Translate this page into:
Fabrication and biocompatibility of neem/chitosan coated silk sutures for infection control and wound healing
⁎Corresponding author. muhammad.shoaib@scme.nust.edu.pk (Muhammad Shoaib Butt),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The surgical site infections cost 0.5 % of the annual budget of the hospitals. Therefore, present research aimed to develop and evaluate antibacterial Neem/chitosan-coated silk braided sutures to reduce the risk of hospital-acquired surgical site infections. The silk braided sutures were coated by dip-coating technique by using 5, 10, 15 and 20 % concentrations of ethanolic Neem extract and chitosan. Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and tensile strength measurements were used to examine the surface morphology, chemical composition, and tensile strength of the coated sutures. At a 20 % concentration, the sutures showed strong antibacterial action against Escherichia coli and Staphylococcus aureus, with inhibition zones of 15 and 19 mm, respectively. Additionally, the sutures demonstrated low cytotoxicity in the MTT assay, good radical scavenging activity, and hemolytic activity of less than 2 % at all concentrations. The sutures for the in vivo investigation were coated at a concentration of 20 % since this concentration showed the best antibacterial action. The rats were incised on both sides of their spine, with the left side stitched with uncoated sutures, and the right side stitched with coated sutures. The wound was inoculated with E. coli and S. aureus and examined for 7 days. The results indicated complete healing of the wound at the site where the coated sutures were applied. On other hand, wound with uncoated suture was still not completely healed and revealed signs of infection. The in vivo study revealed the efficacy of Neem/chitosan-coated sutures in wound healing and infection control.
Keywords
Azadirachta indica
Chitosan
Coated silk sutures
Surgical site infections
Antimicrobial coating
- SSI
-
Surgical Site Infections
- E. coli
-
Escherichia coli
- S. aureus
-
Staphylococcus aureus
- FTIR
-
Fourier Transform Infrared Spectroscopy
- SEM
-
Scanning Electron Microscopy
- PBS
-
Phosphate-buffered saline
- DPPH
-
2,2-Diphenyl-1-picrylhydrazyl
- DMEM
-
Dulbecco’s Modified Eagle Medium
- MTT
-
3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide
Abbreviations
1 Introduction
Surgical site infections (SSIs) are commonly encountered complications in the postoperative setting, with an incidence rate of up to 5 % across all surgical procedures (Cheadle, 2006). Most commercially available sutures lack antimicrobial properties, allowing skin pathogens to enter wounds through capillary action, proliferate, and form biofilms that are difficult to manage (Kathju et al., 2009). The primary bacterial pathogens causing SSIs are Escherichia coli; Staphylococcus aureus, Enterococci,eem and Pseudomonas aeruginosa (Ghafoor, 2016). Currently, all commercially available antimicrobial sutures are coated with triclosan, including Vicryl, Monocryl, and polydioxanone suture (Obermeier, 2014). Triclosan's harmful effects and metal ions' uncertain tissue compatibility highlight the necessity for biocompatible, non-hazardous suture materials that exhibit strong antibacterial activity. (Prabha, 2021).
Considering the aforementioned concerns, several studies have reported on the progress being made in developing chitosan-coated sutures and chitosan-herbal extract coatings (Antunes, 2021; Sudha et al., 2017). Herbal extracts, including aloe vera and curcumin, have previously been employed for the fabrication of antimicrobial sutures in conjunction with an additional polymeric coating agent (Ravishankar et al., 2019; Zhang, 2023). Chitosan is derived from deacetylated chitin in alkaline conditions. Chitosan's antibacterial qualities rely on its molecular weight and degree of deacetylation (Elsabee and Abdou, 013). The potential of chitosan as an antibacterial agent is driving its increasing use in the field of biomaterials engineering (Viju and Thilagavathi).
Azadirachta Indica commonly known as Neem has been used for generations in Ayurvedic and indigenous medicine due to its therapeutic properties, including antimicrobial, anti-inflammatory, antioxidant, anticancer, and immunomodulatory effects (Altayb et al., 2022). These benefits are attributed to various bioactive phyto-constituents (Al-Sarraj, 2021). Neem extract contains numerous antimicrobial compounds such as alkaloids, flavonoids, phenolic compounds, triterpenoids, nimbin, and azadirachtin (Hassan, 2020). Neem has demonstrated strong antibacterial action in earlier studies, with inhibition zones of 12 against S. aureus and 11 mm against E. coli (Jamge et al., 2014). According to another study, Neem and chitosan have combined antibacterial effectiveness against S. aureus and E. coli, with inhibition zones measuring 12.5 mm and 19 mm, respectively (Mohammad Ahamed). Hydrogels and nanofibers loaded with Neem extract have been created for the controlled release of bioactive substances in drug delivery and wound healing applications. Research emphasizes the promising potential of Neem-based formulations in wound care and sustained drug delivery (Hameed, 2022).
To address the rising concern of surgical site infections, developing antimicrobial sutures is essential. These sutures play a crucial role in preventing microorganism proliferation at the surgical site, thereby reducing post-operative infection risk. The sutures must be both biocompatible and antimicrobial. This study uses ethanolic Neem extract combined with chitosan to coat silk braided sutures via dip coating. Scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) were used to assess the effectiveness of the coating. Additionally, studies were conducted in vitro and in vivo to assess the effectiveness of sutures coated with Neem/chitosan.
2 Materials and methods
2.1 Preparation of Neem extract
Azadirachta indica (Neem) leaves were procured from a local nursery in Islamabad, the leaves were washed thoroughly and left to dry. Once the leaves were completely dry, they were grinded into fine powder using an electric blender. Ethanol was used as a solvent for Neem extraction, 4 g of Neem was added into 100 mL of 100 % ethanol. The blend was placed in the shaking water bath for about 30 min at 65 °C, followed by centrifugation (FastGene CAT. NO. NG003) at 5000 rpm for 10 min according to a previously reported study with slight modification (Kumar et al., 2023; X. min Liu, 2022). After filtering the supernatant through Whatman No. 1 filter paper, the filtrate was obtained. The filtrate was placed in a vacuum oven to remove ethanol and then stored at 4 °C. A fresh batch of plant extract was made for the subsequent assays and characterizations.
2.2 Preparation of coating material
Chitosan (Macklin Biochem, China) with degree of acetylation ≥ 95 % was prepared by dissolving 1 g in 1 % acetic acid. The mixture was magnetically stirred at ambient temperature and 1200 rpm until chitosan was dissolved completely. Neem extract was added in aforementioned solution in 5, 10, 15 and 20 % concentrations. The mixture was stirred until a homogenized solution was obtained.
2.3 Dip coating of sutures
Non-biodegradable silk sutures from TRUSILK China, were coated with a Neem/chitosan composite solution using a dip coating machine (PTL-MMB01 OK-M210, China). The solution was placed in a beaker, and the suture was wound on a frame and immersed at an optimal speed for 15 min. The dip coater's temperature was set at 37 °C to dry the sutures. This process was repeated for all concentrations (5, 10, 15, and 20 %). Once dried, the sutures were cut into 1 cm pieces under aseptic conditions inside a laminar flow hood for subsequent experiments.
2.4 Characterizations of Neem/chitosan coated sutures
2.4.1 FTIR analysis
FTIR of Neem/chitosan coated sutures was carried out to study the incidence of functional groups and type of interactions among Neem and chitosan. FTIR of Neem/chitosan composite was performed using Perkin-Elmer Spectrum-100-Spectrophotometer (United States) with wavelength ranges from 4000 cm−1 to 400 cm−1.
2.4.2 Morphological studies
The surface morphology of Neem/chitosan coated sutures was determined by scanning electron microscope (JSM-6490A JEOL, Japan). The sutures were subjected to a process of gold–palladium sputter coating using a sputter coating machine (JEOL JFC-1500, Japan) to improve the conductivity of the samples.
2.4.3 Tensile strength
The tensile strength of the suture helps practitioners form knots. Weak sutures under high knotting force may break easily. It is crucial to know the tensile strength of the coated suture. The Shimadzu AUTOGRAPH AG-X plus assessed mechanical attributes like tensile strength. A 75 cm coated suture was tested between clamps 15 cm apart. The computer measured the tensile strength at the suture break.
2.5 Neem release profile of Neem/chitosan coated sutures
The Neem release profile from Neem/chitosan sutures was evaluated in a phosphate-buffered saline (PBS) solution. At 37 °C, the coated sutures were cut to a length of 3 cm and immersed in 5 mL of PBS (pH 7.4). Subsequently, the residual PBS was removed and replaced with fresh 3 mL of PBS at various time intervals (24, 48, 72, 96, 120, and 144 h). The drained PBS solutions were assessed for the Neem release profile using a UV–VIS spectrophotometer (JENWAY 7315) by determining the absorbance at 380 nm (Altundag et al., 2020). The release study was performed in triplicate, and an average value was computed. The drained PBS solutions were assessed for the Neem release profile using a UV–VIS spectrophotometer (JENWAY 7315) by determining the absorbance at 380 nm (Hashim et al., 2021). The release experiments were performed in triplicate, and an average value was computed.
2.6 Antimicrobial susceptibility test for Neem/chitosan coated sutures
The Disc Diffusion method was used to assess the antibacterial activity of Neem/chitosan-coated surgical sutures. By dissolving 38.2 g of Mueller-Hinton Agar (MHA) provided by Oxoid, Malaysia in 1000 mL of distilled water and autoclaving the resulting combination, sterile MHA was prepared. The MHA was then poured into petri plates and incubated at 37 °C for 24 h. After confirming no unwanted bacterial growth, the plates were inoculated with 0.1 mL of bacterial inoculum from pre-cultured E. coli and S. aureus strains. Sutures, cut into 1 cm pieces, were placed on the inoculated petri dishes, divided into six sections: four with sutures dip-coated with 5 %, 10 %, 15 %, and 20 % Neem/chitosan composite, ciprofloxacin and sterile Whatman filter paper as the positive and negative control respectively. The inhibition zones were measured after a 24 h of incubation. The study was conducted in triplicate, and average results were reported.
2.7 Hemolytic assay of Neem/chitosan composite
The hemolysis assay evaluated the hemocompatibility of Neem/chitosan coated sutures. PBS solution was used to submerge the sutures, and then fresh human blood was extracted and centrifuged. A mixture of 0.5 mL blood and 1 mL PBS was used to isolate RBCs. Then, varied quantities of Neem/chitosan-coated sutures were added to the PBS suspension. PBS and 0.1 % Triton X-100 were utilized as the positive and negative controls, respectively. After two hours of incubation, the tubes were centrifuged for ten minutes at 6000 rpm. Triplicates of the supernatant were added to a 96-well plate, and a UV microplate reader (Labtron LMPR-B10) was used to quantify absorbance at 540 nm. Eq. (1) was used to compute each sample's hemolytic rate.
2.8 Antioxidant activity of Neem/chitosan composite
The ability of the Neem/chitosan composite to scavenge 2,2-Diphenyl-1-picrylhydrazyl was evaluated using a modified protocol. (Altundag et al., 2020). One mg of DPPH was dissolved in 25 mL of methanol to form a DPPH solution. Neem/chitosan composites were prepared at different concentrations, and samples were then placed into a 96-well plate. Using a microplate reader, the absorbance of the plate was assessed following a half-hour dark incubation period. Ascorbic acid and methanol were employed as the positive and negative controls, respectively.. The results were calculated using Equation (2).
2.9 Cytocompatibility of Neem/chitosan coated sutures
The study evaluated the cytotoxicity of coated sutures on mouse 3 T3 fibroblasts in DMEM. The cells were grown in 10 % fetal bovine serum-containing Dulbecco's Modified Eagle Medium (DMEM) at 37 °C. To form eluates, coated sutures were put in tubes with 1.5 mL DMEM and cultured for 24 h at 37 °C at 100 rpm. The eluates were added to the cell culture media after a 24-hour period. Following a 48-hour period, each well received 20 µL of MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide), which induced the production of formazan salt by the action of mitochondrial dehydrogenase. Comparing metabolic activity to 3 T3 cells without sutures was done. Equation (3) was utilized to determine cell viability.
2.10 In vivo investigation of Neem/chitosan coated sutures
A total of 6 Sprague Dawley rats (100–150 g) were used to examine the antimicrobial and wound healing potential of Neem/chitosan-coated sutures. Three rats were allocated for evaluation against E. coli and the other three for S. aureus procured from animal house of the university. The rats were acclimatized for 1 week under a 12-hour light/dark cycle with unrestricted food and water access. The rats were put on fasting 8 h before surgery. Anesthesia was induced using ketamine (80 mg/kg) and xylazine (10 mg/kg). After hair removal from the dorsal region, a 1 cm incision was made on both sides of the spine. One side was stitched with a coated suture, and the other with an uncoated suture as a control. The wounds were inoculated with E. coli and S. aureus 24 h’ post-surgery. To compare wound healing and infection control, daily images of the wound area were taken for 7 days. Among the factors evaluated were the wound's color, look, and signs of infection, as well as regular measures of its length, width, and area. The analysis, which was based on macroscopic observations and photographs, concentrated on the variations in wound healing and infection control between the left (uncoated sutures) and right (coated sutures) sides.
3 Results and discussion
3.1 FTIR
Powdered Neem leaves, pure chitosan, and Neem/chitosan composites at varying concentrations (5 %, 10 %, 15 %, 20 %) were mixed with potassium bromide and compressed into thin pellets for FTIR analysis. A range of 4000 cm−1 to 400 cm−1 was used to record the FTIR spectra. OH and N–H stretching vibrations at 3339 cm−1 and C–H stretching vibrations at 2876 cm−1 are the two peaks in the chitosan spectra shown in Fig. 1(Wang and Wang, 2011). Neem extract spectra reveal a peak at 3399 cm−1, indicating the presence of intermolecular hydrogen bonding and hydroxyl OH groups in Neem leaves (Raja and Devarajan, 2023; Bhaskara Rao and Murugan, 2023). Identification of aliphatic C–C bonds and C-O bond stretching in powdered Neem leaves is made at 1252 cm−1 and 1034 cm−1, respectively. All concentrations of the Neem/chitosan combination show the absorption band at 1400–1420 cm−1, which denotes symmetric stretching of ja functional group. C–C double bond stretching is indicated by a large peak at 1600 cm−1, whereas the presence of C=O bonds is indicated by a peak at 1700 cm−1. The occurrence of difference in individual spectra and in Neem/chitosan composite shows active state of functional groups in these molecules. This can be further correlated with antimicrobial properties of 20 % Neem with chitosan revealed the best antimicrobial properties which confirms that the active components are still active and are enhanced with increasing concentrations (Ali et al., 2019).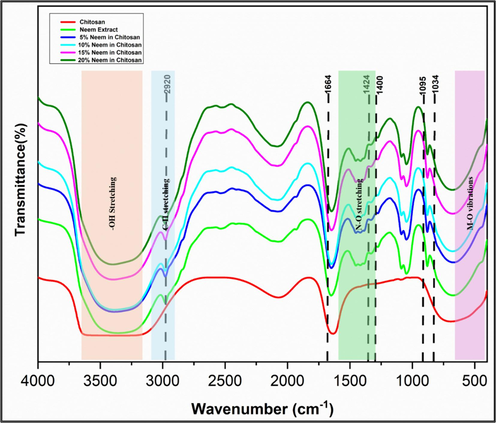
The graph shows the FTIR spectra of Chitosan, ethanolic Neem extract and Neem/chitosan composite with varying concentrations (5%, 10%, 15%, 20%).
3.2 Scanning electron microscopy
As indicated in Fig. 2, SEM results show the difference between uncoated (Fig. 2a) and coated (Fig. 2b) sutures. In contrast to the smooth and uniform surface observed along the entirety of the Neem/chitosan coated sutures, the surface of the uncoated suture exhibited an irregular pattern.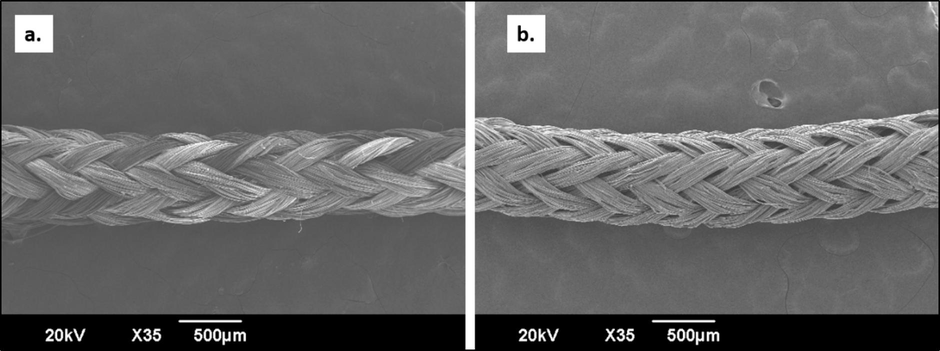
SEM images of uncoated (a) and coated (b) silk braided sutures.
3.3 Tensile strength
For in vivo study, the suture coated with 20 % Neem/chitosan had its tensile strength evaluated. Under a load of 47 N±2, statistical analysis revealed that the uncoated silk braided suture's mean tensile strength was marginally less than the coated suture's. This suggests Neem/chitosan-coated sutures possess superior tensile strength compared to uncoated ones, due to enhanced fiber adhesion from chitosan, increasing resistance to axial load (Fig. 3). Sutures with high friction coefficients struggle to penetrate biological tissue, causing more damage, making it important to understand the frictional properties of suture materials (Viju and Thilagavathi). Results show treated silk braided sutures have a lower friction coefficient than untreated ones, aiding knot formation. The knot area in treated sutures is resilient and less prone to weakening compared to untreated sutures.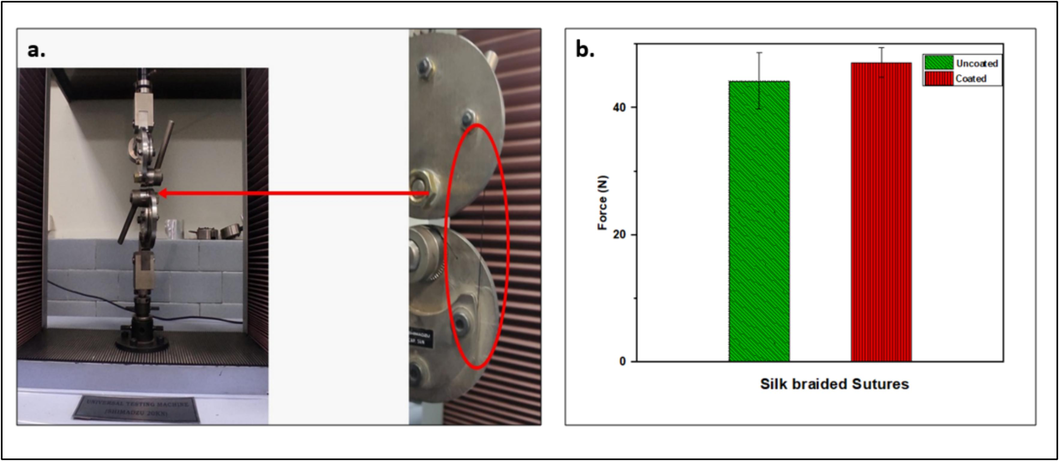
Tensile strength of Neem/chitosan coated suture.
3.4 Neem release profile
The results of Neem release from different concentrations (5, 10, 15 and 20 %) of Neem/chitosan coated sutures are illustrated in Fig. 4. As depicted in the graph, the sutures demonstrated an initial rapid release within the first 25 h, from 25 h onwards till 90 h the rate of Neem release became slower, which was succeeded by a nearly linear and prolonged release pattern over the course of 144 h. The initial burst can be attributed to the rapid release of Neem molecules bound to the surface of the suture (Fu and Kao, 2010). Chitosan has an overall positive charge because of the presence of amine group so it can adhere very well on the negatively charged biological surfaces. The inclusion of bioadhesive polymers such as chitosan extends the duration in which the drug-loaded system remains in place and allows for targeted drug delivery which explains the constant Neem release.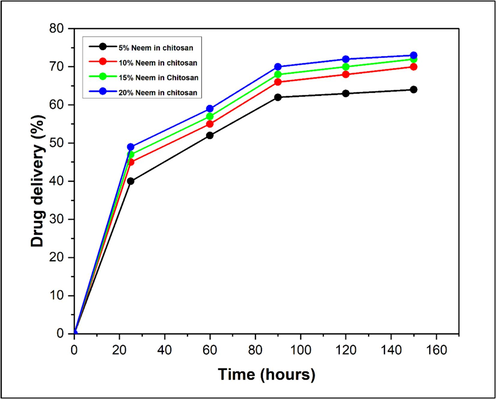
Neem release profile of Neem/chitosan coated suture.
3.5 Antimicrobial activity
The zone of inhibition of Neem against E. coli and S. aureus was measured in millimeters. Results show that Neem/chitosan-coated sutures exhibit significant antibacterial activity against these strains (Fig. 5). Sutures with varying Neem concentrations displayed different antimicrobial rates, with the highest activity at 20 % concentration against S. aureus, showing a ± 19 mm inhibition zone (Fig. 6). E. coli was less susceptible than S. aureus but still showed a notable ± 15 mm inhibition zone at 20 % concentration (Fig. 5a). The inhibition zone increased with higher coating concentrations due to the greater release of Neem particles. Previous studies indicated Neem's considerable antibacterial activity, with zones of ± 11 mm against E. coli and ± 12 mm against S. aureus (Jamge et al., 2014). Another study showed combined Neem and chitosan activity with inhibition zones of ± 12.5 mm against E. coli and ± 19 mm against S. aureus (Mohammad Ahamed).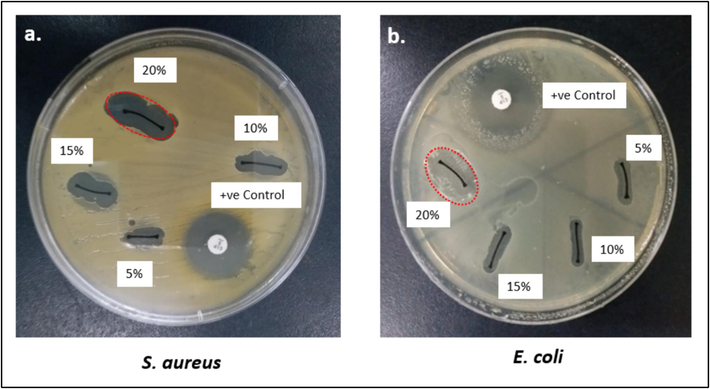
Antimicrobial susceptibility assay of Neem/chitosan sutures at different concentrations (5, 10, 15 and 20%).
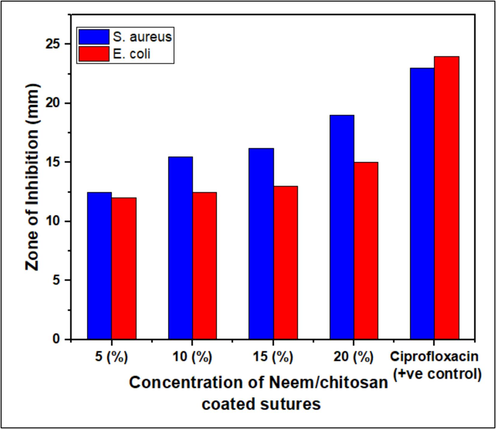
Anti-bacterial activity of Neem/chitosan sutures at different concentrations (5%, 10%, 15% and 20%).
3.6 Hemolytic activity
The hemolytic assay outcomes directly indicate the hemocompatibility of Neem/chitosan-coated sutures. An in vitro analysi of the hemolytic activity of ethanolic Neem extract in chitosan-coated silk braided sutures was conducted on human red blood cells. Using 0.1 % Triton X-100 as the positive control, a 100 % hemolytic activity was attained, while 0 % hemolysis was observed with PBS as the negative control. The Neem/chitosan-coated sutures exhibited minimal hemolysis towards red blood cells. The results show a concentration-dependent increase in hemolysis, with the highest hemolysis rate of 1.9 % at a 20 % concentration, as shown in Fig. 7. This increase can be attributed to the greater Neem release at higher concentrations. (Pan et al., 2006). A previous study found that ethanolic extract from Azadirachta indica caused 4.52 % hemolysis (Hussain, 2023), likely due to the use of pure ethanolic extract in that study. In contrast, the present study used various Neem extract concentrations, peaking at 20 %. Hemolysis below 2 % indicates that Neem/chitosan-coated sutures are safe for surgical site stitching.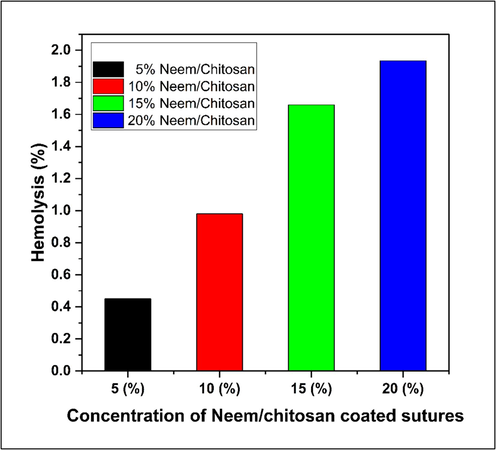
Hemolytic activity of Neem/chitosan coated sutures.
3.7 Antioxidant activity
Free radicals are a primary cause of many diseases, and mitigating their effects is crucial to disease prevention. Antioxidants can stabilize or deactivate free radicals before they attack cells (Alzohairy, 2016). DPPH (1,1-diphenyl-2-picrylhydrazyl) is a stable free radical that accepts an electron, forming a stable diamagnetic molecule (Baliyan, et al., 2022). The Neem/chitosan composite neutralizes DPPH radicals by donating hydrogen. The antioxidant activity of the Neem/chitosan composite was recorded as 18.65, 18.99, 19.5, and 20.2 for concentrations of 5 %, 10 %, 15 %, and 20 %, respectively, as shown in Fig. 8. This indicates a direct correlation between Neem concentration in chitosan and radical scavenging activity, with peak activity at 20 % concentration due to the higher availability of antioxidant particles. Ascorbic acid was used as a positive control. Previous studies have confirmed Neem extract's antioxidant properties. Methanolic Neem leaf extract showed 12.56 % antioxidant activity, while the ethanolic Neem extract varied from 45.03 % at 200 µg/mL to 55.82 % at 1000 µg/mL (Ghimeray et al., 2009; Ahmed et al., 2023). Neem's antioxidant potential is attributed to phenols and flavonoids like Nimbin and Azadirachtin (Sarkar et al., 2021).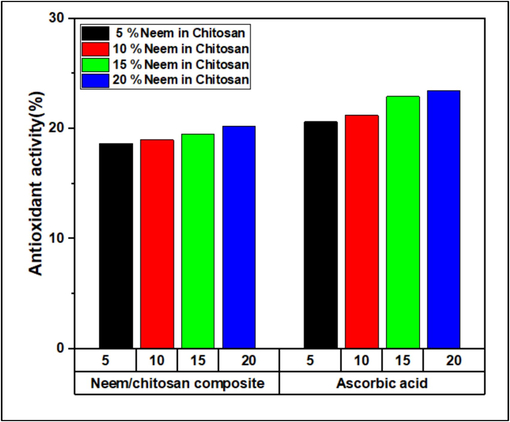
Radical Scavenging activity of Neem/chitosan coated suture and ascorbic acid as positive control.
3.8 Cytocompatibility
Neem/chitosan coated sutures did not elicit a noteworthy cytotoxic response in the 3 T3 fibroblast cells of mice. Following a post-incubation period of 24 h, the viability of the 3 T3 cells displayed percentages of 98, 96, 94.5 and 92 % at concentration 5, 10, 15 and 20 % respectively as depicted in Fig. 9. The control group (without eluate) showed 100 % cell viability. Mammalian cells exhibited above 90 % viability after being incubated with Neem/chitosan sutures, indicating non-toxicity. It is imperative to investigate the cytocompatibility of biomaterials before conducting in vivo study (Kandimalla, 2016). The results of this study demonstrate that the suture materials under test are non-toxic.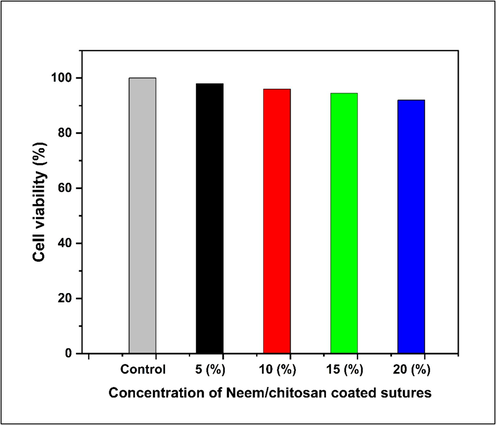
MTT assay showing cell viability of Neem/chitosan coated sutures at various concentrations.
3.9 In vivo evaluation of Neem/chitosan coated sutures
Neem at 20 % concentration in Chitosan was selected to be coated on silk braided sutures. This is because at 20 % concentration of Neem/chitosan coated suture displayed greatest anti-bacterial activity with the highest zone of inhibitions as compared 5, 10 and 15 %, while hemolytic activity was only 1.9 % which is completely safe to use. Maximum antioxidant activity was also seen at 20 %. Fig. 16 shows the wound healing stages of incisions inoculated with S. aureus and E. coli. The left incision was stitched with uncoated suture while the right incision was stitched with Neem/chitosan coated suture. After 24 h, the wounds were inoculated with S. aureus and E. coli. Both sides coated and uncoated showed inflammation after the inoculation of bacteria. To evaluate the signs of infection, erythema (redness) surrounding the wound was noted as a primary indicator of inflammation and potential infection followed by pus formation (Gardner et al., 2001). The wounds were observed for 7 days. The pictures were taken on the 1st; 3 rd and 7th day as illustrated in Fig. 10. Reduced inflammation can be seen on the side stitched with coated suture as compared to the uncoated suture on the 3rd day. On the 7th day, sutures were removed, uncoated side still displayed signs of inflammation, redness and slight pus formation as depicted by the red arrows in Fig. 10, while the coated suture wound was completely healed accompanied by noticeable hair growth at the wound site and an absence of inflammation.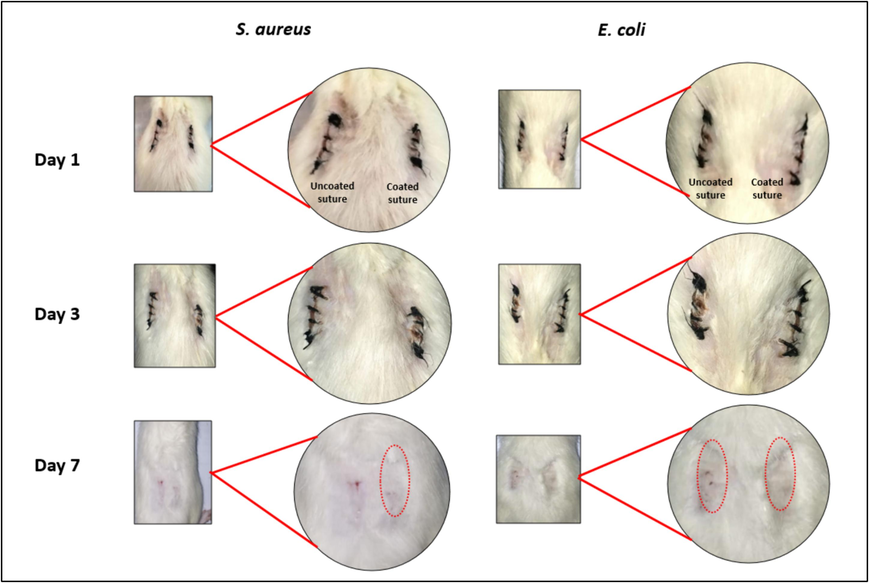
In vivo evaluation of Neem/chitosan coated sutures. The suture on the left side is uncoated and taken as a control and the suture on the right is coated with Neem/chitosan composite. Infection control and increased healing can be seen on the right side (coated sutures) during subsequent days.
4 Conclusion
Neem/chitosan composite was coated on silk braided sutures and assessed for in vivo applications. Various concentrations (5 %, 10 %, 15 %, and 20 %) were analysed to identify the concentration with optimal antimicrobial and antioxidant activities, and low hemolytic activity (<3%). With significant antibacterial potential against E. coli and S. aureus, with inhibition zones of 15 and 19 mm, respectively, and hemolytic activity below 2 %, regarded safe for application, the 20 % ethanolic Neem/chitosan combination was selected for additional research. An in vivo study showed the effectiveness of Neem/chitosan coated sutures in wound healing and infection control. Rats were incised on both sides of the spine, with the left side stitched with uncoated sutures and the right with coated sutures, and wounds were inoculated with E. coli and S. aureus. After 7 days, wounds with coated sutures showed complete healing, whereas wounds with uncoated sutures remained infected. Although promising, additional biocompatibility tests are needed before Neem/chitosan coated sutures can be commercially produced to reduce surgical site infections.
CRediT authorship contribution statement
Urwah Rasheed: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Data curation, Conceptualization. Muhammad Naeem Kiani: Writing – original draft, Visualization, Validation, Methodology, Investigation, Data curation. Muhammad Shoaib Butt: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization. Hina Saeed: Writing – review & editing, Validation. Rumeza Hanif: Writing – review & editing, Resources. Sidra Anwar: Writing – review & editing.
Acknowledgments
This work was supported by the Pakistan Science Foundation (PSF) G-5/2, Islamabad-Pakistan under PSF grant (PSF/Res/C-NUST/Med 521).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed, M., et al., 2023. Studying the antioxidant and the antimicrobial activities of leaf successive extracts compared to the green-chemically synthesized silver nanoparticles and the crude aqueous extract from Azadirachta indica. Process, Page 1644, vol. 11, no. 6, p. 1644, May 2023, doi: 10.3390/PR11061644.
- Antibacterial bi-layered polyvinyl alcohol (PVA)-chitosan blend nanofibrous mat loaded with Azadirachta indica (neem) extract. Int. J. Biol. Macromol.. 2019;138:13-20.
- [CrossRef] [Google Scholar]
- A review on the impacts of Azadirachta indica on multi-drug resistant extended spectrum beta lactamase-positive of Escherichia coli and Klebsiella pneumonia. Adv. Life Sci.. 2021;8(3):228-232.
- [Google Scholar]
- In-vitro and in-silico antibacterial activity of Azadirachta indica (Neem), methanolic extract, and identification of Beta.d-Mannofuranoside as a promising antibacterial agent. BMC Plant Biol.. 2022;22(1):1-14.
- [CrossRef] [Google Scholar]
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus. Turkish J. Biochem.. 2020;45(4):365-372.
- [CrossRef] [Google Scholar]
- Therapeutics role of azadirachta indica (neem) and their active constituents in diseases prevention and treatment. Evid. Based. Complement. Alternat. Med.. 2016;2016
- [CrossRef] [Google Scholar]
- Bioactivity of chitosan-based particles loaded with plant-derived extracts for biomedical applications: emphasis on antimicrobial fiber-based systems. Mar. Drugs. 2021;19(7)
- [CrossRef] [Google Scholar]
- Baliyan, S., et al., 2022. Determination of Antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules vol. 27, no. 4, Feb. 2022, doi: 10.3390/MOLECULES27041326.
- Experimental investigation of drying neem (Azadirachta indica) in an evacuated tube solar dryer: performance, drying kinetics and characterization. SoEn. 2023;253:270-284.
- [CrossRef] [Google Scholar]
- Cheadle, W.G., 2006. “Risk Factors for Surgical Site Infection,” https://home.liebertpub.com/sur, vol. 7, no. SUPPL. 1, Oct. 2006, doi: 10.1089/SUR.2006.7.S1-7.
- Chitosan based edible films and coatings: a review. Mater. Sci. Eng. C. 2013;33(4):1819-1841.
- [CrossRef] [Google Scholar]
- Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv.. 2010;7(4):429.
- [CrossRef] [Google Scholar]
- The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen.. 2001;9(3):178-186.
- [CrossRef] [Google Scholar]
- New biofunctional loading of natural antimicrobial agent in biodegradable polymeric films for biomedical applications. Int. J. Biomater.. 2016;2016
- [CrossRef] [Google Scholar]
- Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta Indica A. Juss grown in foothills of Nepal. African J. Biotechnol.. 2009;8(13):3084-3091.
- [CrossRef] [Google Scholar]
- Development of polymeric nanofibers blended with extract of neem (Azadirachta indica), for potential biomedical applications. Front. Mater.. 2022;9:1042304.
- [CrossRef] [Google Scholar]
- A study of neem leaves: Identification of method and solvent in extraction. Mater. Today Proc.. 2021;42:217-221.
- [CrossRef] [Google Scholar]
- Therapeutic potential of azadirachta indica (neem) and their active phytoconstituents against diseases prevention Shahzad SHARIF Mughal Lahore Garrison Education System. Artic. J. Chem. Chem. Sci. 2020
- [Google Scholar]
- Synergistic antibacterial screening of cymbopogon citratus and azadirachta indica: phytochemical profiling and antioxidant and hemolytic activities. ACS Omega. 2023;8(19):16600-16611.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of Azadirachta indica (Neem) against Pathogenic Microorganisms. J. Acad. Ind. Res.. 2014;3:327.
- [Google Scholar]
- Fiber from ramie plant (Boehmeria nivea): A novel suture biomaterial. Mater. Sci. Eng. c. Mater. Biol. Appl.. 2016;62:816-822.
- [CrossRef] [Google Scholar]
- Chronic surgical site infection due to suture-associated polymicrobial biofilm. Surg. Infect. (larchmt). 2009;10(5):457.
- [CrossRef] [Google Scholar]
- A. Kumar et al., “Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method,” Mol. 2023, Vol. 28, Page 887, vol. 28, no. 2, p. 887, Jan. 2023, doi: 10.3390/MOLECULES28020887.
- Effects of five extraction methods on total content, composition, and stability of flavonoids in jujube. Food Chem.. 2022;X, vol. 14:100287
- [CrossRef] [Google Scholar]
- H. A. Mohammad Ahamed, “Synthesis and Characterization of Neem Chitosan Nanocomposites for Development of Antimicrobial Cotton Textiles,” vol. 7.
- Novel high efficient coatings for anti-microbial surgical sutures using chlorhexidine in fatty acid slow-release carrier systems. PLoS One. 2014;9(7):e101426.
- [Google Scholar]
- Preparation, characterization and anticoagulation of curcumin-eluting controlled biodegradable coating stents. J. Control. Release. 2006;116(1):42-49.
- [CrossRef] [Google Scholar]
- Chitosan-coated surgical sutures prevent adherence and biofilms of mixed microbial communities. Curr. Microbiol.. 2021;78(2):502-512.
- [CrossRef] [Google Scholar]
- Effective utilization of fibre extracted from the waste neem tree twigs—a step towards sustainable practices. Biomass Convers. Biorefinery 2023
- [CrossRef] [Google Scholar]
- In vitro antibacterial efficacy of sutures coated with aloe vera and ciprofloxacin: a comparative evaluation. J. Pharm. Bioallied Sci.. 2019;11(Suppl 2):S164-S168.
- [CrossRef] [Google Scholar]
- Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: an update on molecular approach. 3 Biotech. 2021;11(4):178.
- [CrossRef] [Google Scholar]
- Development of herbal drug loaded antimicrobial silk suture. Indian J. Fibre Text. Res.. 2017;42:286-290.
- [Google Scholar]
- S. Viju and G. Thilagavathi, “Effect of chitosan coating on the characteristics of silk-braided sutures”, doi: 10.1177/1528083711435713.
- Preparation of soluble p-aminobenzoyl chitosan ester by Schiff’s base and antibacterial activity of the derivatives. Int. J. Biol. Macromol.. 2011;48(3):523-529.
- [CrossRef] [Google Scholar]
- Stage-controlled antibacterial surgical sutures based on curcumin@ZIF-8 functional coating for improved wound healing. Prog. Org. Coatings. 2023;184:107829
- [CrossRef] [Google Scholar]







