Translate this page into:
Exploring various extracts and compounds of Grewia velutina as potential anticancer agents: An in vitro and in silico investigations
⁎Corresponding authors. mkhan3@ksu.edu.sa (Merajuddin Khan), khathlan@ksu.edu.sa (Hamad Z. Alkhathlan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study presents a comprehensive investigation into the potential anticancer properties and chemical constituents of an unexplored plant (Grewia velutina) extracts. Three distinct extracts of hexane (GVH), chloroform (GVC) and methanol(GVM) of the plant were used to check their anticancer activity against four prominent cancer cell lines (HepG2, DU145, Hela, A549), and the results were compared with the standard chemotherapeutic agent doxorubicin. Anticancer assays revealed that the GVC extract exhibited highest activity than others, with IC50 values of 61.06 µg/mL, 47.87 µg/mL, 88.76 µg/mL, and 87.99 µg/mL against HepG2, DU145, Hela, and A549 cell lines, respectively and reflected a comparable activity to doxorubicin, highlighting its potential as an effective anticancer agent. With the help of chemical profiling data, molecular docking studies were performed and this study suggested that ionol, present in GVC extract is the most active compound against all the cancerous cells.
Keywords
Grewia velutina
Molecular docking
Chemical profiling
Volatiles
Phytoconstituents
1 Introduction
Various plant species with potential anticancer properties have been explored for the prevention of cancer, particularly the plant species which are already being used as herbal medicines are largely considered for scientific endeavor (Khan et al., 2022a). Over the couple of decades, a considerable number of phytochemicals with slight structural modifications have been approved to combat cancer (Iqbal et al., 2018). Particularly, some specific groups of secondary metabolites like terpenes, flavonoids, alkaloids, lignans, saponins etc., have been proved to be highly effective in selectively inhibiting cancer cell proliferation and inducing their death (Agarwal et al., 2020). For example, a methoxylated flavone i.e., xanthomicrol extracted from Dracocephalum kotschyii Boisst has been found to inhibit the growth of a variety of malignant cells by inhibiting the proliferation of endothelial cell via decreased vascular endothelial growth factor activity (Fattahi et al., 2014). Similarly, in our recent study, we have demonstrated effective anticancer properties of seed extract of Citrullus colocynthis against several cell lines including HepG2, DU145, Hela, and A549 (Khan et al., 2023a). As a result of such medicinal properties, scientists across the globe routinely conduct a thorough initial screening of plant extracts with the hope of discovering potential anticancer agents from plant sources (Khan et al., 2022a).
Among different regions, the Arabian Peninsula is known for its rich variety of plants from different families which have long been used for medicinal purposes. However, most of these natural products, particularly plants-based materials have not been explored scientifically for their potential anti-cancer properties (Khan et al., 2023b). Indeed, extracts of different families of plants from Saudi Arabia with potential anticancer and other medicinal properties including Tiliaceae, Asteraceae, Lamiaceae etc., have been rarely explored for their anticancer potential (Ullah et al., 2020). One such plant is Grewia velutina belonging to the Grewia genus of Tiliaceae family, and is commonly found in tropical and subtropical regions, including some African, Arabian, Asian and Australian regions (Ullah et al., 2012). Due to effective medicinal properties, several species of the genus Grewia have long been utilized for different therapeutic purposes (Qamar et al., 2021, Suguna and Umesha 2022). For example, the roots and barks of G. tiliaefolia are used for the treatment of diarrhea, hypertension, skin, neurodegenerative and inflammatory bowel diseases etc., while the extracts of different parts of G. asiatica have shown effective antimicrobial and antioxidant properties (Shukla et al., 2016, Rajput et al., 2023).
Despite of diverse medicinal applications of different plants species of the genus Grewia, there is no recognized scientific reports on the therapeutic potential of G. velutina. Indeed, to the best of our knowledge, we did not find any single report in the literature about the phytochemical analysis and medicinal properties of this plant. Thus, detail phytochemical analysis and investigation of the anticancer properties of G. velutina is highly desirable. Therefore, in the current study we performed the detail phytochemical analysis of different extracts of G. velutina and assess their anticancer properties against various cancer cell lines such as, HepG2, DU145, Hela, and A549. Moreover, molecular docking study were also performed to identify the anticancer potential of the specific compounds present in different extracts.
2 Materials and methods
2.1 Plant material
The complete aerial components of G. velutina were gathered in May 2020 from the Al-Baha region, situated in the southwestern part of Saudi Arabia. The verification and authentication of the plant material were conducted by Dr. Rajakrishnan Rajagopal, a botanist from the herbarium division of King Saud University. A specific specimen labelled as 24,573 of G. velutina has been preserved within the herbarium division of King Saud University, Riyadh, Saudi Arabia.
2.2 Extraction of G. velutina aerial parts
The plant material of G. velutina aerial parts were extracted according to the method described previously (Khan et al., 2023a). Detailed extraction methodology is given in Supplementary Materials (Scheme S1).
2.3 GC and GC–MS analysis of G. velutina aerial parts extracts
GC–MS and GC–FID analysis of G. velutina extracts were done employing the procedure reported earlier (Khan et al., 2023a). Detailed methodology for the analysis is provided in Supplementary Materials (Scheme S2).
2.3.1 Calculation of linear retention indices (LRIs)
The linear retention indices (LRIs) values of chemical constituents of G. velutina extracts were determined according to the procedure described earlier (Khan et al., 2023a). Detailed methodology is provided in Supplementary Materials (Scheme S3).
2.3.2 Identification of chemical constituents of G. velutina aerial parts extracts
The identification of phytocomponents in the G. velutina extracts (GVH, GVC, and GVM) was achieved employing the methods as described previously (Khan et al., 2023a). Detailed methodology is provided in Supplementary Materials (Scheme S4).
2.4 Evaluation of anticancer activity
Anticancer activity of G. velutina extracts was assessed against the human lung adenocarcinoma cell line (A549), human hepatocarcinoma cell line (HepG2), human cervical cancer cell line (HeLa), and human prostate cancer cell line (DU145) using MTT assay as described earlier (Hansen et al., 1989, Khan et al., 2023a). Detailed methodology is provided in Supplementary Materials (Scheme S5).
2.5 Computational methodology for molecular docking study
The three-dimensional structure of the selected proteins considering various cancer cell lines [HepG2, A549, DU145, Hela] were modeled based on the available structures in the Protein Data Bank (https://www.rcsb.org) and previous studies. SWISS-MODEL (Waterhouse et al., 2018) web server was used to model the missing residues when the available structures comprise some unsolved region. The PDB IDs considered for different proteins are listed in Table-1 with their structures and supporting studies. Autodock tools were used to clean the structure followed by the removing of heteroatoms and to add necessary hydrogen atoms and charges (Baildya et al., 2021). Optimized structures of the major compounds present in the three distinct extracts (GVH, GVC, GVM) as per chemical profiling were used as ligands in the molecular docking study (Table 1).
S. No.
Cancerous cell
Receptor name
PDB Id
Reference
1
HepG2
Cysteine-aspartic acid protease-3 (CASPASE-3)
1GFW
(Siddiqui et al., 2021)
2
A549
Epidermal Growth Factor Receptor (Tyrosine kinase)
1 M17
(Muniaraj et al., 2018)
3
DU145
Vascular endothelial growth factor receptor 2 (VEGFR2)
2QU6
(Husain et al., 2021)
4
Hela
Refined 13pf Hela Cell Tubulin microtubule
6I2I
(Aydın et al., 2021)
A few major compounds (Table 2), present in GVC extract was also considered as GVC extract showed maximum activity against all the cancerous cells (Ghosh et al., 2022). Selected compounds were optimized using Gaussian 09 programme with B3LYP hybrid functional and localized 6-31G(d,p) basis set. Autodock Vina 6. (Seeliger and de Groot 2010) package was used for docking between the selected proteins and ligands. The docking score is a key screening characteristic and plays a crucial role to ensure screening success. PyMOL (Seeliger and de Groot 2010) Discovery Studio (Wang et al., 2015) and LigPlot + (Laskowski and Swindells 2011) platforms were used for docking result analysis and the type of interaction present between the protein and ligand.
S. No.
Name of the compounds
Structure of the compounds
Optimized structure of the compounds
1
10-Methyltetracosane


2
(8S,8aS)-3,8-Dimethyl-4-propan-2-ylidene-1,2,6,7,8,8a-hexahydroazulen-5-one


3
Ethyl-octadecanoate


4
Hexahydrofarnesyl-acetone

5
Ionol


6
Methyl-hexadecanoate


7
Methyl-linoleate


8
Sitosterol


9
Triacontane


10
Tricosane


3 Results and discussion
In order to determine chemical constituents of unexplored G. velutina and to conduct a comparative phytochemical analysis of various solvent extracts, aerial parts of the plant were extracted in three different solvents including hexane (GVH), chloroform (GVC) and methanol (GVM). Resultant extracts were analyzed using detailed GC–MS and GC-FID analysis. Table 3 exhibits the list of compounds identified in three different extracts of G. velutina.
No.
Compound
M.F.
CAS No.
R.T. (min)
LRI
GVH
%
GVC
%
GVM
%
1
3-Methyl-2-buten-1-ol
C5H10O
556–82-1
5.19
771
−
−
0.6
2
α-Pinene
C10H16
80–56-8
10.76
934
1.9
−
−
3
Isobutyl tiglate
C9H16O2
66917–61-1
17.09
1099
−
−
1.1
4
2-Methylacetophenone
C9H10O
577–16-2
18.53
1138
4.0
−
−
5
4-Oxoisophorone
C9H12O2
1125–21-9
18.84
1147
−
−
0.3
6
Ionol
C15H24O
128–37-0
31.04
1516
−
0.8
−
7
γ-Dodecalactone
C12H22O2
2305–05-7
35.84
1686
−
−
0.5
8
Aristolone
C15H22O
6831–17-0
38.62
1791
−
−
0.4
9
Hexahydrofarnesyl acetone
C17H18O2
68607–88-5
39.81
1838
−
1.7
1.7
10
Diisobutyl phthalate
C16H22O4
84–69-5
40.43
1863
−
−
0.6
11
Pentadecanoic acid
C15H30O2
1002–84-2
40.87
1881
−
−
0.9
12
Methyl hexadecanoate
C17H34O2
112–39-0
41.95
1925
3.4
1.6
4.7
13
n-Hexadecanoic acid
C16H32O2
57–10-3
42.76
1959
−
−
0.6
14
Hexadecanoic acid, ethyl ester
C18H36O2
628–97-7
43.57
1993
−
2.2
0.6
15
8,11-Octadecadienoic acid, methyl ester
C19H34O2
56599–58-7
45.88
2090
−
−
0.8
16
(Z)-9-Octadecenoic acid methyl ester
C19H36O2
112–62-9
46.03
2096
−
−
0.9
17
Methyl linoleate
C19H34O2
112–63-0
46.29
2107
−
0.9
3.2
18
Methyl octadecanoate
C19H38O2
112–61-8
46.60
2120
1.7
1.3
0.8
19
Ethyl octadecanoate
C20H40O2
111–61-5
48.22
2188
−
0.7
−
20
Tricosane
C23H48
638–67-5
50.65
2300
−
0.6
−
21
10-Methyltetracosane
C25H52
65820–50-0
53.93
2427
−
0.7
−
22
1-[(2-Hydroxyphenyl)thioxomethyl]pyrrolidine
C11H13NOS
1000100–20-2
54.64
2457
1.0
1.2
17.9
23
2,6,10,14,18-Pentamethyl-2,6,10,14,18-eicosapentaene
C25H42
75581–03-2
57.81
2589
2.3
0.7
−
24
Hexacosane
C26H54
630–01-3
58.51
2600
1.7
1.2
−
25
11-Butyldocosane
C26H54
13475–76-8
61.14
2729
3.0
1.5
−
26
Benzo[h]quinoline, 2,4-dimethyl-
C15H13N
605–67-4
64.25
2859
2.8
2.0
−
27
Nonacosane
C29H60
630–03-5
65.46
2900
13.3
10.0
16.2
28
(8S,8aS)-3,8-Dimethyl-4-propan-2-ylidene-1,2,6,7,8,8a-hexahydroazulen-5-one
C15H22O
6754–66-1
66.31
2945
4.4
7.0
2.2
29
Triacontane
C30H62
638–68-6
67.41
3000
58.9
57.0
41.1
Total identified
98.4
91.1
95.1
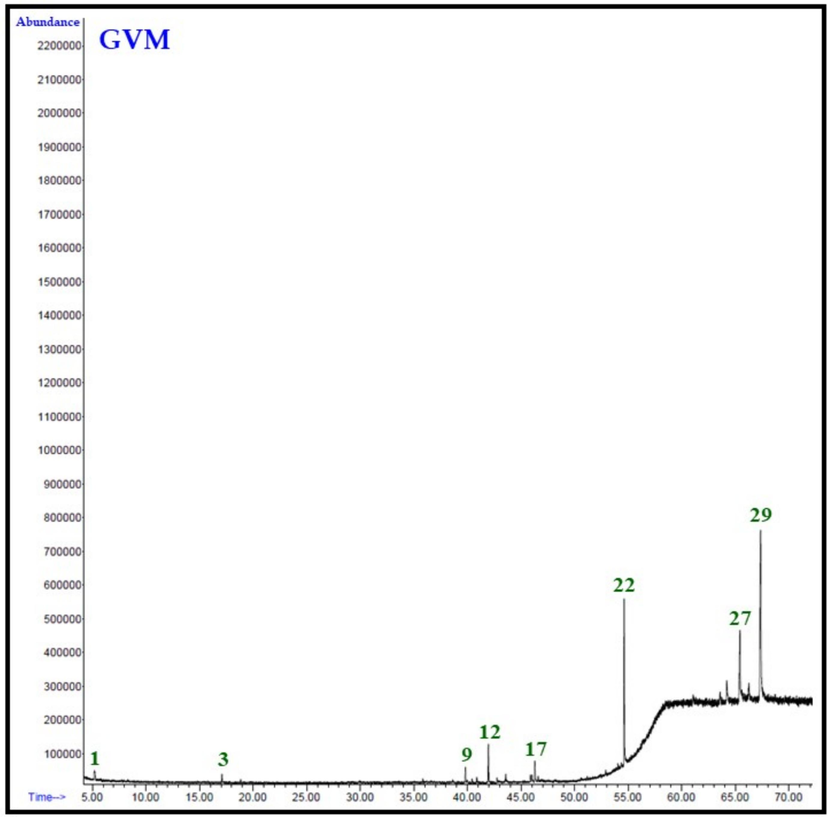
Overall 29 compounds were identified from the GVH, GVC and GVM extracts of G. velutina. Notably, each extract does not contain all the identified (29) compounds, and the number of compounds and their quantities varied in all extracts. For example, GVH extract consists of only 12 compounds, while GVC and GVM contain 17 and 19 different types of phytomolecules, representing 97.4 %, 91.1 %, and 96.2 % of the total extract compositions, respectively. The total ion chromatogram from the GC analysis of each extracts of the aerial parts of G. velutina are given in Fig. 1.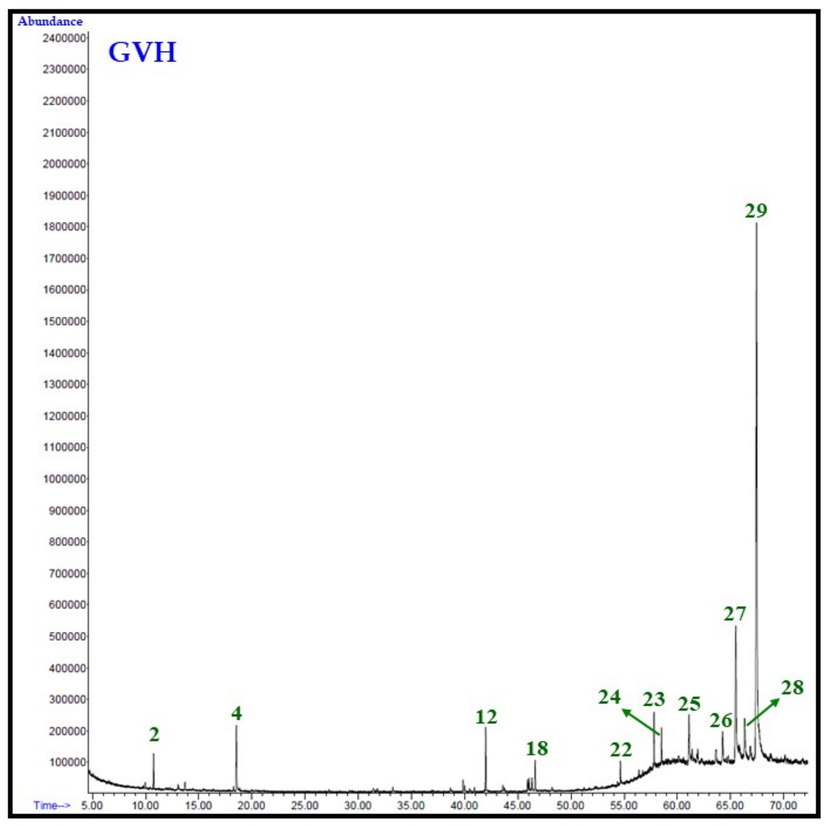
Total ion chromatogram (TIC) of n-hexane (GVH), chloroform (GVC), and methanol (GVM) extracts of aerial parts of G. velutina.

Total ion chromatogram (TIC) of n-hexane (GVH), chloroform (GVC), and methanol (GVM) extracts of aerial parts of G. velutina.
Total ion chromatogram (TIC) of n-hexane (GVH), chloroform (GVC), and methanol (GVM) extracts of aerial parts of G. velutina.
It is interesting to note that in all the three extracts, the major constituent is triacontane, which is present in ∼ 59, 57, and 47 % in GVH, GVC and GVM extracts, respectively. While the second major component in these extracts is nonacosane which is present in the range of 13 to 17 %. Remarkably, another significant constituent found in GVM extract is 1-[(2-Hydroxyphenyl)thioxomethyl]pyrrolidine, accounting for approximately 18 % according to Table 3. Conversely, in GVC and GVH, this compound was detected in mere 1 % of the sample. There were some other notable compounds present in appreciable amounts in these three extracts. For instance, GVH extract contained 2-methylacetophenone, methyl hexadecanoate, 11-butyldocosane, and (8S,8aS)-3,8-dimethyl-4-propan-2-ylidene-1,2,6,7,8,8a-hexahydroazulen-5-one at concentrations ranging from 3 % to 5 %. GVM included methyl hexadecanoate, methyl linoleate, and (8S,8aS)-3,8-dimethyl-4-propan-2-ylidene-1,2,6,7,8,8a-hexahydroazulen-5-one between 2 % to 5 %, while GVC exhibited the presence of hexadecanoic acid ethyl ester, benzo[h]quinoline, 2,4-dimethyl-, and (8S,8aS)-3,8-dimethyl-4-propan-2-ylidene-1,2,6,7,8,8a-hexahydroazulen-5-one within the range of 2 % to 7 %.
It is quite fascinating to note that two compounds, α-pinene (1.9 %) and 2-methylacetophenone (4.0 %), were exclusively present in GVH extract. Conversely, four compounds, namely ionol (0.8 %), ethyl octadecenoate (1.3 %), tricosane (0.6 %), and 10-methyltetracosane (0.7 %), were solely detected in GVC extracts. On the other hand, three compounds, namely n-hexadecanoic acid (0.6 %), 8,11-octadecadienoic acid methyl ester (0.8 %), and (Z)-9-octadecenoic acid methyl ester (0.9 %), were specific to GVM extracts.
During the current study, these three extracts of G. velutina from Saudi Arabia i.e., GVH, GVC and GVM, were subjected to in vitro anticancer evaluation against different types of cancer cell lines including, HepG2 (hepatic cancer cells), DU145 (prostate cancer cells), Hela (cervical cancer cells) and A549 (human lung cancer cells). On the other hand, doxorubicin, which is commonly prescribed anticancer medicine, is used as a positive control for the study (Table 4). Results revealed significant variations in IC50 values, highlighting the potential of G. velutina as a source of bioactive compounds with anticancer properties. Results are expressed as mean ± SD, NA=No activity, NS=Not soluble.
Tested Extracts of G. velutina aerial parts
IC50 (µg/mL)
HepG2
DU145
Hela
A549
GVM
NA
121.27 ± 7.56
NA
NA
GVH
NS
NS
NS
NS
GVC
61.06 ± 1.11
47.87 ± 3.27
88.76 ± 0.91
87.99 ± 0.12
Doxorubicin
0.39 ± 0.012
0.19 ± 0.01
0.43 ± 0.71
0.29 ± 0.16
It is significant to mention that the GVC extract demonstrated notable anticancer activity across all tested cell lines, exhibiting IC50 values of 61.06 ± 1.11 µg/mL (HepG2), 47.87 ± 3.27 µg/mL (DU145), 88.76 ± 0.91 µg/mL (Hela), and 87.99 ± 0.12 µg/mL (A549). These findings suggest the presence of potent cytotoxic compounds within the GVC extract that merit further investigation for potential therapeutic applications. In contrast, the GVM extract displayed selective cytotoxicity only against DU145 (IC50 ∼ 122 µg/mL) cell line, indicating it might be useful in prostate cancer treatment. However, it exhibited no activity against HepG2, Hela, and A549 cell lines. Similarly, the GVH extract exhibited no activity against any of the tested cell lines, indicating a lack of potency against the tested cancer cell lines. It is noteworthy that upon comparing the anticancer activity of the GVC extract with that of standard anticancer agents, it was observed that the anticancer activity of the GVC extract was comparable to that of the standard chemotherapeutic agent doxorubicin at the extract level, particularly against DU145 and HepG2 cell lines. This implies that G. velutina extracts, specifically GVC, may harbor some bioactive compounds with cytotoxic potential.
In order to corelate the anticancer activities of all the extracts with their phytochemical constituents, we compared the type of major constituents present in each extract. Notably, the major constituents (more than 5 % of total amount of constituents) are same in all the extract i.e., triacontane is the most abundant constituent, while nonacosane is the second largest component in GVH and GVC. While, GVM consisted of 1-[(2-hydroxyphenyl)thioxomethyl]pyrrolidine as the second largest component. Since, the highest anticancer activity is exhibited by GVC, there is a minimum chance of the role of major constituents of G. velutina towards the contribution to the anticancer properties of this extract. To further confirm this claim, literature was explored to find reports on the anticancer properties of major components, including triacontane and nonacosane which are present in large quantities in all the extracts. However, no references were found which are directly linked to the anticancer properties of these components. On the other hand, the GVC consists of several minor components which are only unique to this particular extract, such as, ionol, ethyl octadecanoate, tricosane, 10-methyltetracosane. Possibly, these constituents may have played a crucial role in the anticancer properties of GVC. To confirm this assessment, we have performed in silico studies to predict cell permeability of G. velutina constituents.
3.1 Analysis from molecular docking study
The molecular docking results are represented in Table 5 which reflects the Binding affinities of the selected compounds (Fig. 2) against the selected proteins. The more negative binding energy implies best docking score with greater binding affinity (Dutta et al., 2021). Binding pose and binding score are the significant tools to check the ability of a compound to block that protein considering the related disease. As per the docking scores, ionol showed very good activity against the four selected proteins.
S. No.
Compounds Name
Docking Score
HepG2
(PDB Id:1GFW)
A549
(PDB Id:1M17)
DU145
(PDB Id:2QU6)
Hela
(PDB Id:6I2I)
1
10-Methyltetracosane
−7.2
−7.1
−4.1
−5
2
Dimethyl-4-propan
−2.8
−4
−3.8
−3.9
3
Ethyl-octadecanoate
−3.8
−5.1
−2.8
−3.2
4
Hexahydrofarnesyl-acetone
−1.5
−1.8
−3.2
−2.4
5
Ionol
−6.8
−8.8
−7
−7.9
6
Methyl-hexadecanoate
−3.3
−3.2
−1.2
−2.8
7
Methyl-linoleate
−3.2
−3.8
−6.7
−4.0
8
Sitosterol
−2.1
−2.2
−3.1
−3.2
9
Triacontane
−3.5
−3.2
−2.7
−3.3
10
Tricosane
−3.5
−5
−3.1
−4.7

Docked structure of ionol and 10-methyltetracosane with CASPASE-3 (HepG2).
Experimental studies revealed that GVC extract showed highest activity against all the cancerous cells and ionol is present only in GVC extract. GVH extract is totally inactive whereas GVM extract only showed weak activity against DU145. Another compound methyl-linoleate also reflects good binding score (−6.7) against DU145, present in very large amount in GVM extract. 10-methyltetracosane is also very active compound against HepG2 (−7.2) and A549 (−7.1) and it is only present in GVC extract.
Ionol forms H-Bonding with CASPASE-3 whereas non-polar interactions dominate in the 10-methyltetracosane and CASPASE-3 docked structure (Fig. 3). Hydrophobic environment also helps to create a strong protein–ligand interaction ‘Ionol-Tyrosine kinase’ and ‘10-Methyltetracosane-Tyrosine kinase’ docked structure (Fig. 3).
Docked structure of ionol and 10-methyltetracosane with Tyrosine kinase (A549).
Both ionol and methyl-linoleate forms H-bonding with VEGFR but non-polar interaction only present in case of ‘ionol-HeLa’ docked structure (Fig. 4). Overall, H-bonding and non-polar interaction are majorly responsible for ionol, 10-methyltetracosane and methyl-linoleate to block the selected proteins.
Docked structure of ionol and methyl-linoleate with VEGFR (DU145) and HeLa.
Hence, as per the molecular docking study ionol is the most active compound. 10-methyltetracosane and methyl-linoleate are also showed significant activity. In silico study clearly identifies the specific compounds of Grewia velutina, majorly responsible for the anticancer activities as obtained from the experimental observations and the experimental observation is well supported by the in silico study.
4 Conclusion
For the first time, this study provides a comprehensive exploration of the phytochemical composition and anticancer properties of different extracts derived from the aerial parts of G. velutina. These extracts showcased significant variations in their chemical profiles, particularly evident from the minor constituents. Notably, the GVC extract revealed several minor components unique to its composition. The difference was also clearly reflected by the results of anticancer properties i.e., only the GVC extract has showed significant activity, with IC50 values ranging from 47 to 88 µg/mL against all the studied cell lines. Furthermore, the molecular docking studies have identified ionol as the most active compound which is only found in GVC as minor component.
CRediT authorship contribution statement
Merajuddin Khan: Writing – review & editing, Writing – original draft, Validation, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Tanmoy Dutta: Supervision, Software, Formal analysis, Data curation. Mujeeb Khan: Validation, Data curation, Conceptualization. Khaleel Al-hamoud: Visualization, Validation, Methodology, Formal analysis. Shams Tabrez Khan: Visualization, Validation, Data curation. Mahmood M.S. Abdullah: Validation, Formal analysis, Data curation. Hamad Z. Alkhathlan: Supervision, Resources, Project administration, Funding acquisition.
Acknowledgments
This work was funded by the Researchers Supporting Project number (RSPD2024R817), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol. Adv.. 2020;38:107337
- [Google Scholar]
- In–vitro anticancer and antibacterial activities of brominated indeno [1, 2-b] qinoline amines supported with molecular docking and MCDM. ChemistrySelect. 2021;6(13):3286-3295.
- [Google Scholar]
- Screening of potential drug from Azadirachta Indica (Neem) extracts for SARS-CoV-2: An insight from molecular docking and MD-simulation studies. J. Mol. Struct.. 2021;1227:129390
- [Google Scholar]
- Dutta, T., S. Ghorai, A. A. Khan, et al., 2021. Screening of potential anti-HIV compounds from Achyranthes aspera extracts for SARS-CoV-2: An insight from molecular docking study. Journal of Physics: Conference Series, IOP Publishing.
- Xanthomicrol: A comprehensive review of its chemistry, distribution, biosynthesis and pharmacological activity. Mini Rev. Med. Chem.. 2014;14(9):725-733.
- [Google Scholar]
- CO2 activation on transition metal decorated graphene quantum dots: an insight from first principles. Physica E. 2022;135:114993
- [Google Scholar]
- Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods.. 1989;119(2):203-210.
- [Google Scholar]
- Synthesis, in vitro cytotoxicity, ADME, and molecular docking studies of benzimidazole-bearing furanone derivatives. J. Chin. Chem. Soc.. 2021;68(2):362-373.
- [Google Scholar]
- Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother.. 2018;108:752-756.
- [Google Scholar]
- Screening of potential cytotoxic activities of some medicinal plants of Saudi Arabia. Saudi J. Biol. Sci.. 2022;29(3):1801-1807.
- [Google Scholar]
- Diversity of Citrullus colocynthis (L.) Schrad seeds extracts: detailed chemical profiling and evaluation of their medicinal properties. Plants.. 2023;12(3):567.
- [Google Scholar]
- Chemical characterization and chemotaxonomic significance of essential oil constituents of matricaria aurea grown in two different agro-climatic conditions. Plants.. 2023;12(20):3553.
- [Google Scholar]
- LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. ACS 2011 Publications
- [Google Scholar]
- In silico and in vitro studies on Lyngbya majuscula using against lung cancer cell line (A549) Pharmacogn. J.. 2018;10(3)
- [Google Scholar]
- The chemical composition and health-promoting effects of the Grewia species—A systematic review and meta-analysis. Nutrients. 2021;13(12):4565.
- [Google Scholar]
- Neuroprotective activity of novel phenanthrene derivative from Grewia tiliaefolia by in vitro and in silico studies. Sci. Rep.. 2023;13(1):2444.
- [Google Scholar]
- Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des.. 2010;24(5):417-422.
- [Google Scholar]
- Antioxidant, antimicrobial activity and medicinal properties of Grewia asiatica L. Med. Chem.. 2016;12(3):211-216.
- [Google Scholar]
- Cytotoxicity of Moringa oleifera fruits on human liver cancer and molecular docking analysis of bioactive constituents against caspase-3 enzyme. J. Food Biochem.. 2021;45(5):e13720.
- [Google Scholar]
- Phytochemical composition, pharmacological properties, and therapeutic activities of genus: Grewia. Journal of Pharmacognosy and Phytochemistry.. 2022;11(4):263-272.
- [Google Scholar]
- A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci.. 2020;27(10):2706.
- [Google Scholar]
- Ethnic uses, pharmacological and phytochemical profile of genus Grewia. J. Asian Nat. Prod. Res.. 2012;14(2):186-195.
- [Google Scholar]
- Interaction of α-cyperone with human serum albumin: Determination of the binding site by using Discovery Studio and via spectroscopic methods. J. Lumin.. 2015;164:81-85.
- [Google Scholar]
- SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res.. 2018;46(W1):W296-W303.
- [Google Scholar]
Appendix A
Supplementary material
The following supporting information can be downloaded at: www.mdpi.com/xxx/s1, Figure S1: title; Table S1: title; Video S1: title. Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103427.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







