Translate this page into:
Extraction and purification of an antimicrobial bioactive element from lichen associated Streptomyces olivaceus LEP7 against wound inhabiting microbial pathogens
⁎Corresponding author. sureshssraja2020@gmail.com (Raja S.S. Suresh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The ever increasing spectrum of microbial disease and emergence of life threatening antibiotic resistant pathogen necessitates more intensive search for microbial antagonistic agents from diverse symbionts and habitats. Lichens from various niches and its associated cohabitates promise derivation of such antimicrobial compounds. In the present study conventionally and genetically identified Streptomyces olivaceus LEP7, one among 36 isolates from tree bark inhabiting lichen Leptogium sp., was assessed for its synthesis of antimicrobial bioactive compounds. Screening of antimicrobial activity was performed as per standard procedures. In vitro production by shake flask culture, solvent based extraction, TLC and GC–MS based separation, purification and identification revealed the presence of cyclopentene, an effective antimicrobial compound against wound inhabiting pathogens.
E. coli, P. aeroginosa, S. aureus, Klebsiella sp., Acinetobacter sp., and Candida sp. MIC value of partially purified compound was 7.81 μg/ml against E. coli and P. aeroginosa. In addition, TLC profile of the active extract displayed four different spot with Rf values of 0.64, 0.78, 0.93 and 1.00. This initial lead will add to antimicrobial arsenal on further research.
Keywords
Lichen
Streptomyces olivaceus
Antimicrobial activity
Cyclopentene
1 Introduction
Actinomycetes are Gram-positive filamentous bacteria with substrate and aerial mycelia. They are rich source of bio-active secondary metabolites like antibiotics, enzymes, enzyme inhibitors, antioxidants, and others having restorative importance (Barka et al., 2016). Fifty percent of approximately 22,000 microbial biologically potent secondary metabolites and 40% of 160 microbes originated antibiotics are from actinomycetes (Berdy, 2012). However, with often originating new uncharacterized etiological agent and reemergence of old agents with more threatening multiple drug resistance, there is an immediate need for discovery and development of new drugs. In order to combat this challenge, exploration of new strategies or a revisit of earlier methods is required. Search of special ecological niches along with new methods of isolation of novel species of actinobacteria may lead to the identification of new products (Xu et al., 2010). Marine habitats (Wagner et al., 2014), animal gut (Li et al., 2014), insects (Kim et al., 2014) and plants (Janso and Carter, 2010) are continuously assessed for novel actinomycetes members which produce equally novel bioactive compounds. Of utmost importance is the exploration of often neglected and poorly studied habitats for the derivation of novel bioactive producing taxa (Okoro et al., 2009; Hong et al., 2009). One such ignored niche is lichens which hold a prime place from ecological and biological point of view. Lichens do have valuable bioactive elements and such elements from lichen associated actinomycetes should provide novelty for the benefit of human kind.
Lichens inhabit around 8% surface of the earth (Ahmadjian, 1995) with nearly 18,450 species (Boustie and Grube, 2005; Feuerer and Hawksworth, 2007). Though ubiquitous, lichens are slow-growing organisms and hence serve as a niche for numerous microorganisms, actinomycetes in particular. Cardinale et al. (2006) have isolated seven different actinomycete strains and it’s belonging to five genera “Streptomyces, Streptosporangium, Curtobacterium, Cellulomonas, and Micromonospora” from 11 distinct lichen samples. Gonzalz et al., 2005 explored 337 actinomycetes from twenty five lichens acquired in temperate and cold regions and designated these strains to 11 different genera. In contrast, Parrot et al. (2015) have isolated ten actinomycetes species from marine and coastal ecosystem lichens. Similarly, according to Coley (1988) slow developing organisms inhabiting low-resource environments generate maximum amount of defensive metabolites in order to protect themselves from innumerable consumers. Thus on its part lichens are producing more than a 1000 diverse potent secondary metabolites. Lichens and their metabolites possess multiple biological activities such as antiviral (Dülger et al., 1997), antibacterial (Aslan et al., 2001), anticancer, allergenic, plant growth inhibitors, antiherbivores, and enzyme inhibitors (Dülger et al., 1998; Huneck, 1999). Furthermore, new antimicrobial drugs from lichen accompanying Streptomyces spp., including uncialamycin, cladoniamides A-G and angucycline, have been documented (Davies et al., 2005; Williams et al., 2008; Motohashi et al., 2010). Thus these symbionts of lichen and actinomycetes are naturally an abode for beneficial bioactive compounds and our aim is to assess bacterial partner’s role in producing such antimicrobial elements.
2 Materials and methods
2.1 Sample collection and surface sterilization
Lichen samples were attained from the tree barks of the botanical garden, Nilgiris, Tamilnadu. The collected lichen samples were washed in water, followed by surface sterilization with 70% ethanol for 30 s, 4% sodium hypo chloride for 3 min and lastly it was washed with sterile distilled water (Araújo et al., 2000). After sterilization 3 g of sample was homogenized with 30 ml of sterile distilled water in a surface sterilized motor and pestle.
2.2 Isolation of actinomycetes
The homogenized lichen samples were used for isolation of actinomycetes (Deng et al., 2015). One ml of homogenized sample was serially diluted up to 10-5 dilution.
100 µl of sample from each dilution was inoculated into starch casein nitrate (SCN) agar and incubated at 28 °C for 5 days. After incubation, the colonies on SCN agar plates were re-streaked in a fresh plate containing SCN medium to obtain a pure culture.
2.3 Screening of antimicrobial activity of actinomycete isolates
2.3.1 Screening by cross streak method
The cross streak procedure was accomplished to assess antimicrobial activity of actinomycetes (Ganesan et al., 2017). The Muller Hinton Agar (MHA) plates were prepared and the actinomycetes (LEP 01 to LEP 36) were streaked vertically and the test organisms, Staphylococcus aureus ATCC 25904, Escherichia coli ATCC 25922, Pseudomonas aeuroginosa ATCC 27584, Klebsiella sp. ATCC 700834, Acinetobacter sp. ATCC 55587, and Candida sp. ATCC 28528, were streaked perpendicular to actinomycete isolates. Inhibition of pathogen growth after incubation was recorded.
2.3.2 Screening by agar well diffusion method
The secondary screening was performed by agar well diffusion method (Nurkanto and Julistiono, 2014), in which the test organisms were swabbed on MHA plates. The well was prepared in the swabbed plates and 50 µl of the isolate LEP7 extract was added in the well and incubated for 24 h at 37 °C.
2.4 Minimum inhibitory concentration (MIC)
Determination of MIC values using the 96-well plate or broth micro-dilution is the most accurate method of determining the MIC (Duraipandiyan and Ignacimuthu, 2009). The 0.5 McFarland bacterial and fungi culture were prepared along with doubling dilutions of the extracted compound. Hundred microliters of a sterile MH broth was added to each well followed by addition of 50 µl of diluted extract compound. Five microliters of the both bacterial and fungal suspension was inoculated to each well. All the micro-titre plates were incubated at 37 °C for 24 h. The MIC was determined based on least concentration of the extracts that inhibited bacterial and fungal growth.
2.5 Molecular characteristics of isolate LEP7
The DNA was separated from LEP7 and Polymerase Chain Reaction (PCR) amplification performed with 16S rRNA primer 8F AGAGTTTGATCCTGGCTCAG and 1541R AAGGAGGTGATCCAGCCGCA (Ganesan et al., 2017). The DNA sequence was assessed through ABI PRISM BigDyeTM Terminator Cycle Sequencing Kits with AmpliTaq DNA polymerase (FS enzyme) (Applied Biosystems). The amplified PCR products were subjected to electrophoresis in an ABI 3730xl sequencer (Applied Biosystems) by re-suspending them in distilled water.
2.6 Production of bioactive metabolites from LEP7 strain
The isolate LEP7 sample was inoculated in the starch casein media and it was incubated with shaker at 29 °C for two days, then the 2% of pre-inoculation media was inoculated into the 8 L of production media and incubated at 29 °C with periodical shaking for 8 to 12 days (Singh et al., 2012).
2.7 Extraction of bioactive metabolites
The extraction was accomplished as per the procedure designated by Tanvir et al. (2016).The filtrate of LEP7 culture and the solvent ethyl acetate was mixed at 1:1 proportion and agitated overnight. Then the separation was done in the separating funnel and the upper layer containing bioactive compounds was collected. Finally the collected upper layer was concentrated in vacuum rotatory evaporator to separate bioactive compound from the ethyl acetate.
2.8 Separation of crude extract by thin layer chromatography (TLC)
The concentrated extracts were used for TLC analysis. TLC profile of the extract was performed on glass plate (dimensions 12 cm height and 4 cm width) coated with stationary phase silica gel (5 g silica and 0.1 g calcium sulphate in 10 ml distilled water). The concentrated extract was loaded on the TLC plate and plate was placed inside TLC chamber containing mobile phase (ethyl acetate: hexane (1:1)). The plates were observed under normal white light and UV light and also by exposure to iodine vapours (Kameník et al., 2010).
2.9 GC–MS analysis
GC–MS analysis of extract was performed using Perkin-Elmer GC Clarus 500 system and Gas chromatography mass spectrometry (GC–MS) fortified with an Elite-I, fused silica capillary column (30 mm × 0.25 mm 1D × 1 ìMdf, consists of 100% Dimethyl poly siloxane). The electron ionization system with ionizing energy of 70 eV was used for GC–MS detection. Mass spectrum was determined at 70 eV; with a scan interim of 0.5 s and the fractions between 45 and 450 Da. The final results were interpreted with National Institute of Standard and technology (NSIT) library.
3 Results and discussion
3.1 Actinomycetes isolation
The actinomycetes were isolated and identified with the aid of morphological characters based on the colony appearance, sporulation and pigment on the SCN agar medium. Total of thirty six actinomycete strains were isolated. All the pure cultures were designated as LEP1 to LEP36. Among 36, the strains LEP7 showed rapid growth and produced secondary metabolites on SCN agar medium. These isolates were preserved in starch casein agar medium at 29 °C. Most of the publications reveal that the optimum temperature and the suitable culture medium for the isolation of the actinomycetes were 28 °C and starch casein agar medium (Ganesan et al., 2017). Cardinale et al. (2006) have documented seven isolates of actinomycetes, associated with 5 genera “Micromonospora, Streptomyces, Cellulomonas, Curtobacterium, and Streptosporangium”, from various lichen samples. Parrot et al. (2015) have isolated ten actinomycetes species from coastal lichens. These findings are in accordance with our results wherein Streptomyces spp., predominates other actinomycetes.
3.2 Preliminary screening for antimicrobial activity
The isolate LEP 7 were subjected to primary screening by cross streak method to determine its ability to produce antimicrobial compounds against tested wound infection causing microbial pathogens. The isolate LEP7 showed a substantial antagonistic activity against the tested microbial pathogens. The observed results are depicted in Fig. 1. Earlier reports of Liu et al. (2017) and Jiang et al. (2015) supports our results that the actinomycetes particularly Streptomyces strains isolated from different sources exhibits antimicrobial activity. This finding reaffirms the fact that the actinomycetes isolated from the lichens exhibit antimicrobial activity against tested wound infection causing microbial pathogens.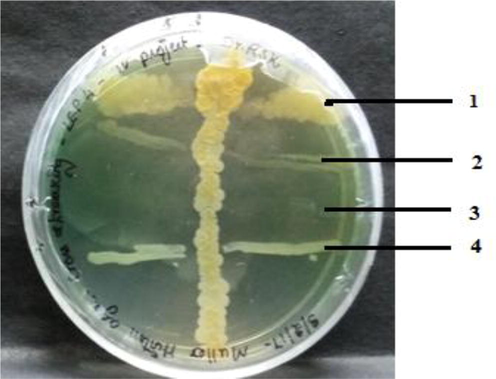
Cross streaking of LEP7 against microbial pathogens.
3.3 Morphological characterization of isolate LEP7
The colony morphology of the prospective isolate LEP 7 as per colony colour, mycelium nature, colony margin, colony appearance, Gram’s reaction and development of the colony on SCN agar are presented in Table 1 and Fig. 2.
Morphological Characteristics on Starch casein nitrate agar
Colony morphology
Aerial mycelium
Substrate mycelium
Gram reaction’s
Dry with pale
Pale white
Yellow
Gram positive log rods
Enzyme activities
Observations
Amylase
+
Protease
+
Chitinase
–
Catalase
+
DNase
+
Hydrolysis of esculin
+
Lecithin hydrolysis
–
H2S production
–
Nitrate reduction
+
Urea hydrolysis
+
Lipid hydrolysis
–
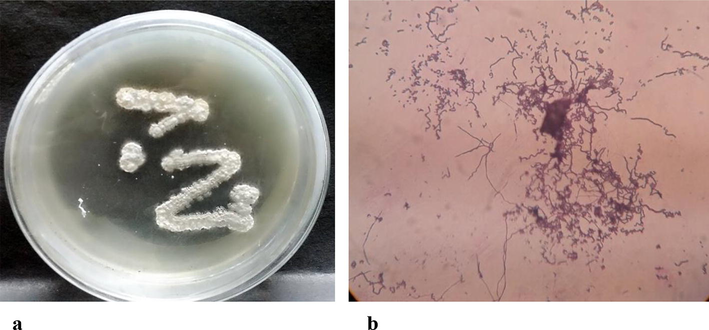
Growth and colony morphology of actinomycetes isolate LEP 7. (a)Isolate LEP7 on Starch casein nitrate agar (b) Microscopic appearance of spore (1000×).
3.4 Molecular identification of isolate LEP7
The PCR amplified 16S rDNA product showed that the isolate has the highest percentage of similarity with Streptomyces olivaceous and the evolutionary tree shown in Fig. 3 represent the phylogenetic affiliation of isolate LEP7 with other Streptomyces isolates. The results of molecular identification of isolate LEP7 by 16S rDNA gene sequencing has revealed Streptomyces olivaceous with 99% homology value (Fig. 3).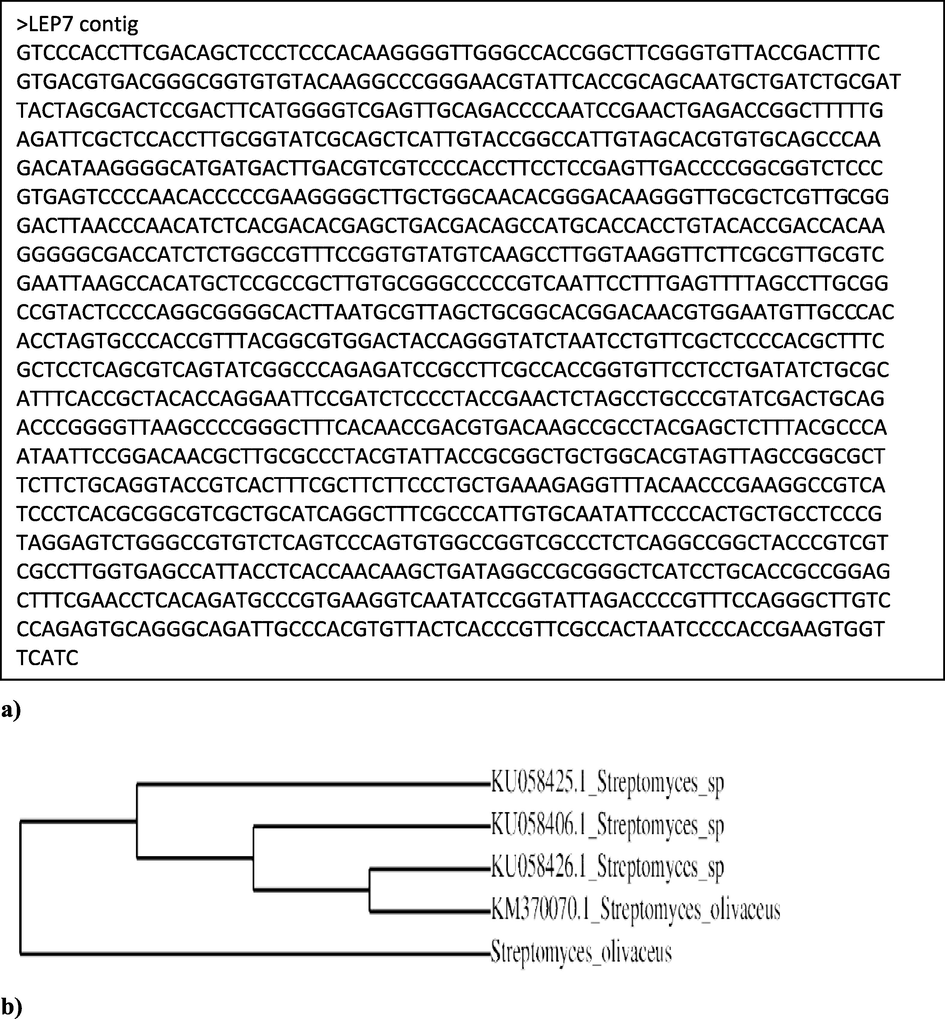
a) 16S rRNA sequence of isolate LEP7. b) Phylogenetic tree showing the relationship between the isolate LEP7 and identified as Streptomyces olivaceus.
3.5 Antimicrobial activity by agar well diffusion method
The culture free extract of isolate LEP7 was tested against all the wound infection causing microbial pathogens. The isolate LEP7 showed an effective zone of inhibition against E. coli, S. aureus, P. aeruginosa and Klebsiella sp. (Table 2 and Fig. 4). The zone of inhibition was observed between 6 and 21 mm. However, no zone of inhibition was observed against Acinetobacter sp., upto the concentration of 25 µl extracts. The results of antimicrobial properties of the isolate LEP7 supported are in accordance with the earlier results of Valan et al. (2012).
Tested pathogens
Zone of Inhibition(mm)/Concentration(µl)
5
15
25
50
Bacterial pathogens
S. aureus ATCC 25904
6.00 ± 0.2
8.00 ± 0.2
11.00 ± 0.2
13.00 ± 0.1
E. coli ATCC 25922
7.00 ± 0.4
10.00 ± 0.4
15.00 ± 0.4
21.00 ± 0.7
P. aeruginosa ATCC 25922
5.00 ± 0.2
7.00 ± 0.2
13.00 ± 0.6
17.00 ± 1.0
Klebsiella sp. ATCC 700834
6.00 ± 0.1
9.00 ± 0.4
12.00 ± 0.3
16.00 ± 0.5
Acinetobacter sp. ATCC 55587
ND
ND
ND
7.00 ± 0.2
Fungal pathogen
Candida sp. ATCC 28528
ND
ND
8.00 ± 0.2
11.00 ± 0.1
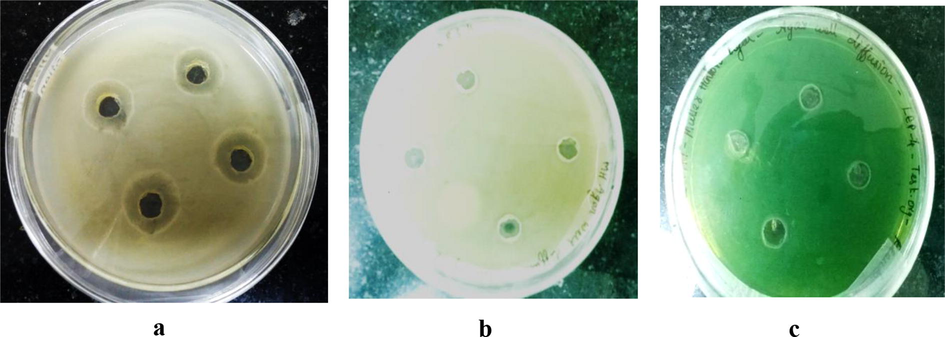
Antibacterial activity for isolate LEP7 against microbial pathogens by agar well diffusion. (a) E. coli ATCC 25922 b) S. aureus ATCC 25904 c) P. aeruginosa ATCC 27584.
3.6 Determination of MIC
The MIC endpoint was recorded after six hours of incubation and the lowest concentration at which there is no visible growth of the pathogenic strain was recorded as MIC value. The MIC values of tested bacterial pathogens are presented in Table 3.
Tested pathogens
MIC value (µg)
Bioactive extract
Streptomycin
Bacterial pathogens
S. aureus ATCC 25904
25.00
10.00
E. coli ATCC 25922
12.00
10.00
P. aeruginosa ATCC 25922
50.00
10.00
Klebsiella sp. ATCC 700834
25.00
10.00
Acinetobacter sp. ATCC 55587
>100
15.00
Fungal pathogen
Nystatin
Candida sp. ATCC 28528
>100
10.00
3.7 Production and extraction bioactive compound
The production was carried out for 15 days at 28 °C using starch casein nitrate broth as a production medium. The clear filtrate containing the active metabolite was then extracted with ethyl acetate at the level of 7:4 (v/v). The crude extracts were dissolved in ethyl acetate and it was used for further TLC and GC–MS analysis. Most of the literature studies state that ethyl acetate is the prominent solvent used for extraction of compound from fermentation medium and also the solvent for the extraction of antimicrobial bioactive compounds (Abdelfattah et al., 2016).
3.8 Purification of the bioactive compound
3.8.1 Thin layer chromatography
TLC profile of the active extract was accomplished with the mixture of hexane and ethyl acetate in 1:1 ratio and it gave the discrete profiling pattern. Using this mobile phase, concentrated crude extract was run in silica plate and four different spots were visualized on TLC from crude metabolite with Rf values of 0.64, 0.78, 0.93 and 1.00 respectively. The active fraction with Rf value 0.93 spot was further analyzed with GC–MS.
3.8.2 GC–MS analysis
The dynamic fraction of ethyl acetate extract was analyzed with GC–MS. The GC–MS result is shown in Fig. 5. GC–MS analysis detected different peaks and molecules. The most abundant compound was observed with retention time of 2.456. The compound was related with mass spectrum with NIST library constituents and it was found to be a cyclopentene. The compound cyclopentene (C6H8O) was identified from the bioactive extract of S. olivaceus LEP7 (Fig. 5). The unique new antimicrobial elements from lichen accompanying Streptomyces spp., containing uncialamycin, cladoniamides A-G, and angucycline, have been recorded by Davies et al. (2005), Williams et al. (2008) and Motohashi et al. (2010).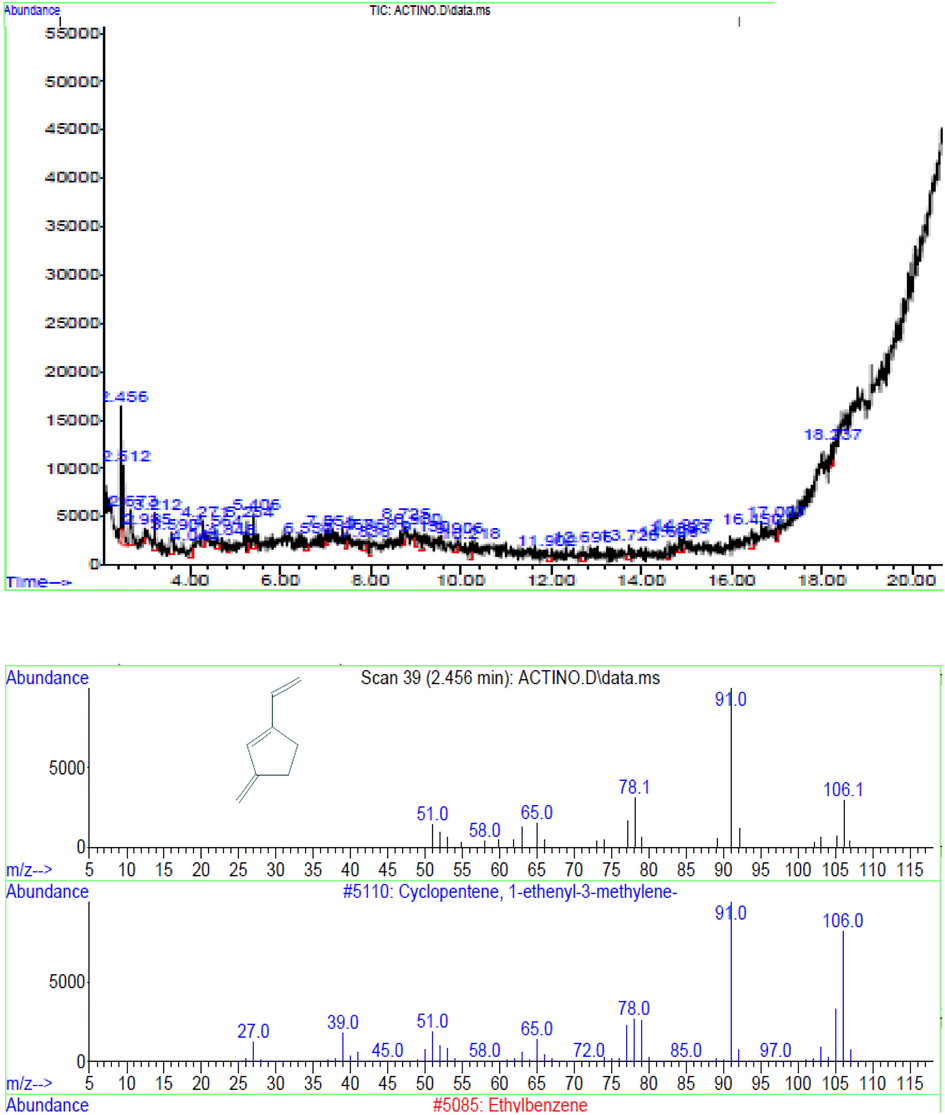
GC–MS analysis of the partially purified active fraction (a) GC chromatogram, b) Mass spectrum of respective retention time of the compound.
4 Conclusions
The findings in the current study reaffirms the fact that new niches like lichen need to be explored intensively for the bioactive element producing actinomycetes. Resource poor niches provide cohabitants, lichens and actinobacteria, the required environmental induction which will ensure a novel antimicrobial agaent. Our study provides a lead which with well-designed approach will result in new antimicrobial drug which is the need of the hour. Significant antimicrobial activity of the Streptomyces olivaceus against the tested wound infection causing microbial pathogens and derivation of cyclopentene from the Streptomyces olivaceus is the first step in this direction.
Acknowledgement
The authors extend their appreciation to the Researchers Supporting Project Number (RSP-2019/116) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation and characterization of marine-derived actinomycetes with cytotoxic activity from the Red Sea coast. Asian Pac. J. Trop. Biomed.. 2016;6:651-657.
- [Google Scholar]
- Isolation of endophytic actinomycetes from roots and leaves of maize (Zea mays L.) Braz. Arch. Biol. Technol.. 2000;43(4):1-5.
- [Google Scholar]
- A Study of Antimicrobial Activity of Some Lichens. Bull. Pure Appl. Sci.. 2001;20:23-26.
- [Google Scholar]
- Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev.. 2016;80:1-43.
- [Google Scholar]
- Thoughts and facts about antibiotics: where we are now and where we are heading. J. Antibiot.. 2012;65:385-395.
- [Google Scholar]
- Lichens-a promising source of bioactive secondary metabolites. Plant Genet. Resour.. 2005;3:273-287.
- [Google Scholar]
- Molecular analysis of lichen-associated bacterial communities. FEMS Microbiol. Ecol.. 2006;57:484-495.
- [Google Scholar]
- Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia. 1988;74:531-536.
- [Google Scholar]
- Isolation and characterization of halophilic bacteria and archaea from salt ponds in Hangu Saltworks, Tianjin, China. Chin. J. Oceanol. Limn.. 2015;33(4):862-866.
- [Google Scholar]
- Cetraria islandica (L.) Ach. Likenin Antimikrobiyal Aktivitesi. Tr. J. Biol.. 1998;22:111-118.
- [Google Scholar]
- Usnea florida (L) wig. Likeninin Antimikrobiyal Aktivitesi. Tr. J. Biol.. 1997;21:103-108.
- [Google Scholar]
- Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J. Ethnopharmacol.. 2009;123(3):494-498.
- [Google Scholar]
- Biodiversity of lichens, including a world-wide analysis of checklist data based on Takhtajan’s floristic regions. Biodivers. Conserv.. 2007;16:85-98.
- [Google Scholar]
- Antimicrobial activity of some actinomycetes from Western Ghats of Tamil Nadu, India. Alexandria J. Med.. 2017;53:101-110.
- [Google Scholar]
- Actinomycetes isolated from lichens: evaluation of their diversity and detection of biosynthetic gene sequences. FEMS Microbiol. Ecol.. 2005;54:401-415.
- [Google Scholar]
- Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs. 2009;7:24-44.
- [Google Scholar]
- The Significance of lichens and their metabolites. Naturwissenschaften. 1999;86:559-576.
- [Google Scholar]
- Biosynthetic potential of phylogenetically unique endophytic actinomycetes from tropical plants. Appl. Environ. Microbiol.. 2010;76:4377-4386.
- [Google Scholar]
- Influence of Titanium Carbide on the Three- Body Abrasive Wear Behaviour of Glass-Fabric Reinforced Epoxy Composites. Ameri. J. BioSci.. 2015;3:171-177.
- [Google Scholar]
- Ultra-high-performance liquid chromatography fingerprinting method for chemical screening of metabolites in cultivation broth. J. Chromatogr. A.. 2010;1217(51):8016-8025.
- [Google Scholar]
- Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem. Sci.. 2014;5:4333-4338.
- [Google Scholar]
- An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol.. 2014;32:834-841.
- [Google Scholar]
- Diversity, antimicrobial activity, and biosynthetic potential of cultivable actinomycetes associated with lichen symbiosis. Microb. Ecol.. 2017;74:570-584.
- [Google Scholar]
- New angucycline and a new butenolide isolated from lichen-derived Streptomyces spp. A. J. Antibiot.. 2010;63:545-548.
- [Google Scholar]
- Screening and study of antifungal activity of leaf litter actinomycetes isolated from Ternate Island, Indonesia. Asian Pac. J. Trop. Med.. 2014;7:S238-S243.
- [Google Scholar]
- Diversity of culturable actinomycetes in hyper-arid soils of the Atacama Desert, Chile. Antonie Van Leeuwenhoek. 2009;95:121-133.
- [Google Scholar]
- Littoral lichens as a novel source of potentially bioactive actinobacteria. Sci. Rep.. 2015;5:15839.
- [Google Scholar]
- Isolation and partial characterization of actinomycetes with antimicrobial activity against multidrug resistant bacteria. Asian Pac. J. Trop. Biomed.. 2012;2:S1147-S1150.
- [Google Scholar]
- Rare actinomycetes Nocardia caishijiensis and Pseudonocardia carboxydivorans as endophytes, their bioactivity and metabolites evaluation. Microbiol. Res.. 2016;185:22-35.
- [Google Scholar]
- Actinomycetes from Western Ghats of Tamil Nadu with its antimicrobial properties. Asian Pac. J. Trop. Biomed.. 2012;2(2):S830-S837.
- [Google Scholar]
- Dermacozines H-J isolated from a deep-sea strain of Dermacoccus abyssi from Mariana Trench sediments. J. Nat. Prod.. 2014;77:416-420.
- [Google Scholar]
- Cladoniamides A-G, tryptophan-derived alkaloids produced in culture by Streptomyces uncialis. Org. Lett.. 2008;10:3501-3504.
- [Google Scholar]
- Microbial Resources Science (2nd ed.). Beijing: Academic Press; 2010.







