70% ethanol. The concentration of the extract was found to be 4.56 ± 0.8 g. Ethanol was used as solvent for propolis extraction because large number of compounds can be extracted using this solvent and higher yield of propolis has been obtained in previous studies by using ethanol. Also, higher percentage of antibacterial activity has been obtained by using ethanolic extraction when compared to other solvents (Da Silva et al., 2006; Baltrušaitytė et al., 2007).
3.2 Silver nanoparticle synthesis using ethanolic propolis extract and UV–Visible spectrophotometry analysis
Synthesis of silver nanoparticles was confirmed by the change of color from yellow to dark brown color (Fig. 1). As a confirmation of this synthesis process a stringent absorption peak was obtained at 430 nm. This peak depicted the characteristics of synthesized silver nanoparticles which are produced by change in its surface plasmon resonance. As discussed by previous reports silver nitrate is reduced to elemental silver by using reductase as a catalyst result in the formation of dark brown color due to change in plasmon resonance of silver nitrate (Augustine and Rajarathinam, 2012; Borase et al., 2014; Kothai and Jayanthi, 2015a,b). Thus, the change of color from yellow to dark brown clearly indicates the synthesis of silver nanoparticles in this study.

- Synthesis of silver nanoparticles using propolis extract (AgNPs). The color change from yellow to dark brown between the initial and final stage of synthesis.
3.3 Subculturing of clinical pathogens
The collected samples were inoculated overnight and next day, a loop full of culture were streaked on specific media E. coli in MacConkey agar, Staphylococcus aureus in Baird Parker agar and Aspergillus niger in potato dextrose agar. The plates were incubated at 37 °C overnight. Lactose fermenting colonies were observed in the MacConkey agar medium indicated that it is E. coli. black colonies with lecithinase activity were obtained in Baird parker agar medium that indicated it is Staphylococcus aureus. Aspergillus niger was grown on potato dextrose agar medium.
3.4 Antimicrobial activity of propolis
Significant differences were observed in zone of clearance for the two different bacteria with respect to various concentrations for propolis extract (Table 1, Fig. 2, Fig. 3 and Fig. 4). This may be due to the differences in chemical composition of propolis which is influenced by different regions and temperature zones from where the propolis is collected (Alencar et al., 2007). These differences may also be due to seasonal nature and seasonal nature as discussed in a previous study by Bankova et al. (1998). Another important factor is that the chemical constituents of propolis increases the bacterial membrane permeability and also inhibit the mobility and growth of bacteria (Mirzoeva et al., 1997). Among the four micro-organisms tested, Staphylococcus aureus showed higher sensitivity to propolis nanoparticle extract when compared to other bacteria and fungi. The structural difference in the cell wall bacteria results in the more sensitivity of Gram-positive bacteria (Popova et al., 2007). Besides these factors, the extraction methods, osmotic effect and origin of the matrix are also responsible for the differences in zone of inhibition (Machado et al., 2016).
| Concentration of propolis silver nanoparticles (mg/ml) | Zone of Clearance (mm) | ||
|---|---|---|---|
| S. aureus | E. coli | A. niger | |
| 100 | 1.8 ± 0.01 | 1.2 ± 0.03 | 1.0 ± 0.06 |
| 75 | 1.5 ± 0.02 | 1.0 ± 0.04 | 0.8 ± 0.03 |
| 50 | 1.4 ± 0.01 | 0.8 ± 0.02 | 0.7 ± 0.03 |
| 25 | 1.2 ± 0.05 | 0.8 ± 0.06 | 0.7 ± 0.02 |
| 12.5 | 1.2 ± 0.07 | 0.1 ± 0.08 | 0.5 ± 0.06 |
| Silver nitrate (Control) | 0.8 ± 0.03 | 0.6 ± 0.01 | 0.4 ± 0.01 |
| Distilled water (Control) | 0.0 | 0.0 | 0.0 |
| Propolis Extract (Control) | 0.4 ± 0.03 | 0.4 ± 0.04 | 0.4 ± 0.06 |
Values are depicted as average ± standard deviation.
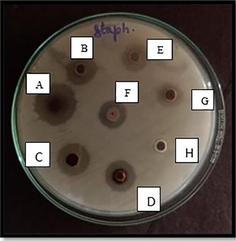
- Antimicrobial Well diffusion method for Staphylococcus aureus (Muller Hinton Agar) in various concentrations. Well A- 100 mg/ml, B- 75 mg/ml, C- 50 mg/ml, D- 25 mg/ml, E-12.5 mg/ml, F- Propolis extract, G- Silver nitrate, H- Distilled water.

- Antimicrobial Well diffusion method for E.coli (Muller Hinton Agar) in various concentrations. Well A- 100 mg/ml, B- 75 mg/ml, C- 50 mg/ml, D- 25 mg/ml, E-12.5 mg/ml, F- Propolis extract, G- Silver nitrate, H- Distilled water.
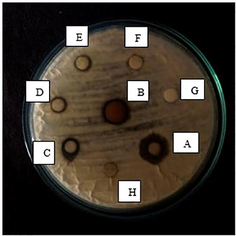
- Antimicrobial Well diffusion method for Aspergillus niger (Potato dextrose agar) in various concentrations. Well A- 100 mg/ml, B- 75 mg/ml, C- 50 mg/ml, D- 25 mg/ml, E-12.5 mg/ml, F- Propolis extract, G- Silver nitrate, H- Distilled water.
3.5 Coating of propolis silver nanoparticles on surgical sutures and analysis of its antimicrobial activity
The sutures were coated with the synthesized propolis silver nanoparticles using the slurry dipping technique using sodium alginate as immobilization agent because alginate acts as a mucoadhesive agent and highly water soluble. The coated sutures were studied for their anti-microbial activity against E. coli and Staphylococcus aureus. A clear zone of clearance was found around the coated sutures where there was no zone obtained by uncoated sutures (Fig. 5). This indicated an effective antimicrobial activity. E. coli and S. aureus are multi drug resistant bacteria that cause many human infections (Wisplinghoff et al., 2004). Enhanced bacteriostatic activity of propolis silver nanoparticle coated sutures was clearly evident from clear zone of clearance around the coated sutures. Other methods like X ray lithography, electro plating, plasma spray and electrodeposition were also used for coating of the nanoparticles on different surfaces.
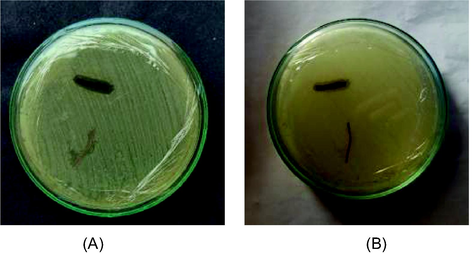
- Antimicrobial activity of surgical sutures. (A)- Zone against Escherichia coli, (B). Zone against Staphylococcus aureus grown in Muller Hinton Agar.
3.6 Fourier-Transform Infrared (FTIR) analysis of the coated sutures
FTIR spectra was taken to get clear idea about the nature of biological molecules present in the sutures (Kothai and Jayanthi, 2015a,b). For coated sutures, the various absorbance bands were obtained which showed the stretching vibrations for chemical groups like C–N, N–H, C–O and C⚌O. Also, many functional groups like aliphatic primary amine, oxime, aliphatic ether, secondary alcohol group and alkane whereas the uncoated suture showed very few bands and functional group which clearly depicted that there was absence of the propolis silver nanoparticles in the uncoated suture (Table 2 and 3).
| Wave number | Stretch | Functional group |
|---|---|---|
| 3364 | N–H stretching | Aliphatic primary amine |
| 2947 | N–H stretching | Amine salts |
| 1680 | C–N stretching | Oxime |
| 1142 | C–O stretching | Aliphatic ether |
| 1089 | C–O stretching | Secondary alcohol |
| 840 | C⚌C stretching | Alkane |
| Wave number | Stretch | Functional group |
|---|---|---|
| 3278 | C–H Stretching | Alkyne |
| 2958 | C–H Stretching | Alkane |
| 1650 | C⚌O Stretching | δ lactose |
| 1232 | C–N Stretching | Amine |
3.7 Scanning electron microscopic (SEM) analysis of the sutures
The structural characteristics of the synthesized propolis silver nanoparticles were analyzed by the obtained SEM images and it showed that all the nanoparticles were of same shape and size. They are spherical in shape with approximately 20 nm in size. Also, the surface of coated sutures was rough whereas the uncoated sutures had a smooth even surface (Fig. 6). These results confirmed the presence of propolis nanoparticle coated sutures. The results are in accordance with the previous reports by Mannan and Pawar (2014).
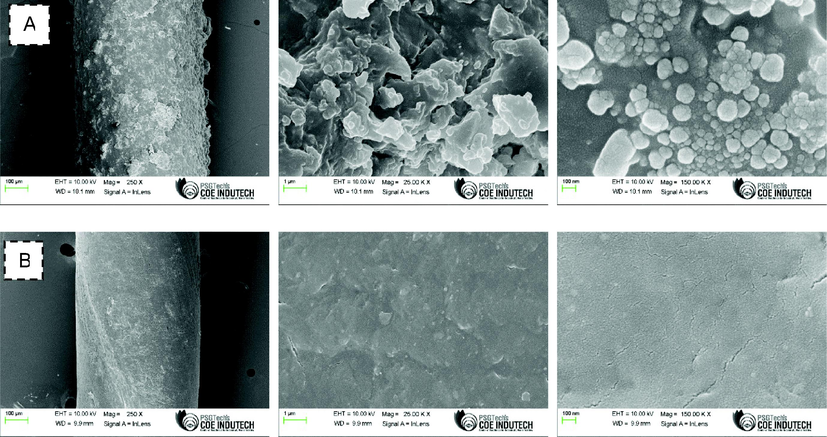
- Scanning electron microscopy (SEM) analysis. A: coated suture; B: Uncoated suture. Images were captured under various magnifications.
4 Conclusions
In conclusion, propolis remains a wonder product which is obtained naturally and acts as a boon to the mankind in terms of production of bioactive compounds and higher antimicrobial properties. Propolis loaded sutures are beneficial in enhancing the wound healing and thereby preventing the surgical site infections. Along with the synergistic effect of a silver nanoparticles, propolis can enhance the bioactive properties of the coated sutures. Thus, it could be novel approach towards the use of biological materials as antimicrobial agents in clinical practice for surgical site infections.
Acknowledgements
The authors acknowledge Dr. G. R. Damodaran college of science, Coimbatore, Tamil Nadu, India for providing laboratory facilities to carry out the research work. The authors would like to acknowledge Sree Balaji Dental College and Hospital, Pallikarani,Chennai,India for providing financial support and facilities for the completion of this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical composition and biological activity of a new type of Brazilian propolis: red propolis. J. Ethnopharmacol.. 2007;113(2):278-283.
- [Google Scholar]
- Augustine, R., Rajarathinam, K., 2012. Synthesis and characterization of silver nanoparticles and its immobilization on alginate coated sutures for the prevention of surgical wound infections and the in vitro release studies.
- Antibacterial Activity of Honey and Beebread of Different Origin Against S. aureus and S. epidermidis. Food Technol. Biotechnol.. 2007;45(2)
- [Google Scholar]
- Seasonal variations of the chemical composition of Brazilian propolis. Apidologie. 1998;29(4):361-367.
- [Google Scholar]
- Recent progress in pharmacological research of propolis. Phytother. Res.. 2001;15(7):561-571.
- [Google Scholar]
- Characterization of silk sutures coated with propolis and biogenic silver nanoparticles (AgNPs); an eco-friendly solution with wound healing potential against surgical site infections (SSIs) Turk. J. Med. Sci.. 2020;50(1):258-266.
- [Google Scholar]
- Plant extract: a promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol.. 2014;173(1):1-29.
- [Google Scholar]
- Review of the biological properties and toxicity of bee propolis (propolis) Food Chem. Toxicol.. 1998;36(4):347-363.
- [Google Scholar]
- Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities. Food Chem.. 2006;99(3):431-435.
- [Google Scholar]
- Population analysis of susceptibility to propolis in reference strains of Staphylococcus aureus and Escherichia coli. J. Venomous Anim. Toxins. 1997;3:287-294.
- [Google Scholar]
- The composition and plant origins of propolis: a report of work at Oxford. Bee world. 1990;71(3):107-118.
- [Google Scholar]
- Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria turcica. J. Nanomater.. 2020;2020:1-7.
- [Google Scholar]
- Silver nanoparticles stabilized with propolis show reduced toxicity and potential activity against fungal infections. Future Microbiol.. 2020;15(7):521-539.
- [Google Scholar]
- A study on propolis of stingless bees reared from the most commercial hub of Chennai, Tamilnadu, India. Int. Res. J. Environ. Sci.. 2015;4(7):39-47.
- [Google Scholar]
- Environmental impact on stingless bee propolis (Tetragonula iridipennis) reared from two different regions of Tamilnadu-a comparative study. Int. J. ChemTech Res.. 2015;7(7):3081-3088.
- [Google Scholar]
- Solvent removal induces a reversible β-to-α switch in oligomeric Aβ peptide. J. Mol. Biol.. 2016;428(2):268-273.
- [Google Scholar]
- Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci.. 2019;20(2):449.
- [Google Scholar]
- Comparative Study of Chemical Composition and Biological Activity of Yellow, Green, Brown, and Red Brazilian Propolis. Evid.-Based Complementary Altern. Med.. 2016;2016:1-11.
- [Google Scholar]
- Anti-infective coating of gentamicin sulphate encapsulated PEG/PVA/chitosan for prevention of biofilm formation. Int. J. Pharm. Pharm. Sci.. 2014;6:571-576.
- [Google Scholar]
- Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26(2):83-99.
- [Google Scholar]
- Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol. Res.. 1997;152(3):239-246.
- [Google Scholar]
- Chemical characteristics of poplar type propolis of different geographic origin. Apidologie. 2007;38(3):306-311.
- [Google Scholar]
- Biosynthesis and characterization, antioxidant and antimicrobial activities of selenium nanoparticles from ethanol extract of Bee Propolis. J. Nanomed. Nanotechnol.. 2019;10(522):2.
- [Google Scholar]
- Increased resin collection after parasite challenge: a case of self-medication in honey bees? PLoS ONE. 2012;7(3)
- [Google Scholar]
- In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res.. 2003;158(4):353-357.
- [Google Scholar]
- Caffeoylquinic Acids Are Major Constituents with Potent Anti-Influenza Effects in Brazilian Green Propolis Water Extract. Evid.-Based Complementary Altern. Med.. 2011;2011:1-7.
- [Google Scholar]
- Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules. 2013;18(8):9623-9640.
- [Google Scholar]







